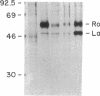
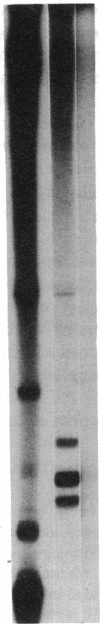
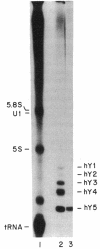
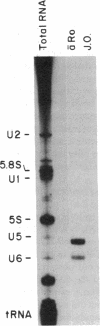
Abstract
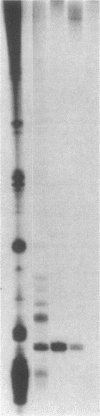
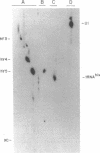
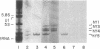
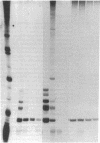
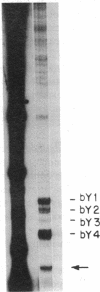
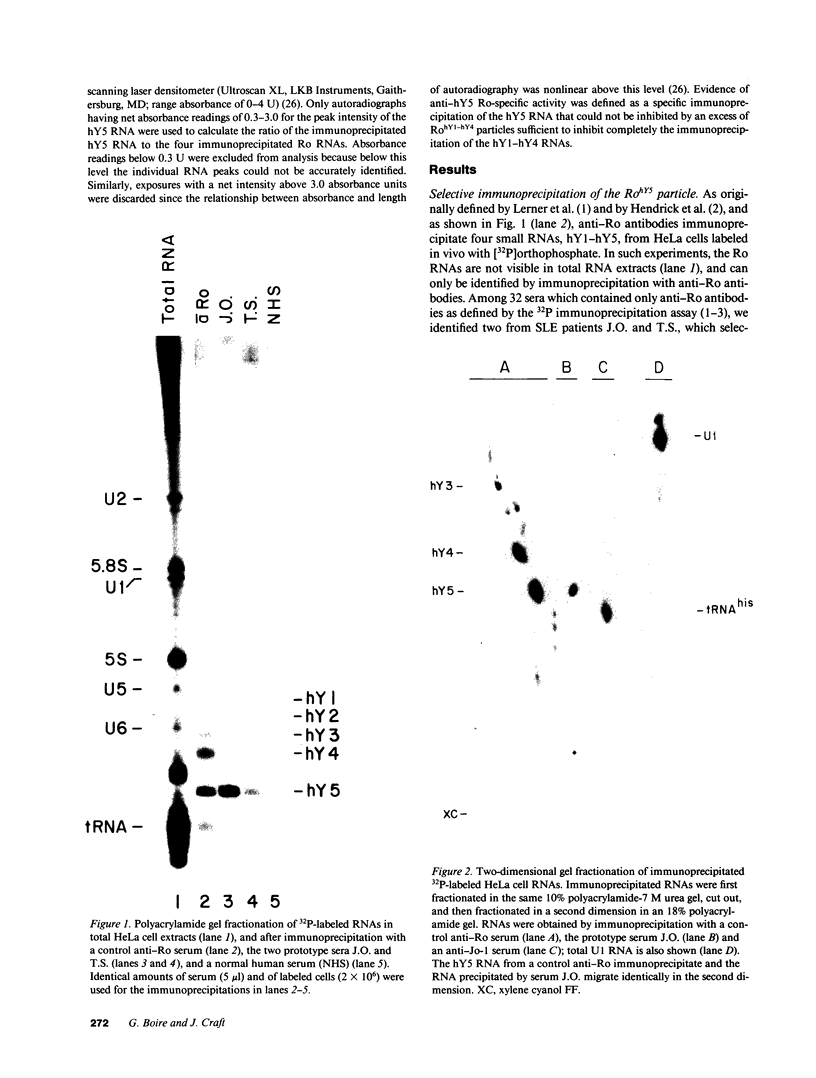
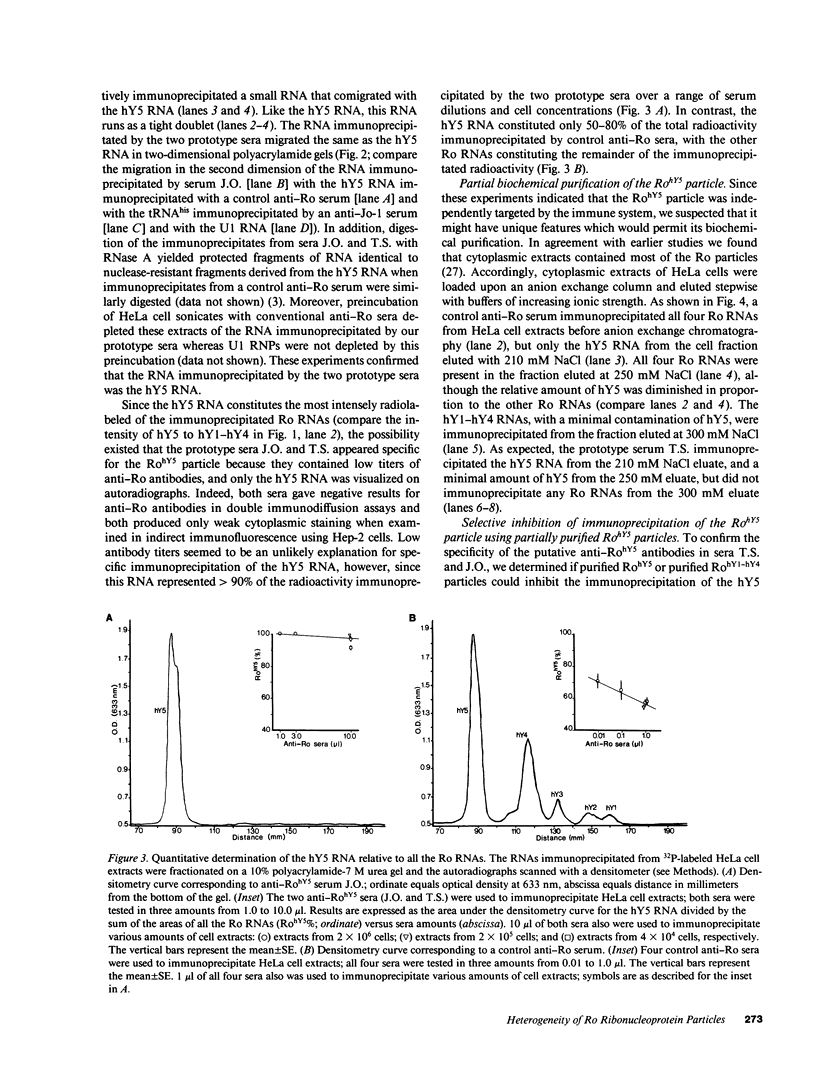
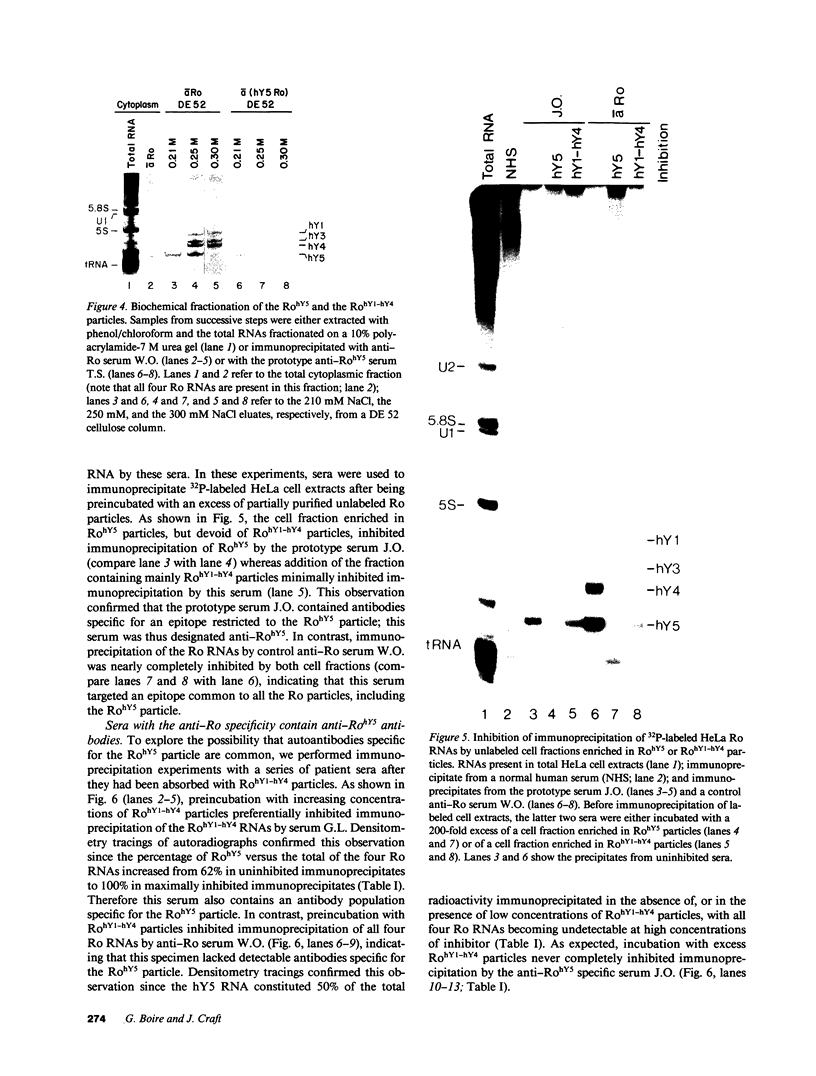
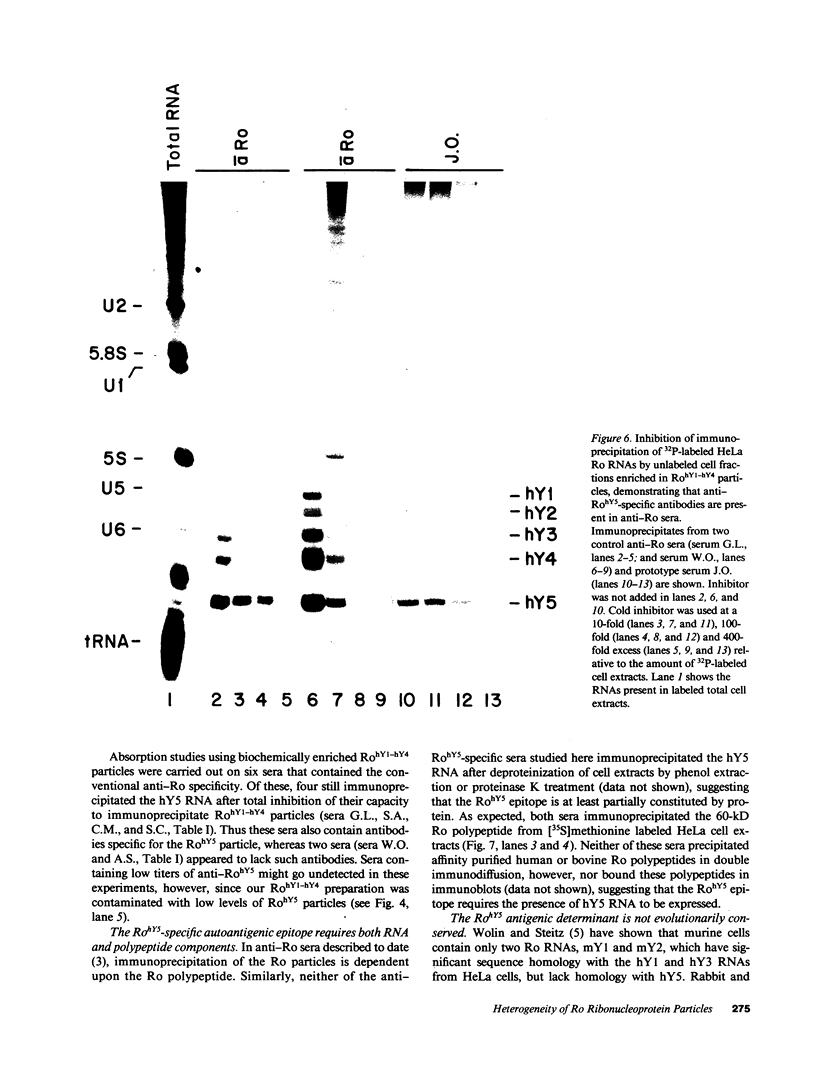
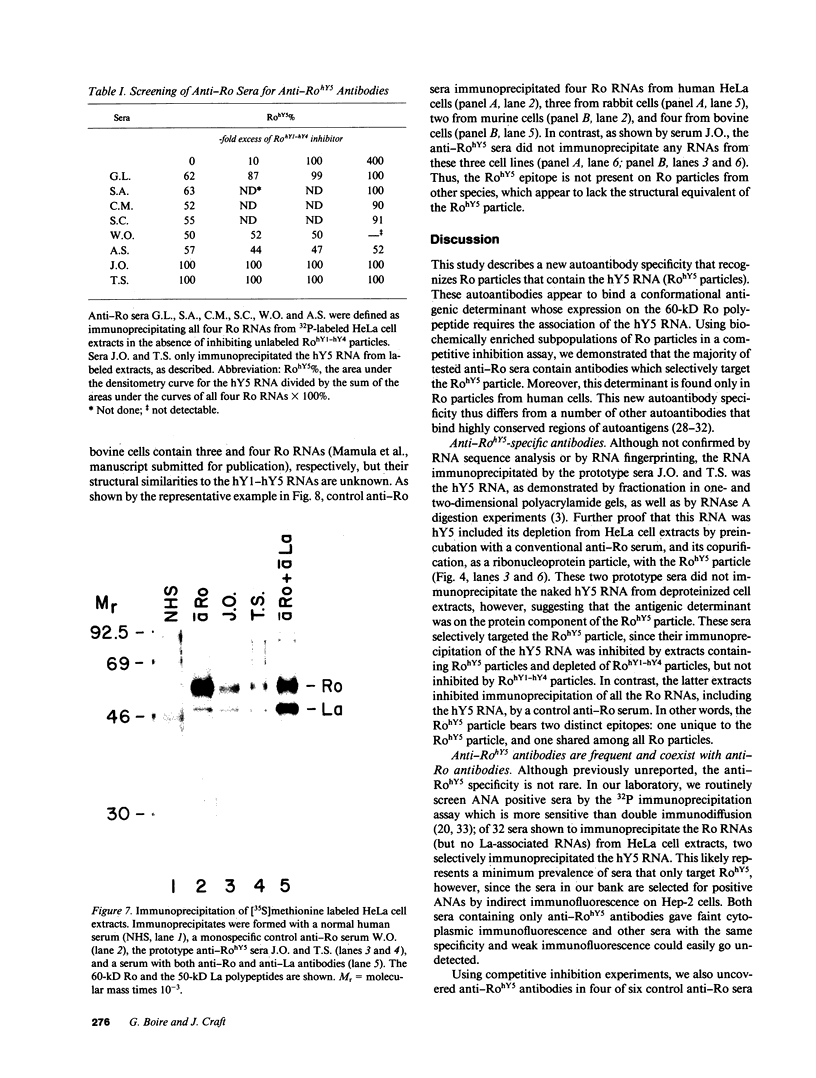
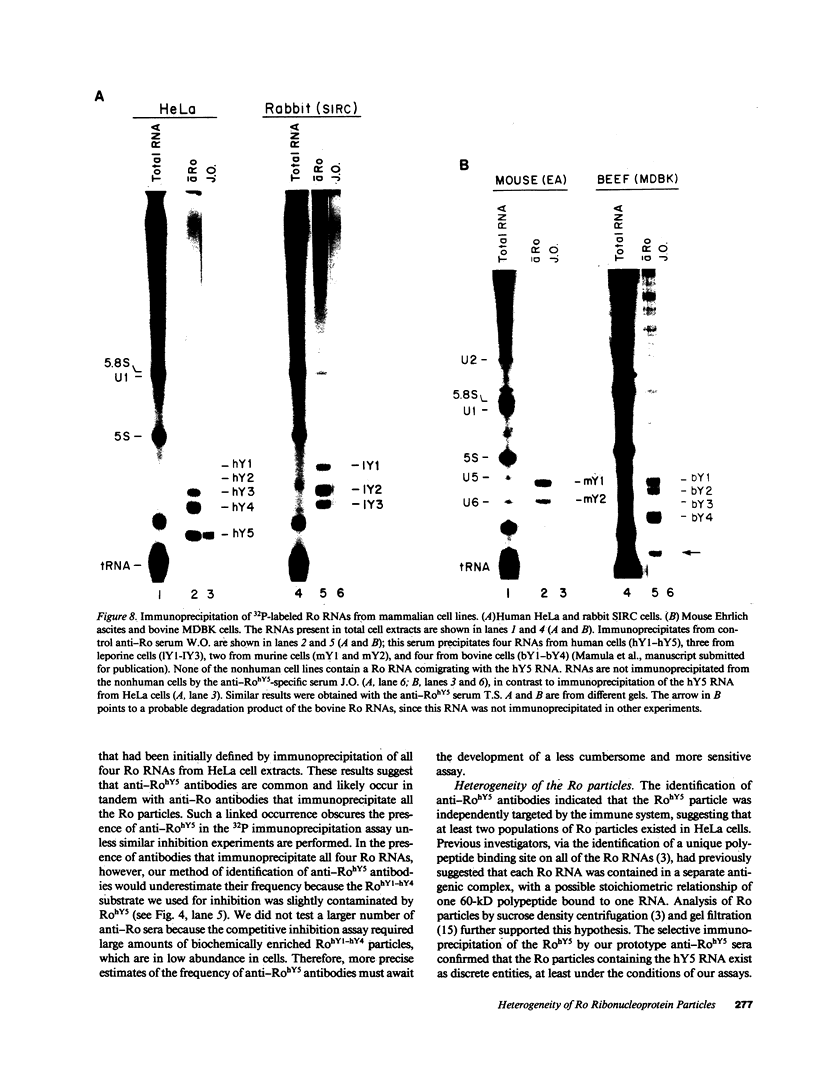
Anti-Ro autoantibodies found in sera from patients with systemic lupus erythematosus and related diseases precipitate four RNAs (hY1-hY5) from human cell extracts. We identified two patient sera that selectively immunoprecipitated from such extracts the Ro particle containing the hY5 RNA (RohY5 particle). Using cell fractions either enriched in or depleted of RohY5 particles, we have shown that these sera contain autoantibodies that target an antigenic determinant on the 60-kD Ro polypeptide that is expressed only on RohY5 particles and is absent on the Ro particles containing the hY1-hY4 RNAs (RohY1-hY4 particles). In a competitive inhibition assay using a cell fraction enriched in RohY1-hY4 particles but depleted of RohY5 particles, four of six control anti-Ro sera were also shown to contain antibodies reactive with the epitope specific for the RohY5 particle. Thus anti-RohY5 antibodies frequently occur in tandem with anti-Ro antibodies, but are not detected unless inhibition assays are performed. Finally, anti-RohY5 specific sera do not immunoprecipitate any Ro particles from various nonhuman cell lines. In contrast to other autoantibodies in systemic lupus and related diseases that bind conserved regions on conserved polypeptides, this observation suggests that a portion of the anti-Ro response targets a nonconserved epitope on a conserved autoantigen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh M., Maddison P. Resolution of the identity of certain antigen-antibody systems in systemic lupus erythematosus and Sjögren's syndrome: an interlaboratory collaboration. Arthritis Rheum. 1979 Jul;22(7):796–798. doi: 10.1002/art.1780220719. [DOI] [PubMed] [Google Scholar]
- Bell D. A., Komar R., Chodirker W. B., Block J., Cairns E. A comparison of serologic reactivity among SLE patients with or without anti-Ro (SS-A) antibodies. J Rheumatol. 1984 Jun;11(3):315–317. [PubMed] [Google Scholar]
- Ben-Chetrit E., Chan E. K., Sullivan K. F., Tan E. M. A 52-kD protein is a novel component of the SS-A/Ro antigenic particle. J Exp Med. 1988 May 1;167(5):1560–1571. doi: 10.1084/jem.167.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Chan E. K., Tan E. M. Human autoantibody-reactive epitopes of SS-B/La are highly conserved in comparison with epitopes recognized by murine monoclonal antibodies. J Exp Med. 1987 Dec 1;166(6):1627–1640. doi: 10.1084/jem.166.6.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft J., Mimori T., Olsen T. L., Hardin J. A. The U2 small nuclear ribonucleoprotein particle as an autoantigen. Analysis with sera from patients with overlap syndromes. J Clin Invest. 1988 Jun;81(6):1716–1724. doi: 10.1172/JCI113511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J. S., Sontheimer R. D., Gilliam J. N. Molecular characteristics of SS-B/La and SS-A/Ro cellular antigens. J Invest Dermatol. 1985 Feb;84(2):86–90. doi: 10.1111/1523-1747.ep12274950. [DOI] [PubMed] [Google Scholar]
- Deutscher S. L., Harley J. B., Keene J. D. Molecular analysis of the 60-kDa human Ro ribonucleoprotein. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9479–9483. doi: 10.1073/pnas.85.24.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. A. Association between the Ro and La antigenic determinants: immunodiffusion analysis of human spleen extract. J Immunol. 1985 Sep;135(3):1707–1713. [PubMed] [Google Scholar]
- Elkon K., Bonfa E., Llovet R., Danho W., Weissbach H., Brot N. Properties of the ribosomal P2 protein autoantigen are similar to those of foreign protein antigens. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5186–5189. doi: 10.1073/pnas.85.14.5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman M. S., Nakamura M., Mimori T., Gelpi C., Hardin J. A. Detection of antibodies to small nuclear ribonucleoproteins and small cytoplasmic ribonucleoproteins using unlabeled cell extracts. Arthritis Rheum. 1985 Dec;28(12):1356–1361. doi: 10.1002/art.1780281207. [DOI] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Alexander E. L., Bias W. B., Fox O. F., Provost T. T., Reichlin M., Yamagata H., Arnett F. C. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjögren's syndrome. Arthritis Rheum. 1986 Feb;29(2):196–206. doi: 10.1002/art.1780290207. [DOI] [PubMed] [Google Scholar]
- Harmon C. E., Deng J. S., Peebles C. L., Tan E. M. The importance of tissue substrate in the SS-A/Ro antigen-antibody system. Arthritis Rheum. 1984 Feb;27(2):166–173. doi: 10.1002/art.1780270207. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Wolin S. L., Rinke J., Lerner M. R., Steitz J. A. Ro small cytoplasmic ribonucleoproteins are a subclass of La ribonucleoproteins: further characterization of the Ro and La small ribonucleoproteins from uninfected mammalian cells. Mol Cell Biol. 1981 Dec;1(12):1138–1149. doi: 10.1128/mcb.1.12.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmerson R., Blankenfeld R. Clonal analysis of the BALB/c secondary B cell repertoire specific for a self-antigen, cytochrome c. J Immunol. 1988 Mar 15;140(6):1762–1769. [PubMed] [Google Scholar]
- Jemmerson R., Margoliash E. Specificity of the antibody response of rabbits to a self-antigen. Nature. 1979 Nov 29;282(5738):468–471. doi: 10.1038/282468a0. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. Antibody complementarity and antibody structure. J Immunol. 1988 Oct 1;141(7 Suppl):S25–S36. [PubMed] [Google Scholar]
- Kato N., Hoshino H., Harada F. Nucleotide sequence of 4.5S RNA (C8 or hY5) from HeLa cells. Biochem Biophys Res Commun. 1982 Sep 16;108(1):363–370. doi: 10.1016/0006-291x(82)91875-7. [DOI] [PubMed] [Google Scholar]
- Kieber-Emmons T., Kohler H. Evolutionary origin of autoreactive determinants (autogens). Proc Natl Acad Sci U S A. 1986 Apr;83(8):2521–2525. doi: 10.1073/pnas.83.8.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Hardin J. A., Steitz J. A. Two novel classes of small ribonucleoproteins detected by antibodies associated with lupus erythematosus. Science. 1981 Jan 23;211(4480):400–402. doi: 10.1126/science.6164096. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Boyle J. A., Mount S. M., Wolin S. L., Steitz J. A. Are snRNPs involved in splicing? Nature. 1980 Jan 10;283(5743):220–224. doi: 10.1038/283220a0. [DOI] [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Mimori T., Hinterberger M., Pettersson I., Steitz J. A. Autoantibodies to the U2 small nuclear ribonucleoprotein in a patient with scleroderma-polymyositis overlap syndrome. J Biol Chem. 1984 Jan 10;259(1):560–565. [PubMed] [Google Scholar]
- Nisonoff A., Margoliash E., Reichlin M. Antibodies to rabbit cytochrome c arising in rabbits. Science. 1967 Mar 10;155(3767):1273–1275. doi: 10.1126/science.155.3767.1273. [DOI] [PubMed] [Google Scholar]
- Reddy R., Tan E. M., Henning D., Nohga K., Busch H. Detection of a nucleolar 7-2 ribonucleoprotein and a cytoplasmic 8-2 ribonucleoprotein with autoantibodies from patients with scleroderma. J Biol Chem. 1983 Feb 10;258(3):1383–1386. [PubMed] [Google Scholar]
- Rosa M. D., Hendrick J. P., Jr, Lerner M. R., Steitz J. A., Reichlin M. A mammalian tRNAHis-containing antigen is recognized by the polymyositis-specific antibody anti-Jo-1. Nucleic Acids Res. 1983 Feb 11;11(3):853–870. doi: 10.1093/nar/11.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scopelitis E., Biundo J. J., Jr, Alspaugh M. A. Anti-SS-A antibody and other antinuclear antibodies in systemic lupus erythematosus. Arthritis Rheum. 1980 Mar;23(3):287–293. doi: 10.1002/art.1780230304. [DOI] [PubMed] [Google Scholar]
- Scott J. S., Maddison P. J., Taylor P. V., Esscher E., Scott O., Skinner R. P. Connective-tissue disease, antibodies to ribonucleoprotein, and congenital heart block. N Engl J Med. 1983 Jul 28;309(4):209–212. doi: 10.1056/NEJM198307283090403. [DOI] [PubMed] [Google Scholar]
- Sontheimer R. D., Maddison P. J., Reichlin M., Jordon R. E., Stastny P., Gilliam J. N. Serologic and HLA associations in subacute cutaneous lupus erythematosus, a clinical subset of lupus erythematosus. Ann Intern Med. 1982 Nov;97(5):664–671. doi: 10.7326/0003-4819-97-5-664. [DOI] [PubMed] [Google Scholar]
- Tan C. K., Sullivan K., Li X. Y., Tan E. M., Downey K. M., So A. G. Autoantibody to the proliferating cell nuclear antigen neutralizes the activity of the auxiliary protein for DNA polymerase delta. Nucleic Acids Res. 1987 Nov 25;15(22):9299–9308. doi: 10.1093/nar/15.22.9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Water J., Gershwin M. E., Leung P., Ansari A., Coppel R. L. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988 Jun 1;167(6):1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. Genes for two small cytoplasmic Ro RNAs are adjacent and appear to be single-copy in the human genome. Cell. 1983 Mar;32(3):735–744. doi: 10.1016/0092-8674(83)90059-4. [DOI] [PubMed] [Google Scholar]
- Wolin S. L., Steitz J. A. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Harley J. B., Reichlin M. Molecular properties of the Ro/SSA antigen and enzyme-linked immunosorbent assay for quantitation of antibody. J Clin Invest. 1984 Aug;74(2):625–633. doi: 10.1172/JCI111460. [DOI] [PMC free article] [PubMed] [Google Scholar]