Abstract
Multibody modelling is underutilised in craniofacial analyses, particularly when compared to other computational methods such as finite element analysis. However, there are many potential applications within this area, where bony movements, muscle forces, joint kinematics and bite forces can all be studied. This paper provides an overview of recent, three-dimensional, multibody modelling studies related to the analysis of skulls. The goal of this paper is not to offer a critical review of past studies, but instead intends to inform the reader of what has been achieved with multibody modelling.
Keywords: biomechanics, computational modelling, jaw, multibody dynamics analysis, muscle, muscle activation, skull
Introduction
Movements of the head and jaw are functionally complex. Multiple muscle groups interact to produce forces on bony structures that are constrained at joints with multiple degrees of freedom. It is difficult, and often impossible, to measure directly the many physical variables that define bone and muscle movements, and when data is experimentally determined they are often limited or incomplete. Virtual modelling can be used to clarify relationships between structure and function, and may be the only practical way to study variables such as muscle tensions, joint loading and specific kinematics. Multibody dynamics analysis (MDA) provides a means to analyse movements and forces associated with the neck, head and lower jaw. Once confined to engineering, multibody modelling is now becoming more accessible to anatomists and biomechanists, either through multidiscipline interactions or by the use of specialist multibody software designed for simulating anatomical structures (e.g. adams lifemod, anybody, simm). Here MDA is introduced and an overview given of its applications within craniofacial biomechanics. The multibody descriptions will be confined to rigid body mechanics, whereby a structure undergoes so little deformation that it has no effect on gross body motion. The complexities, in terms of derivation of dynamic equations and matrices linked to multibody analyses, are beyond the scope of this paper, and the reader is urged to consult specialist literature dealing solely with the dynamics of multibody systems for more information (Shabana, 2005).
A multibody system is defined as a collection of rigid and/or flexible bodies that are constrained by kinematic joints and contacts, and eventually acted upon by a set of internal and/or external forces. Landmark data represent the anatomical features of interest in the mechanical system being analysed, which in masticatory analyses typically include muscles, ligaments, temporomandibular joints, and the mandible and cranium. Representative material properties can be specified at and between the landmarks that define muscle, ligament and joint behaviour. In essence, the dynamic behaviour of a multibody system is described by solving equations of motion that relate back to Newton's laws in classical mechanics. Re-formulations and advances on these classical methods allow for easier solutions of systems with multiple, interacting bodies; for example Newton–Euler and Euler–Lagrange approaches (for more information see for example Hahn, 2002; Shabana, 2005). Musculoskeletal and, indeed, dynamic problems in general can be divided into two groups: forward and inverse dynamics. Simply, forward dynamics computes rigid body motion based on applied muscle forces (either experimentally derived or previously predicted), whereas inverse dynamics utilises body motions and external forces to calculate the muscle forces. Inverse dynamics musculoskeletal modelling does, however, have inherent problems, most notably that the number of unknowns (e.g. muscle and joint forces) exceeds the number of equilibrium equations derived to solve for the unknowns. This is commonly known as a redundancy or an indeterminacy problem associated with muscle recruitment (e.g. Glitsch & Baumann, 1997; Koolstra & van Eijden, 2001; Rasmussen et al. 2001; Bei & Fregly, 2004; de Zee et al. 2007), whereby there are many possible muscle recruitment combinations that satisfy a particular motion or force criteria. For a solution to this problem one turns to optimisation, where a unique muscle recruitment combination that relates to some biological or neural condition is sought, for example minimisation of energy or joint loading. The exact answer to this optimisation problem is not known, but studies have shown satisfactory comparisons between actual and predicted muscle activation patterns using such criteria (Koolstra et al. 1988; Iwasaki et al. 2003).
Mathematical modelling
There are a good range of published articles involving mathematical models of the skull and lower jaw, a selection of which will be presented here. Two-dimensional static analyses are normally associated with peak bite force estimates and appear with relative frequency in the literature (e.g. Greaves, 1978; Throckmorton & Throckmorton, 1985; Sinclair & Alexander, 1987; Cleuren et al. 1995; Herrel et al. 1998; Spencer, 1998). Such analyses can provide useful comparative data on bite performance and the mechanical efficiency of the jaw muscles, in terms of muscle force to bite force calculations. However, this overview will focus on recent, complex, three-dimensional (3D) modelling studies associated with rigid body movements and the muscle forces that drive them. The majority of these 3D mathematical modelling studies are related to the human masticatory system, and are likely a result of available anatomical data and the scope for clinical implications. Although less common, examples of 3D non-human multibody analyses are covered.
The studies covered in this overview are grouped together based on their primary aims. As a consequence, the overview discusses investigations that aim to predict muscle activities, investigations on specific muscle parameters and function, investigations of jaw motion, and finally investigations on novel or alternative uses of multibody dynamics analysis.
Predicting muscle activity
One application of an MDA is to predict muscle activation patterns, particularly in cases where electromyography (EMG) data are unavailable. One of the first published studies to investigate this in three dimensions was that of Osborn & Baragar (1985). They presented a computer-assisted 3D model of the human jaw that was used to analyse muscle activations during normal, bilateral biting. Thirteen independent muscle units were constructed on each side of the skull where attachment positions were taken from a human specimen. The 3D direction of the muscles and the maximum forces that they could generate, the bite force, and two joint reaction forces were incorporated into the model. As with all studies that include detailed muscle anatomy and aim to predict muscle activity, there is the issue of muscle indeterminacy: that is, there is not one unique muscle force combination for a particular functional task. To find a single muscle recruitment pattern during a bilateral biting simulation, two optimisation criteria were assessed by Osborn & Barager: minimisation of the sum of the muscle forces and minimisation of the joint reaction forces.
This study showed that by minimising the sum of the muscle forces, the predicted muscle recruitment patterns corresponded well with observations in human subjects. They rejected an optimisation process that aimed to minimise the joint reaction forces. Their study identified two types of jaw muscles, namely power muscles and control muscles. The superficial masseter, medial pterygoid, and some portions of temporalis were grouped as power muscles that function to produce bite force, whereas the oblique temporalis and lateral pterygoid were specified as control muscles.
With the aim of predicting muscle activations, muscle forces and temporomandibular joint (TMJ) reaction forces during clenching and dynamic tasks, de Zee et al. (2007) created a 3D musculoskeletal model of the human mandible. This computer model was developed within anybody modelling software (AnyBody Technology, Aalborg, Denmark), and was based on computed tomography (CT) scan data of the cranium and mandible. The use of this particular modelling software had the advantage of combining a multibody dynamics solver and an automated optimisation program that could be used to predict a unique muscle activation pattern for a given task. anybody uses a min/max objective function (Rasmussen et al. 2001), which is equivalent to the minimisation of muscle effort as used in other studies (Iwasaki et al. 2003). The mandibular fossa was modelled as a planar constraint, and the masticatory muscles were represented by 24 Hill-type muscle actuators (Hill, 1938; Zajac, 1989). Figure 1 is a representation of the model.
Fig. 1.
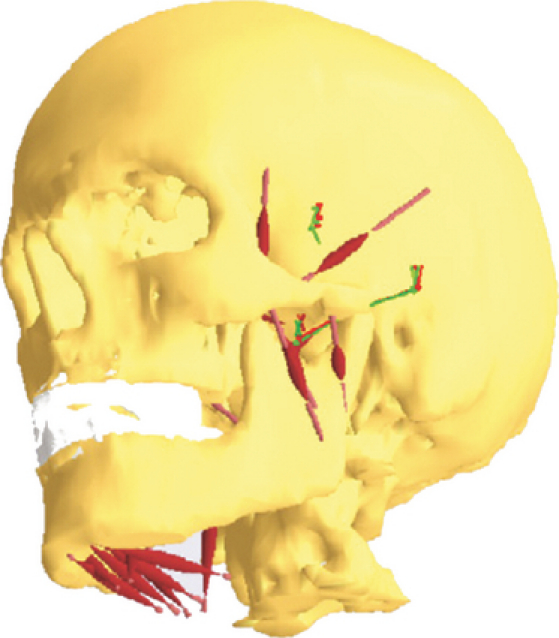
The multibody model described in de Zee et al. (2007).
Over all muscle groups the predicted muscle activations did not compare well to in vivo EMG data. Some muscle groups in particular (i.e. the lateral pterygoid) showed a very poor agreement with experimental data, whereas other groups (i.e. the masseter) showed better agreement. The authors suggest that there is ‘not a straightforward relationship’ between EMG amplitude (as recorded experimentally) and actual muscle force output, which may contribute to differences between the experimental and computational results. When carrying out isometric contraction tasks, such as clenching, the relationship between EMG amplitude and force output is likely to be more linear, and de Zee et al. (2007) did find the highest correlation coefficient between recorded and predicted muscle activities during such tasks.
In an attempt to eliminate any modelling errors associated with the use of average datasets, Iwasaki et al. (2003) developed seven patient-specific models of the human masticatory system and tested two optimisation methods for predicting muscle activation patterns during biting. Here, the optimisation objectives were minimisation of joint loads and the minimisation of muscle effort (or minimisation of the sum of the squared muscle forces). The 3D computer models were derived from radiographs of patients from whom EMG data were recorded. Computer-predicted and experimentally recorded ipsilateral/contralateral (also known as working/balancing) muscle activation ratios were compared, with varying success. Both optimisation methods used to predict muscle activity showed, at times, a good agreement with in vivo comparisons, but at other times the match between predicted and recorded activities was not as convincing. Minimisation of muscle effort proved most successful in predicting ipsilateral/contralateral muscle ratios in five of seven patients during molar biting, whereas the simulated results from one patient best matched the minimisation of joint loads, and another patient performed equally well with both minimisation methods. Although patient-specific models were developed by Iwasaki et al., they still suggested that the difficulty in accurately representing the anatomy could account for error in the predicted results. Also, the specific location, direction and magnitude of the moments applied to the teeth were highlighted as possible causes of error in the muscle force predictions.
Koolstra & van Eijden (1992) also constructed seven patient-specific models of the human masticatory system for the purposes of predicting muscle activations. A combination of magnetic resonance image (MRI) data, experimental measurement of maximum bite force and surface EMG was used to construct and validate the models. The muscle activity was predicted during peak unilateral bites at several positions along the tooth row using optimisation to find a unique muscle recruitment pattern. The optimisation objective stated that for a particular bite force and direction, the relative activity of the most active muscle should be as small as possible. Predicted activity trends for the masseter and temporalis muscle groups (the only muscles with available EMG data) compared reasonably well to those recorded in vivo; however, the standard deviations in the results over the seven subjects were large, meaning exact comparison and accuracy were difficult to evaluate.
A different approach to that discussed was applied by Curtis et al. (2010a) to predict muscle activity patterns in the reptile Sphenodon. The model was developed using adams multibody software (MSC Software Corporation, Santa Ana, CA, USA) and was based on micro-CT data and anatomical dissections. The geometry of the jaw joints was reconstructed from the CT data and contact was specified between the articulating surfaces of the joint (see Fig. 2). A full biting cycle was simulated that incorporated unilateral crushing of a modelled food item. The methods described for predicting muscle recruitment patterns did not apply optimisation objectives as is standard in other studies that seek to determine a unique muscle activation combination, but instead the mechanical efficiency of each muscle was determined from its origin and insertion locations. This approach was termed ‘dynamic geometric optimisation’ (DGO), where muscle forces were linked to the movements of the lower jaw and were dependent on their line-of-action. For example, a more horizontally positioned muscle relative to the occlusal plane is more efficient in moving the jaw forwards and backwards compared to a vertically aligned muscle, and therefore during these kinds of motions would be activated to a greater extent. Results of this approach showed that predicted muscle activations within the individual muscle groups compared well to in vivo EMG data (Gorniak et al. 1982). Variations did occur between in vivo and in silico activities, but modifications to food properties (size and resistance) altered predicted muscle activities, and showed a strong agreement with the experimental data.
Fig. 2.
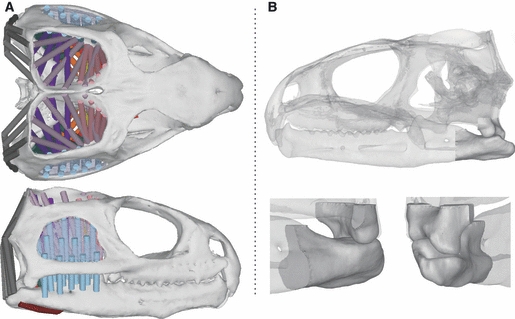
The multibody model of the reptile Sphenodon discussed by Curtis et al. (2010a,b);. (A) The distribution of muscle elements over the skull, and (B) the solid contact geometries of the jaw joints.
Muscle parameters and function
Multibody modelling can also be used to investigate the role of muscle parameters, such as length–tension relationships, on muscle function. Also utilising dynamic modelling software adams, Peck et al. (2000) constructed a 3D model of the human skull where relationships between muscle tensions and articular morphology were assessed during wide jaw opening. Temporomandibular joints (TMJ) were represented by ellipsoidal, canted condylar shapes that rotated and slid against frictionless, curvilinear surfaces (Fig. 3). Specialist design variables and optimisation procedures within the multibody software were used to determine muscle forces and muscle force–length parameters. For example, in their modelled muscles, passive tension was proportional to peak active muscle tension multiplied by an appropriate factor. This factor was unknown, and as such was set as a design variable within adams. Simply, the factor was incrementally and automatically varied in a series of analyses until passive length–tension curves were found that enabled the jaw to reach a state of equilibrium (under an experimentally determined external force) that corresponded to a maximum interincisal gape of 50 mm. Design variables were also utilised to determine the level of active muscle tone necessary to hold the jaw in a realistic rest position (passive tension + tone = 3–5 mm interincisal separation) (Brill & Tryde, 1974). Here, tone was set as the design variable that was incrementally varied in a series of analyses until the required rest position was achieved.
Fig. 3.
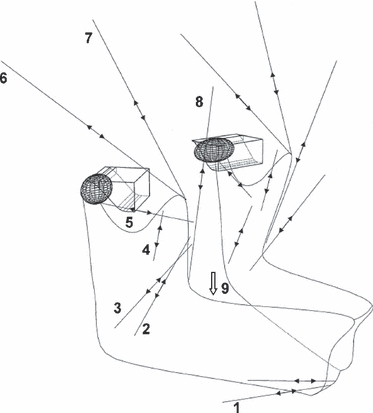
Basic model described in Peck et al. (2000) representing muscle actuators and TMJ contact geometries.
Peck et al. (2000) state that a small force of only 5 N should be enough to open the human jaw fully, but found that passive tensions within the muscles needed to be very low to permit this large gape. They conclude that during normal, sub-maximal gape activities, the passive tension within the muscles would be even lower, suggesting that passive muscle tensions have little constraining effect on the trajectories of jaw motion during such functions. However, these small passive tensions within the muscles did not offer enough resistance to support the jaw in a realistic interincisal resting position of 3–5 mm. Low-level muscle activity in the jaw-closing muscles was necessary to achieve this, but so small was this extra force that it did not restrict muscle-driven jaw opening.
Langenbach & Hannam (1999) also studied the human masticatory system in their 3D modelling study which assessed the effect of optimal fibre length on normal jaw movements. Optimal fibre length is strongly linked with passive tension of the muscles, as studied by Peck et al. (2000). All aspects of the study were developed within multibody dynamics analysis software adams, where muscle activation profiles (Møller, 1966) were used to drive the simulations. Two tests were conducted, one where the muscles were assigned an optimal fibre length that coincided with an interincisal distance of 2 mm, and one with an interincisal distance of 12 mm. An optimal fibre length that coincided with an interincisal distance of 2 mm produced a realistic resting jaw position, but did over-constrain all other normal jaw movements, suggesting that this optimal fibre length was too short. With an optimal fibre length coinciding with an interincisal separation of 12 mm, all simulated jaw movements were comparable to physiological movements, suggesting that this length was a good representation of that in human masticatory muscles. However, a resting interincisal separation of 14.8 mm occurred with this optimal fibre length, which is substantially larger than one would expect. It was suggested that some active tension within the muscles would occur in alert individuals, with Langenbach & Hannam showing that minimal muscle activity (< 0.2% of a muscles maximum activity) produced a realistic interincisal gape of 2.2 mm. This finding agreed with that of Peck et al. (2000).
In a study that intended to gain an understanding of the general function of muscles, Hannam et al. (2008) used a 3D computational model to simulate unilateral chewing in humans. The model was constructed using open source software artisynth (Fels et al. 2006), where each TMJ was modelled as a point coinciding with the anatomical centre of the condyle, and constrained by frictionless surfaces. An elastic, spherical food bolus (10 mm diameter) was positioned between the right first molars, and collapsed when any applied force reached 30 N. Simulations were carried out in artisynth, while all post-processing was conducted with alternative software (matlab, The Mathworks Inc., Natick, MA, USA). Findings of this study revealed that asymmetrical activity was needed in the mylohyoid and lateral pterygoid muscles to enable the jaw to reach appropriate locations during late opening and closing. It was shown that the working side lateral pterygoid was needed to slow backwards motion of the ipsilateral condyle during closing, whereas prolonged activity in the contralateral lateral pterygoid was required to delay the return of the condylar-point on that side.
Again, with the goal of understanding muscle function and the range of jaw movements possible within humans, Koolstra & van Eijden (2001) developed a dynamic mathematical model of the human masticatory system. The model consisted of a lower jaw that was accelerated by forces and accompanying torques produced by a combination of muscle forces, joint surfaces, bite-points and ligaments (Fig. 4). Unlike most studies that consider a specific problem as static, here all forces and torques generated by the muscles (Fm), joints (Fj) and bite-points (Fb) were not in equilibrium, and a certain ‘rest’ force and torque (Fr) remained, according to:
where F expresses a 6 degrees-of-freedom force tensor (as represented in Fig. 4). Fr controls the future direction of movement and can be adapted through Fm by applying an adequate instantaneous activation pattern. Optimisation methods were used to determine Fr, which effectively chose the muscle activation combination that moved the jaw system through the shortest possible route from point A to point B (i.e. start position to end position). Two simulations were conducted by Koolstra & van Eijden (2001). First, the jaw was opened from a closed position in combination with some laterodeviation. The second simulation involved ‘border’ movements, in which desired motion positions were far beyond those normally feasible, therefore generating maximal jaw movements. Maximum interincisal separation, protrusion, retrusion and laterodeviation of 28 mm, 10 mm, 0.5 mm and 14 mm were achieved during the border movement simulations. This defined a maximum working space of the human masticatory system. Results from this study also identified co-contraction amongst different muscle groups. During retrusion movements, for example, one would anticipate activity in the digastric, mylohyoid and posterior temporalis, but these muscles will also try to open the jaw, which resulted in co-activation of the jaw-closing posterior deep masseter and the medial pterygoid. Such analyses of muscle recruitment are unique within computational modelling and highlight the potential for future applications when investigating relationships between motions and muscle recruitment.
Fig. 4.
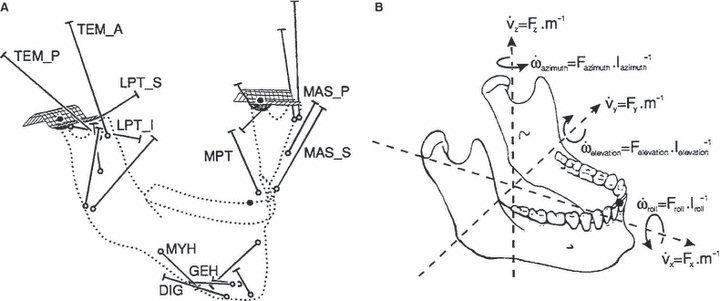
(A) Overview of the model described by Koolstra & van Eijden (2001) showing the muscle actuators and the contact surfaces of the TMJs. (B) Forces and torques controlling the linear and angular accelerations of the lower jaw.
The motivation behind a modelling study by Slager et al. (1997) was to determine the mechanism by which the human jaw system prevents damage to its dental elements. The aim of this modelling study was to identify which specific muscle parameters would prevent the teeth smashing into each other if a hard, brittle food item suddenly fractured. The influence of co-contraction, force–length properties, and force–velocity properties of the muscles on the velocity of impact after sudden food fracture was explored by modifying the properties of the muscles to either include or omit the specific muscle characteristic under investigation. Results suggested that the force–velocity characteristic of the jaw-closing muscles was the primary factor limiting the impact velocity of the dental elements in the jaw. When force-velocity properties were not included in the muscle models, the impact velocity of the teeth was seen to increase by a factor of 2–4 m s−1. The active and passive force–length properties played no role (or an extremely small role) in the control of impact velocity, whereas the co-contraction of the jaw opening muscles may have played some role, although modelling assumptions (i.e. a static hyoid bone) may interfere with this particular finding.
The way in which muscles are represented within 3D computational modelling studies was investigated by Curtis et al. (2008) in their multibody analysis of a macaque skull. The skull and mandible were constructed from micro-CT datasets and imported into adams multibody software. The anterior and posterior temporalis, deep and superficial masseter, and medial and lateral pterygoid muscle groups were represented as single straight-line elements, multiple straight-line elements, or with the temporalis modelled as a curved structure wrapping over the skull (Fig. 5). Analyses were carried out at gape angles of 5°–30° at the second molar position, and also at a constant gape of 15° on the first premolar, first molar, and second molar positions. The way in which the muscles were modelled did not have a major effect on bite force and joint force in this study. A bite force to joint force ratio of 1 : 1.3 was recorded, with bite forces reaching a maximum at the back of the jaw. A significant negative relationship between gape angle and bite force was also noted.
Fig. 5.
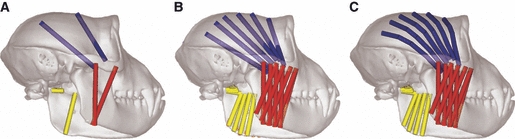
Multibody models of a macaque skull described in Curtis et al. (2008). (A) Single straight-line muscle elements representing whole muscle groups, (B) multiple straight-line muscle elements representing whole muscle groups, and (C) a wrapping temporalis muscle model.
Simulation of jaw motion
Multibody modelling can be used simply to understand jaw movements and the constraint of soft tissue structures. The finding that passive tension within muscles must be very small to achieve maximum gapes (e.g. as suggested by Peck et al. 2000; see Muscle parameters and function section above) was disputed by Koolstra & van Eijden (2004). Instead, they carried out a 3D biomechanical modelling study in which they analysed the coupling effect of head and jaw movements, which they hypothesised would allow for larger gapes to be reached. Their model functioned the same as that described earlier (Koolstra & van Eijden, 2001). The lower jaw was opened as far as possible with the head in a neutral position, and then the simulation was repeated with the head in an extended position (extended by 10° and 20°). With the head in a neutral, upright position, a gape of 20° was reached; this was equivalent to an interincisal separation of 2.85 cm. A 10° and 20° extension of the head during jaw opening increased maximum gapes to 26° and 32° respectively (an interincisal separation of 3.84 cm and 4.64 cm respectively). An explanation given for this increased gape was related to a change in sarcomere length and moment arm associated with an extended head position. Koolstra & van Eijden (2004) noted that the digastric could produce 67% and 89% of its maximum force at head extensions of 10° and 20° respectively, compared to just 44% in the neutral head position. As the jaw opened, the moment arm of the jaw opening muscles decreased, and this decrease was less with simultaneous head extension.
In another study by Koolstra & van Eijden (1995) two functional hypotheses were assessed using a 3D computational model of the human jaw system: the first being that jaw movements occurred primarily by the interaction between muscle forces and articular geometries, and the second that ligaments were of minor importance in guiding the jaw during regular closing movements. Dynamic systems simulation program tutsim (Meerman Automation, Neede, Netherlands) was used in this modelling study. The simulations were simple in nature, consisting of a 10 N force applied to the individual muscle groups to assess the jaw movements they produced. Results of this study showed that the more posterior the line-of-action of a muscle, the slower it would close the jaw, but in general each muscle group moved the lower jaw in a similar manner (in terms of translations of the condyles and closing of the jaw). This suggests the geometry of the articular surfaces plays a significant and dominant role in guiding condylar movements. All jaw movements occurred without a modelled ligament, implying that the ligament has little bearing on standard jaw-closing movements.
In what was possibly the first non-human, animal-specific, 3D biomechanical modelling study of the masticatory system, Langenbach et al. (2002) analysed the biomechanical function of a miniature pig skull (Fig. 6). As with other discussed studies, adams multibody software was used to develop the model in which muscle activity data were used to drive the simulations. Results of this study showed joint loads to be small during standard jaw opening and closing, only increasing significantly during biting. Contact forces at the balancing side joint were double that of the working side joint, although the symmetric muscle activity applied in the simulations may not be comparable to that which occurs in reality. The model functioned as expected, with maximum jaw opening, laterodeviation of the lower jaw, and the timing of the various parts of the chewing cycle comparing well to in vivo data. During normal motions the joint ligament did not play a significant role in guiding condylar movements, suggesting that its main function is to prevent undesirably large movements not consistent with normal jaw motion.
Fig. 6.
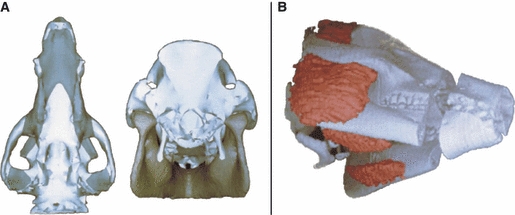
Three-dimensional reconstruction and modelling discussed by Langenbach et al. (2002). (A) Reconstruction of the skull and mandible from CT scans. (B) Superimposition of the bone and muscle reconstructions.
Moazen et al. (2008) also carried out a 3D multibody biomechanical analysis of a non-human subject, this time the skull of a lizard. The primary aims of this study were to predict peak bite and joint forces, and to assess the function of the temporal ligaments. Micro-CT datasets were used to construct geometries of the skull, onto which detailed musculature was represented (Fig. 7). Peak muscle forces were applied to the modelled muscles to predict bite forces, and the temporal ligament was modelled with varying properties of stiffness to assess its role during feeding. Simulations revealed that posterior bites were 72% larger than anterior bites, whereas the quadrosquamosal joint force decreased by 17% and the quadromandibular force by 10% with a posterior bite. The temporal ligament was seen to contribute to jaw stability at low gapes only, whereas ligament stiffness did not affect its loading significantly. Bite forces also compared reasonable well with experimental bite force measurements for lizards.
Fig. 7.
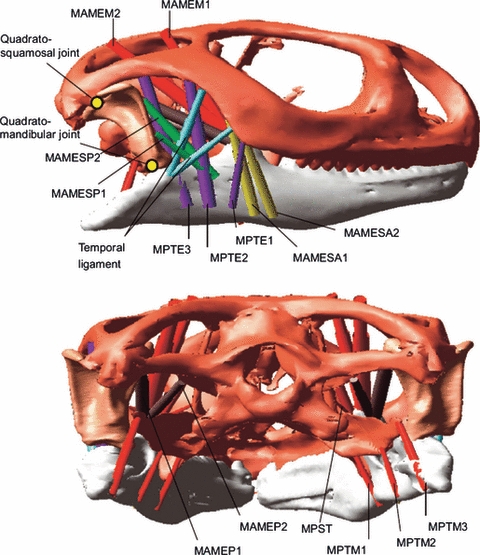
Multibody model of a lizard skull presented by Moazen et al. (2008).
Other potential uses for multibody modelling
A novel use of multibody modelling is presented in Langenbach et al. (2006). A model similar to that already discussed (see Langenbach et al. 2002 in the Simulation of jaw motion section above) was modified to allow forces and torques at the anterior symphysis of the lower jaw to be assessed. The lower jaw was split mid-sagittally into two sections, which were then re-connected with a rigid pin joint at the centre of the symphyseal region and orientated orthogonally to the dental occlusal plane. As with their previous study, adams multibody dynamics modelling software was used, this time to simulate one right-sided unilateral chewing cycle. Results confirmed three previously postulated loading patterns associated with the symphysis of the lower jaw. Jaw opening was associated with medial transverse bending at the symphysis, relating largely to the bilateral tensions in the lateral pterygoid. Lateral transverse bending, also referred to as wishboning, occurred at the late stage of the power stroke, and was associated with the actions of the deep and superficial masseter. Finally, the third loading pattern was dorsolateral shear and was linked to the biting force as the lower teeth contacted the food bolus. This study highlights additional potential uses for multibody modelling, where forces and torques are assessed on regions of the skull other than the jaw joints and teeth.
In a very recent multibody modelling study, Curtis et al. (2010b) investigated a possible feedback system that could help regulate joint pressures and bite forces. The basic model used in this study was the same as that discussed in the Predicting muscle activity section above (Curtis et al. 2010a), but was modified to include a feedback control mechanism between the jaw joints and the jaw-closing muscles. A full biting cycle was simulated in the skull of the reptile Sphenodon, during which the joint reaction forces were continuously monitored, and if the working and balancing side joint forces became uneven, the muscle activities were altered until equilibrium was reached across both joints. Muscle activity predictions resulting from this feedback study compared well to in vivo EMG data, and revealed that if this feedback mechanism, or a similar mechanism that could also control muscle activities, was not present, potentially damaging forces would be transmitted through the jaw joints. Analysis of feedback sources and monitoring joint reaction forces would be virtually impossible experimentally, highlighting the potential of multibody modelling in addressing such questions.
Computational modelling is of course sensitive to the model input parameters. To help understand some of these potential error sources, Sellers & Crompton (2004) conducted a sensitivity analysis on the human jaw system. As with other studies, straight-line force vectors represented the major masticatory musculature, i.e. the temporalis, masseter, and medial and lateral pterygoids. The TMJs were modelled as a pair of dampened springs to allow rotation and a small amount of sliding, while a food particle was modelled as a high resistance spring (10 00 000 N m−1). Figure 8 is a representation of the model described by Sellers & Crompton. With a food particle positioned between each tooth on the left side of the jaw, the peak bite force resulting from 256 different muscle activation combinations (eight muscles each with either maximum or zero activation) was derived. For the sensitivity analysis the muscle attachment locations were varied, which resulted in over 64 000 individual simulations.
Fig. 8.
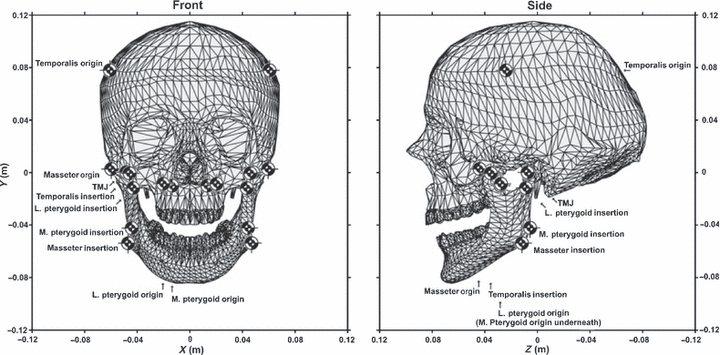
Schematic view of the model used in Sellers & Crompton (2004) showing the locations of the muscle attachment points and the TMJ.
Peak bite force reached 1080 N in this study, which was deemed slightly on the large side; however, large variability can be expected as bite forces are heavily reliant on applied muscle forces, which themselves can vary substantially between individuals. It was noted that at the first molar bite-point, the working side TMJ load was approximately half that of the balancing side, with total TMJ loads being higher when biting on the incisors. Some results were seen as insecure, with the sensitivity analysis revealing that retractive forces were extremely sensitive to temporalis positioning. These authors suggest that future simulations should split the temporalis into several sections rather than representing it as one straight-line force vector, as it was here. However, Sellers & Crompton did conclude that most variables studied in their sensitivity analysis did not affect results significantly, which can be viewed as a positive finding, as it adds robustness to these types of multibody simulations.
Summary
An overview is presented of a selection of multibody modelling studies related to the skull. It shows that multibody modelling has been used to investigate muscle characteristics such as passive muscle tension and optimum fibre length, as well being used to predict muscle activations during jaw function. General movements of the jaw are also investigated in past multibody studies, where the correlation between jaw movements and recorded muscle activation profiles are assessed, along with investigations into the relationship between head position and maximum gape. Both human and non-human studies are covered throughout this overview, providing an insight into the latest research on skulls utilising multibody modelling techniques.
Although a critical review of these studies was not provided, it is clear that there is still much that can be improved upon to close the gap between real physiological function and these virtual reconstructions. Although geometries of the bony structures can be derived accurately from CT scans, it is more difficult to represent the soft tissue structures. Muscle attachment locations, size, fibre pennation and fibre length are all likely to vary between subjects, and representing this variability in silico is difficult. These variables may account to some extent for differences in predicted and experimentally recorded muscle activations. I envisage that future applications of multibody modelling will attempt to gain more insights into these variables and will ultimately aim to create a fully validated system. In addition to this, multibody modelling can be used to understand the function and biomechanics of skulls where no physiological data can be obtained, such as in rare or extinct animals.
Acknowledgments
I would like to thank all colleagues at the University of Hull, Hull York Medical School (HYMNS), and University College London (UCL). I would also like to thank the Biotechnology and Biological Sciences Research Council (BBSRC) who provide funding for related research.
References
- Bei Y, Fregly BJ. Multibody dynamic simulation of knee contact mechanics. Med Eng Phys. 2004;26:777–789. doi: 10.1016/j.medengphy.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill N, Tryde G. Physiology of mandibular positions. In: Kawamura Y, editor. Frontiers of oral physiology: physiology of mastication. Basal: S. Karger; 1974. [DOI] [PubMed] [Google Scholar]
- Cleuren J, Aerts P, De Vree F. Bite and joint force analysis in Caiman crocodilus. Belg J Zool. 1995;125:79–94. [Google Scholar]
- Curtis N, Kupczik K, O’Higgins P, et al. Predicting skull loading: applying multibody dynamics analysis to a macaque skull. Anat Rec. 2008;291:491–501. doi: 10.1002/ar.20689. [DOI] [PubMed] [Google Scholar]
- Curtis N, Jones MEH, Evans SE, et al. Predicting muscle activation patterns from motion and anatomy: modelling the skull of Sphenodon (Diapsida: Rhynchocephalia) J R Soc Interface. 2010a;7:153–160. doi: 10.1098/rsif.2009.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis N, Jones MEH, Evans SE, et al. Feedback control from the jaw joints during biting: an investigation of the reptile Sphenodon using multibody modelling. J Biomech. 2010b doi: 10.1016/j.jbiomech.2010.08.001. doi: 10.1016/j.jbiomech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Fels S, Vogt F, van den Doel K, et al. University of British Columbia; 2006. Artisynth: a biomechanical simulation platform for the vocal tract and upper airway. Technical Report TR-2006-10 (In Computer Science. [Google Scholar]
- Glitsch U, Baumann W. The three-dimensional determination of internal loads in the lower extremity. J Biomech. 1997;30:1123–1131. doi: 10.1016/s0021-9290(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Gorniak GC, Rosenberg HI, Gans C. Mastication in the tuatara, Sphenodon punctatus (Reptilia: Rhynchocephalia): structure and activity of the motor system. J Morphol. 1982;171:321–353. doi: 10.1002/jmor.1051710307. [DOI] [PubMed] [Google Scholar]
- Greaves WS. The jaw lever system in ungulates: a new model. J Zool. 1978;184:271–285. [Google Scholar]
- Hahn H. Rigid Body Dynamics of Mechanisms 1: Theoretical Basis. Berlin: Springer; 2002. [Google Scholar]
- Hannam AG, Stavness I, Lloyd JE, et al. A dynamic model of jaw and hyoid biomechanics during chewing. J Biomech. 2008;41:1069–1076. doi: 10.1016/j.jbiomech.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Herrel A, Aerts P, De Vree F. Static biting in lizards: functional morphology of the temporal ligaments. J Zool (Lond) 1998;244:135–143. [Google Scholar]
- Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- Iwasaki LR, Petsche PE, McCall WD, Jr, et al. Neuromuscular objectives of the human masticatory apparatus during static biting. Arch Oral Biol. 2003;48:767–777. doi: 10.1016/s0003-9969(03)00171-7. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TMGJ. Application and validation of a three-dimensional mathematical model of the human masticatory system in vivo. J Biomech. 1992;25:175–187. doi: 10.1016/0021-9290(92)90274-5. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TMGJ. Biomechanical analysis of jaw-closing movements. J Dent Res. 1995;74:1564–1570. doi: 10.1177/00220345950740091001. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TMGJ. A method to predict muscle control in the kinematically and mechanically indeterminate human masticatory system. J Biomech. 2001;34:1179–1188. doi: 10.1016/s0021-9290(01)00053-7. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TMGJ. Functional significance of the coupling between head and jaw movements. J Biomech. 2004;37:1387–1392. doi: 10.1016/j.jbiomech.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Koolstra JH, van Eijden TMGJ, Weijs WA, et al. A three-dimensional mathematical model of the human masticatory system predicting maximum possible bite forces. J Biomech. 1988;21:563–576. doi: 10.1016/0021-9290(88)90219-9. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, Hannam AG. The role of passive muscle tensions in a three-dimensional dynamic model of the human jaw. Arch Oral Biol. 1999;44:557–573. doi: 10.1016/s0003-9969(99)00034-5. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, Zhang F, Herring SW, et al. Modelling the masticatory biomechanics of a pig. J Anat. 2002;201:383–393. doi: 10.1046/j.0021-8782.2002.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenbach GEJ, Zhang F, Herring SW, et al. Dynamic mechanics in the pig mandibular symphysis. J Anat. 2006;209:69–78. doi: 10.1111/j.1469-7580.2006.00584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazen M, Curtis N, Evans SE, et al. Rigid-body analysis of a lizard skull: modelling the skull of Uromastyx hardwickii. J Biomech. 2008;41:1274–1280. doi: 10.1016/j.jbiomech.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Møller E. The chewing apparatus. An electromyographic study of the action of the muscles of mastication and its correlation to facial morpholog. Acta Physiol Scand. 1966;69:1–299. [PubMed] [Google Scholar]
- Osborn JW, Baragar FA. Predicted pattern of human muscle activity during clenching derived from a computer assisted model: symmetric vertical bite forces. J Biomech. 1985;18:599–612. doi: 10.1016/0021-9290(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Peck CC, Langenbach GEJ, Hannam AG. Dynamic simulation of muscle and articular properties during human wide jaw opening. Arch Oral Biol. 2000;45:963–982. doi: 10.1016/s0003-9969(00)00071-6. [DOI] [PubMed] [Google Scholar]
- Rasmussen J, Damsgaard M, Voigt M. Muscle recruitment by the min/max criterion – a comparative numerical study. J Biomech. 2001;34:409–415. doi: 10.1016/s0021-9290(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Sellers WI, Crompton RH. Using sensitivity analysis to validate the predictions of a biomechanical model of bite forces. Ann Anat. 2004;186:89–95. doi: 10.1016/S0940-9602(04)80132-8. [DOI] [PubMed] [Google Scholar]
- Shabana AA. Dynamics of Multibody Systems. New York: Cambridge University Press; 2005. [Google Scholar]
- Sinclair GS, Alexander MR. Estimates of forces exerted by the jaw muscles of some reptiles. J Zool Lond. 1987;213:107–115. [Google Scholar]
- Slager GEC, Otten E, van Eijden TMGJ, et al. Mathematical model of the human jaw system simulating static biting and movements after unloading. J Neurophysiol. 1997;78:3222–3233. doi: 10.1152/jn.1997.78.6.3222. [DOI] [PubMed] [Google Scholar]
- Spencer MA. Force production in the primate masticatory system: electromyographic tests of biomechanical hypotheses. J Human Evol. 1998;34:25–54. doi: 10.1006/jhev.1997.0180. [DOI] [PubMed] [Google Scholar]
- Throckmorton GS, Throckmorton LS. Quantitative calculations of temporomandibular joint reaction forces – I. The importance of the magnitude of the jaw muscle forces. J Biomech. 1985;18:445–452. doi: 10.1016/0021-9290(85)90279-9. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanical and motor control. Clin Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]
- de Zee M, Dalstra M, Cattaneo PM, et al. Validation of a musculo-skeletal model of the mandible and its application to mandibular distraction osteogenesis. J Biomech. 2007;40:1192–1201. doi: 10.1016/j.jbiomech.2006.06.024. [DOI] [PubMed] [Google Scholar]


