Abstract
Alcoholic beverages are one of the most popular drinks in the world, but ethanol can induce significant liver pathology. We have found that ethanol treatment results in autophagy activation, which is at least in part due to the inhibition of mTOR signaling by reactive oxygen species. Autophagy is important in limiting liver injury and hepatocyte apoptosis by removing damaged mitochondria and accumulated lipid droplets, the two most important culprits of ethanol pathogenesis. The selectivity of ethanol-induced autophagy toward these two targets without affecting other cellular substances, such as long-lived proteins, is remarkably in line with its protective effects. However, we still do not quite understand how this selectivity is determined and how the selection process is accomplished, although evidence from other studies indicates that mitophagy involves distinct molecular steps of mobilization of the autophagy machinery and of preparation of mitochondria for recognition. The avoidance of mistargeting to other cellular components may involve additional mechanisms related to how autophagosomes might form in relation to their targets. Ethanol-induced selective mitophagy and lipophagy thus provides an excellent model to study these events in a pathophysiology-relevant context. Most importantly, the understanding of the mechanisms can bring forward new therapeutic modalities to improve the disease outcome.
Key words: selective autophagy, mitophagy, lipophagy, ethanol, liver
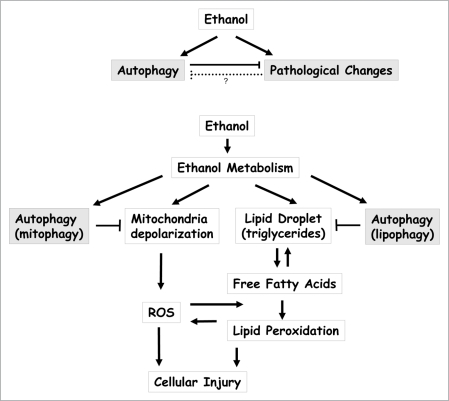
The mechanism of alcoholic liver injury is complicated but may be reversible at an early stage. Signs such as hepatic steatosis and hypoglycemia can be transient, and only minor hepatocyte death may be induced in acute binge drinking. These changes become progressively severe and eventually irreversible in chronic abuse, with signs of fibrosis, carcinogenesis and liver failure. While much effort has been devoted to the understanding of the toxic effects of ethanol and its damaging mechanisms, limited information is available regarding the host defense mechanisms against these effects. Characterization of the protective role of autophagy in this setting is thus of particular interest (Fig. 1A).
Figure 1.
Autophagy mitigates ethanol-induced liver pathogenesis. (A) Acute treatment of ethanol triggers a positive autophagy flux, which would help to protect against ethanol-induced pathologenesis. Inhibition of autophagy leads to greater liver injury and hepatocyte apoptosis. The long-term effect of chronic use of ethanol on autophagy is less clear, but ethanol may induce pathological changes that can compromise the ability of cells to launch or to execute autophagic function, although direct evidence is still lacking. (B) Two major pathological effects of ethanol in hepatocytes are mitochondria depolarization and steatosis, which can form a vicious cycle in promoting ROS generation and lipid peroxidation, culminating in cell death. By selectively removing depolarized damaged mitochondria and lipid droplets, autophagy can effectively arrest this disease process, thus protecting hepatocytes and reducing liver injury.
A variety of criteria indicate that autophagy activated in the acute ethanol condition leads to positive increases in autophagy flux or lysosomal degradation. It has been noted, however, in mice chronically fed with ethanol-containing food, there is a reduction in lysosomal function and an increase in ubiquitinated protein aggregates. Although direct evidence has yet to be sought, the functional status of autophagy may alter during the development of alcoholic liver disease from the acute to the chronic status. Gradual loss of autophagy function during the disease process may occur and can in turn contribute to the exacerbation and the final presentation of the disease. This raises an interesting issue from the clinical point of view that an early intervention with autophagy-enhancing agents may help to block this vicious cycle and promote recovery following the cessation of alcohol drinking (Fig. 1A).
What is unique for acute ethanol-induced autophagy is the strong selectivity toward mitochondria and lipid droplets. We have observed no changes in the basal level of long-lived protein degradation in ethanol-treated hepatocytes. Ethanol does not affect starvation-induced long-lived protein degradation, either. This is important in the context of ethanol-induced liver pathology and the protective role of autophagy as mitochondria damage, oxidative stress and lipid peroxidation all seem to contribute to the pathogenesis (Fig. 1B). In this context, removing damaged mitochondria and accumulated lipid droplets is most pertinent for protecting host cells from injury, whereas degrading long-lived proteins would be important for other scenarios, such as nutrient deprivation. Although autophagy had been thought to be mainly a bulk degradation process, selective degradation of specific cellular components has now been widely recognized. A clear demonstration of such a process in an in vivo system relevant to mammalian pathology by this study further enhances the understanding of the role of selective autophagy in modulating disease processes.
While we can understand the potential importance of mitophagy and lipophagy in ethanol-induced pathogenesis, we do not quite understand how the selectivity is determined and how the selection process is accomplished, including mobilization of autophagy machinery and target recognition. These events are generally applicable to selective autophagy toward all types of substrates, but are often not clearly differentiated. The difficulty is of course that they are often interconnected. Nevertheless, in our opinion, it is important to distinguish events that lead to the mobilization of autophagy machinery, the recognition of specific targets and the assurance of the selectivity with minimal engulfment of other cellular components.
Ethanol-induced mitophagy can be inhibited by antioxidants. Reactive oxygen species (ROS) could be generated during ethanol metabolism and/or due to mitochondria dysfunction. But ROS seem to be involved only in the mobilization of autophagy machinery, at least in part via inhibition of mTOR signaling. ROS may not be important for the recognition of the engulfed targets by the autophagosomal membranes as shown in a model of mitophagy triggered by a mitochondria uncoupler, carbonyl cyanide m-chlorophenylhydrazone (CCCP). In this case, the recognition of the mitochondria to be removed needs a priming process in which mitochondria are modified via the function of Parkin, an E3 ligase, resulting in ubiquitination of certain mitochondria proteins, which can be bound to the autophagosome-associated p62/SQSTM1. Conversely, these molecules do not seem to be engaged in the mobilization of the autophagy machinery. The two separate processes are nevertheless coupled; Nix, a BH3-only Bcl-2 family protein, is important for CCCP-induced mitochondria depolarization, which in turn leads to generation of ROS, and Parkin recruitment to the depolarized mitochondria. How this type of coordination occurs during lipophagy is not known, but similar mechanisms of target recognition based on ubiquitinated molecules and p62-type adaptors may also exist.
The understanding of these mechanisms can bring forward new therapeutic modalities to improve the disease outcome. While enhancing autophagy via general autophagy-inducing agents, such as rapamycin, has led to the reduction of cellular injury in ethanol intoxication and in other scenarios, this approach may have limitations. Specific enhancement of selective autophagy relevant to particular settings may be the ultimate choice for a better control of individual disease processes.
Punctum to: Ding WX, Li M, Chen X, Ni HM, Gao W, Lu B, et al. Autophagy reduces acute ethanol-induced hepatotoxicity and steatosis in mice. Gastroenterology. 2010;139:174052. doi: 10.1053/j.gstro.2010.07.041.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/14347