Abstract
Background:
The optimal treatment of desmoid tumours is controversial. We evaluated desmoid management in Dutch familial adenomatous polyposis (FAP) patients.
Methods:
Seventy-eight FAP patients with desmoids were identified from the Dutch Polyposis Registry. Data on desmoid morphology, management, and outcome were analysed retrospectively. Progression-free survival (PFS) rates and final outcome were compared for surgical vs non-surgical treatment, for intra-abdominal and extra-abdominal desmoids separately. Also, pharmacological treatment was evaluated for all desmoids.
Results:
Median follow-up was 8 years. For intra-abdominal desmoids (n=62), PFS rates at 10 years of follow-up were comparable after surgical and non-surgical treatment (33% and 49%, respectively, P=0.163). None of these desmoids could be removed entirely. Eventually, one fifth died from desmoid disease. Most extra-abdominal and abdominal wall desmoids were treated surgically with a PFS rate of 63% and no deaths from desmoid disease. Comparison between NSAID and anti-estrogen treatment showed comparable outcomes. Four of the 10 patients who received chemotherapy had stabilisation of tumour growth, all after doxorubicin combination therapy.
Conclusion:
For intra-abdominal desmoids, a conservative approach and surgery showed comparable outcomes. For extra-abdominal and abdominal wall desmoids, surgery seemed appropriate. Different pharmacological therapies showed comparable outcomes. If chemotherapy was given for progressively growing intra-abdominal desmoids, most favourable outcomes occurred after combinations including doxorubicin.
Keywords: desmoid tumour, desmoid-type fibromatosis, familial adenomatous polyposis, management
Familial adenomatous polyposis (FAP) is a dominantly inherited cancer predisposition syndrome, caused by mutations in the adenomatous polyposis coli (APC) gene. Carriers of the mutated APC gene develop hundreds to thousands of adenomatous polyps in the colon and rectum, leading to a nearly 100% cancer risk by the age of 40 years (Lynch et al, 2008). By performing a prophylactic colectomy, the risk of death due to colorectal cancer is decreased. Among FAP patients, a spectrum of extra-colonic manifestations is often observed, including duodenal cancer and desmoid tumours. These manifestations are currently the most common causes of death after colorectal cancer (Arvanitis et al, 1990).
Desmoid tumours or aggressive fibromatoses are histologically benign proliferations of fibro-aponeurotic tissue (Goldblum and Fletcher, 2002). In the general population, the incidence of desmoids is about 3 per million per year, and the tumours are mainly located in the extremities or in the abdominal wall (Fallen et al, 2006). Of all patients presenting with a desmoid tumour, at least 7.5% has FAP or will develop FAP later in life (Nieuwenhuis et al, 2010, submitted for publication). In the total FAP population, desmoid tumours develop in about 10–30% and are usually located in the mesentery of the small bowel (Fallen et al, 2006). Desmoids range from small, indolent, or even regressive tumours to large and progressive growing neoplasms causing obstruction of vital organs. Desmoid tumours do not metastasise, although they can present as multifocal disease.
Treatment of FAP-related desmoid tumours is controversial (Sleijfer, 2009). As desmoid tumours are rare, and show a variable disease course, the effectiveness of treatment is difficult to determine. There are no randomised controlled trials. Usually, extra-abdominal and abdominal wall desmoid tumours are removed surgically, but two recently published reports argued a wait-and-see policy for patients in which surgery would result in major functional or cosmetic defects (Bonvalot et al, 2008; Stoeckle et al, 2009). For intra-abdominal desmoid tumours, surgery is not recommended because surgical resection is complicated or impossible in most cases, and because of high recurrence rates (Rodriguez-Bigas et al, 1994). Furthermore, there is evidence that tissue damage is a risk factor for desmoid development (Clark et al, 1999; Bertario et al, 2001). The most frequently used pharmacological therapies include non-steroidal anti-inflammatory drugs (NSAIDs), hormonal agents, biological agents, and cytotoxic chemotherapy (Janinis et al, 2003; Tolan et al, 2007). Currently, most guidelines recommend a stepwise approach, starting with NSAIDs (preferably sulindac). If this is not effective, hormonal therapy is added, most commonly consisting of tamoxifen or toremifene. Fast growing desmoid tumours not responsive to these agents are treated by cytotoxic chemotherapy or surgery (http://www.nccn.org; Janinis et al, 2003; Latchford et al, 2006; Melis et al, 2008; Casali and Blay, 2010).
In the present study, we retrospectively evaluated long-term outcome of Dutch FAP patients with desmoid tumours, undergoing surgical, and pharmacological therapies. First, we assessed the effectiveness of surgical vs non-surgical strategies for intra- and extra-abdominal desmoid tumours, and second, we assessed the effectiveness of various pharmacological modalities in desmoid tumour treatment.
Materials and methods
Patients
The FAP database of the Netherlands Foundation for the Detection of Hereditary Tumours was used for the study. This national database comprises medical data on over a thousand FAP patients. Patients gave written consent to register their personal and medical information. A total of 78 patients with desmoid tumours were identified. Patient characteristics, genetic data, and medical information were retrieved from the database. The study was approved by the Medical Ethics Committee.
Desmoid localisation was defined as ‘at least intra-abdominal’ or ‘extra-abdominal and in the abdominal wall’. For all patients, the type of primary therapy for the first desmoid tumour was determined. If surgery was performed due to the severity of desmoid symptoms or with the aim of removing the desmoid tumour, patients were categorised into the ‘surgery’ group. All patients who received conservative treatment (wait-and-see or medication), and patients whose desmoid tumour was detected coincidentally during another surgical procedure, without resection, were categorised into the ‘non-surgery’ group.
Time from diagnosis of the desmoid tumour to progression of desmoid tumour growth was calculated. Progression of desmoid tumour growth was defined as tumour growth causing clinical symptoms. Also, for each patient, the status of desmoid growth at the end of follow-up was assessed and categorised into either ‘regression or stabilisation of tumour growth’ or ‘progression of tumour growth’.
Data analysis
Baseline characteristics between the groups were analysed by univariate analysis (Student's t-test for numerical variables, χ2-test for categorical variables). Progression-free survival (PFS) was calculated by the Kaplan–Meier method. Univariate analysis was performed using the log-rank test. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) version 16.0 (Chicago, IL, USA).
Results
Group description
Between January 1978 and January 2010, 78 FAP (34 males) patients had developed at least one desmoid tumour. Desmoid localisations were as follows: 49 (62.8%) intra-abdominal, 13 (16.7%) involving both the mesentery and the abdominal wall, 13 (16.7%) abdominal wall only, 2 (2.6%) trunk, and 1 (1.3%) head/neck. The median size of the desmoids was 7 cm, ranging from 1 to 24 cm. Fifty-six patients were treated at a tertiary referral centre, 22 in a local hospital. The median follow-up period from diagnosis of desmoid to the last observation was 8 years, ranging from 0 to 29 years (Table 1).
Table 1. Characteristics and follow-up data of FAP-related mesenterial desmoid tumours, according to primary surgical treatment vs non-surgical treatment.
|
Primary treatment
|
|||
|---|---|---|---|
| Surgery (N=36) | No surgery (N=26) | P-value | |
| Sex, male | |||
| N (%) | 16 (44) | 13 (50) | 0.665 |
| Age at first DT (years) | |||
| Median, min–max | 30, 15–54 | 35, 14–51 | 0.396 |
| Size first DT (cm) | |||
| Median, min–max | 9.5, 1–20 | 6.5, 2–24 | 0.568 |
| DT progression, N (%) | 26 (72) | 13 (50) | 0.074 |
| Time to first DT progression (months) | |||
| Median, min–max | 13, 1–189 | 24, 2–229 | 0.913 |
| Total follow-up from diagnosis of first DT to last observation (years) | |||
| Median, min–max | 8, 0–29 | 7, 0–28 | 0.762 |
| Age at last follow-up (years) | |||
| Median, min–max | 41, 23–67 | 42.5, 18–79 | 0.606 |
| Status at last follow-up, N (%) | |||
| Alive | 21 (58) | 18 (69) | 0.783 |
| Lost to follow-up | 1 (3) | 1 (4) | |
| Dead due to DT | 9 (25) | 5 (19) | |
| Dead due to other cause | 5 (14) | 2 (8) | |
| DT status at last follow-up, N (%) | |||
| Regression/stable | 25 (69) | 20 (77) | 0.515 |
| Progression/variable | 11 (31) | 6 (23) | |
Abbreviations: DT=desmoid tumour; FAP=familial adenomatous polyposis.
Surgery vs non-surgical management for intra-abdominal desmoid tumours
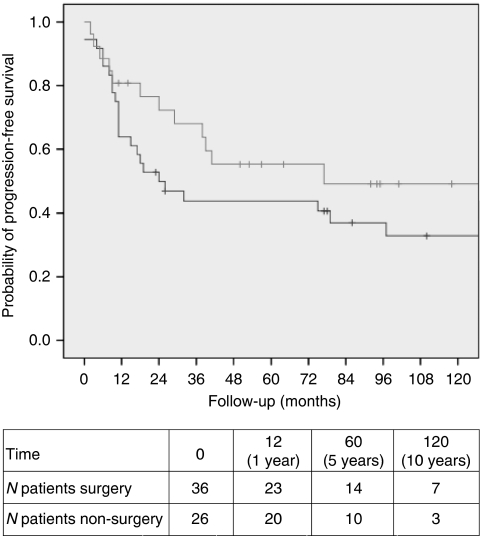
The group with ‘at least intra-abdominal’ desmoid tumours consisted of 62 patients. In 36 patients, primary treatment consisted of surgery with the intention to remove the intra-abdominal desmoid tumour. Twelve of these patients received desmoid-targeted medication immediately after surgery. Primary treatment was non-surgical in 26 patients (17 wait-and-see policy and 9 medication). The surgery and non-surgery groups were comparable for sex, age at first desmoid, size of first desmoid tumour, and duration of follow-up (Table 1). None of the intra-abdominal desmoid tumours could be resected entirely. The probability of PFS for the surgery group was 63.9%, 43.8%, and 32.9% after 1, 5 and 10 years, respectively. In the non-surgical group, these percentages were 80.8%, 55.3%, and 49.1%, respectively (log-rank, P=0.163) (Figure 1).
Figure 1.
Progression-free interval after primary surgical (black line) and non-surgical (grey line) treatment for mesenterial desmoid tumours in FAP patients (log-rank test, P=0.163).
When considering desmoid status at the last observation, the majority of desmoid tumours had become stable or regressive in both the surgery and non-surgery groups (69% and 77%, respectively, P=0.515). In the surgery and non-surgery groups, 25% and 19%, respectively, died from desmoid disease (P=0.783) (Table 1).
Extra-abdominal and abdominal wall desmoid tumours
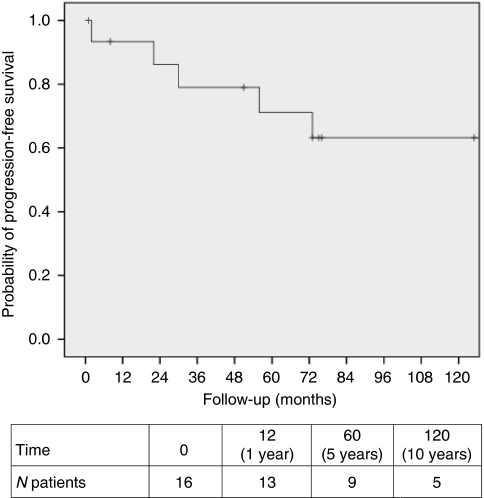
Sixteen patients had extra-abdominal desmoid tumours (Table 2). Thirteen patients had a desmoid tumour in the abdominal wall (3 male patients and 10 female patients), two male patients had desmoids at the thoracic wall and back, and one female patient had a desmoid tumour localised in the muscles of the neck. Fourteen (87.5%) of the tumours were treated surgically, with seven R1/2 (microscopically/macroscopically irradical), six Rx (unknown surgical margin), and one R0 (radical) excision. One, 5, and 10 years after primary surgery, 93.3%, 71.1%, and 63.2% of patients were free of progression. In most patients, progression was observed within 6 years after primary surgery (Figure 2). None of the patients died from desmoid disease. When considering desmoid status at the last observation, three quarter of the desmoid tumours had stabilised or regressed.
Table 2. Characteristics and follow-up data of extra-abdominal and abdominal wall desmoid tumours.
| Extra-abdominal and abdominal wall DT (N=16) | |
|---|---|
| Sex, male, N (%) | 5 (31) |
| DT localisation, N (%) | |
| Abdominal wall | 13 (81) |
| Trunk | 2 (13) |
| Head/neck | 1 (6) |
| Age at first DT (years) | |
| Median, min–max | 30.5, 8–57 |
| Size of first DT (cm) | |
| Median, min–max | 5, 2–13 |
| Primary treatment, N (%) | |
| Surgery | 13 (81) |
| Surgery and medication | 1 (6) |
| Medication | 1 (6) |
| Wait-and-see | 1 (6) |
| DT progression, N (%) | 5 (31) |
| Time to DT growth months | |
| Median, min–max | 30 (2–73) |
| Status last follow-up, N (%) | |
| Alive | 13 (81) |
| Dead due to DT | 0 |
| Dead due to other cause | 3 (19) |
| Desmoid status at last follow-up, N (%) | |
| Regression/stable | 12 (75) |
| Progression/variable | 4 (25) |
Abbreviation: DT=desmoid tumour.
Figure 2.
Progression-free interval after primary treatment for extra-abdominal and abdominal wall desmoid tumours in FAP patients.
Effectiveness of pharmacological treatment
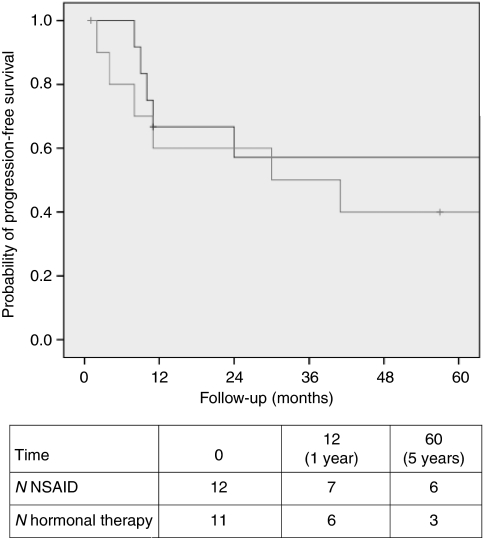
Various pharmacological agents were used, including NSAIDs (sulindac and celecoxib) and hormonal medications (tamoxifen, toremifene, LHRH-agonists, and anastrozole). For all patients who received medical treatment as primary therapy, irrespective of previous surgery, survival rates were calculated. After 5 years of follow-up, the PFS rates were similar after treatment with NSAIDs and hormonal medications including combination therapy, as shown in Figure 3 (log-rank, P=0.111). A small subset of patients had received other drugs, including prednisone, interferon, and colchicines. After these medications, both positive as well as negative effects on desmoid tumour growth were reported, but patient numbers were too small to perform statistical analysis.
Figure 3.
Progression-free interval after NSAIDs (n=12, black line), and hormonal therapy or combination therapy (n=11, grey line), irrespective of previous surgery (log-rank test, P=0.111).
Cytotoxic chemotherapy and imatinib
Ten patients received cytotoxic chemotherapy, and three patients had treatment with imatinib. Effects and complications of these therapies are summarised in Table 3. The most frequently used chemotherapy was doxorubicin, in combination with other agents such as DTIC, carboplatin, and ifosfamide. Effects of chemotherapy were variable. Four patients eventually had regression or stabilisation of tumour growth, and five patients had progression of tumour growth. One patient died only a few days after the first session of chemotherapy due to a massive pulmonary embolism, caused by pressure of the desmoid tumour on the large veins. Another patient bled to death due to fistulas and abscesses after chemotherapy. Furthermore, three patients developed severe complications as fistulas and abscesses (after 5, 18, and 60 months, respectively) besides the known spectrum of side effects associated with cytotoxic chemotherapy.
Table 3. Description of treatment outcome of patients who received cytotoxic chemotherapy and/or targeted agents as desmoid treatment.
| Sex | Site DT | Age (years) | Treatment | Effect on desmoid growth | Follow-up (months) |
|---|---|---|---|---|---|
| Male | Mesentery | 45 | Irresectable DT, etoposide and ifosfamide, tamoxifen tamoxifen and LHRH-agonist anastrozole | Quick regression DT, necrosis in DT Stabilisation, after 5 years progression Progression | 5 70 5 |
| Male | Head, abd. wall and mesentery | 15–17 | R2 resection DT head, RT mes. DT, sulindac, toremifene doxorubicine and DTIC, R2 resection mes. DT sulindac, toremifene, R2 resection abd. wall DT all medication stopped | Progression mes. DT Stabilisation, after 2 years abd. wall DT Both periods of progression and regression, after 2 years growth DT head, DT mes. and abd. wall stable Stabilisation | 35 38 100 36 |
| Male | Mesentery | 29 | R2 resection, sulindac, toremifene doxorubicine and carboplatin R2 resection, sulindac, tamoxifen | Progression Regression <25% Stabilisation | 11 7 50 |
| Male | Mesentery | 29 | R2 resection, sulindac, tamoxifen, toremifene doxorubicine and ifosfamide, sulindac, toremifene imatinib | Progression Stabilisation, after 8 months progression Stabilisation, but fistulas and abscesses at DT | 38 8 10 |
| Female | Abd. wall, trunk, breasts, neck | 25–40 | Multiple R2 resections, tamoxifen, sulindac, LHRH-agonists, anastrozole, radiotherapy imatinib | Progression and multiple new DT Progression Progression | 11 10 |
| Male | Mesentery | 32 | R2 resection doxorubicine and DTIC | Progression | 19 |
| Regression, death not due to DT | 184 | ||||
| Male | Mesentery | 30 | Chemotherapya and radiotherapy, R2 resection colchicine, LHRH-agonists, anti-estrogens, prednison, IFN | Stabilisation for 4 years Progression; after colchicine multiple abscesses; death due to DT | 51 58 |
| Female | Mesentery and abd. wall | 24 | Naproxen, toremifene doxorubicine and DTIC | Progression Progression, death due to DT | 16 3 |
| Female | Mesentery | 33–35 | Sulindac, anti-estrogens, DT irresectable liposomal doxorubicine | Progression Death pulmonary embolism, due to compression of DT on the large veins | 24 0 |
| Female | Mesentery | 35–37 | Wait-and-see, sulindac, celecoxib, tamoxifen, toremifene carboplatin and doxorubicine imatinib fulvestrant | Progression Necrosis in DT, fistulas and abscesses Stabilisation, after 1 year progression Stabilisation, after 2 years progression and death due to DT | 37 7 12 19 |
| Male | Mesentery | 47 | Irresectable DT, sulindac, tamoxifen vinblastin and methotrexat | Progression Progression, death due to desmoid | 4 18 |
Abbreviations: abd. wall=abdominal wall; DT=desmoid tumour; DTIC, dacarbazine; IFN=interferon; LHRH, luteinizing hormone releasing hormone; Mes.=mesenterial; RT=radiotherapy.
Details and type of chemotherapy are not available.
In the three patients receiving imatinib (of which two also had received chemotherapy), variable outcomes were seen, but the follow-up intervals were limited.
Radiotherapy and embolisation
A total of five patients were treated by radiation therapy. Three patients had radiation therapy for intra-abdominal desmoids. In one of them, the tumour size decreased, enabling surgery. The two other patients had stable desmoids during 6 years and progression within 1 year, respectively. Two patients had radiation therapy for extra-abdominal desmoid tumours: a patient with trunk desmoids had progression within a few months, and another patient had regression of an abdominal wall desmoid after irradiation; however, this patient developed serious radiation enteritis.
In two patients, the desmoid tumours were treated by embolisation. However, in both patients this treatment failed as the desmoids did not have large supplying vessels.
Discussion
The present study demonstrates that for intra-abdominal desmoid tumours, similar PFS rates were observed after surgical treatment and a more conservative approach. None of the intra-abdominal desmoids could radically be resected by surgery, but at the end of the follow-up period, two thirds of the intra-abdominal desmoids showed regression or stabilisation of tumour growth. About one fifth of the patients died due to complications of an intra-abdominal desmoid tumour. Most patients with abdominal wall and extra-abdominal desmoid tumours were treated surgically. The PFS rates were greater than after surgery of intra-abdominal desmoids, and at the end of the follow-up period, in 75% of the patients the tumours had stabilised or decreased in growth. None of these patients died from desmoid disease. Evaluation of pharmacological agents showed comparable PFS probabilities after NSAIDs and hormonal agents including combination of both medicines. Effects of chemotherapy were variable, with doxorubicin-based regimens being most effective.
The optimal treatment of intra-abdominal desmoids is unknown. Previous studies reported high recurrence rates after surgery and a low success rate of radical removal of desmoid tumours (Rodriguez-Bigas et al, 1994; Heiskanen and Järvinen, 1996). On the other hand, favourable outcomes have been reported after surgery performed by experienced surgeons in carefully selected patients (Latchford et al, 2006). In our series, none of the desmoids could be radically resected and the PFS was similar after surgery compared to conservative treatment. Based on these findings, a conservative approach appears to be the preferred choice in patients with large stable or slowly growing desmoids. Only in cases of progressively growing desmoids, with complications such as obstruction of the small bowel, surgical treatment might be an option. In such patients, minimal surgery (intestinal bypass) could be performed. In patients with obstruction of the ureter, stenting of the ureter might be indicated. These conclusions support the current guidelines on the treatment of desmoid tumours (http://www.nccn.org) (Casali and Blay, 2010).
Extra-abdominal and abdominal wall desmoid tumours are generally more suitable for surgical therapy than mesentery desmoids. Previous studies reported mainly good outcomes after surgery, although recurrence was common after excision (Clark et al, 1999; Melis et al, 2008). Recent reports proposed a wait-and-see policy for patients in which major surgical defects are expected, because spontaneous regression or tumour stabilisation is not uncommon (Bonvalot et al, 2008; Fiore et al, 2009; Stoeckle et al, 2009). In our series, the majority of extra-abdominal and abdominal wall desmoids was resected, with overall favourable outcomes, despite only few radical resections. Based on this and previous studies, surgery seems to be safe for extra-abdominal and abdominal wall desmoid tumours. In patients in which surgery would result in serious defects, a wait-and-see strategy should be considered.
Commonly used pharmacological agents are NSAIDs and hormonal agents. One systematic review showed favourable outcomes after using NSAIDs and hormonal agents, although the results might be confounded by successful case reports (Janinis et al, 2003). Another prospective study showed the effectiveness of high-dose tamoxifen (120 mg) and sulindac (300 mg) in 9 out of 13 patients (69%), compared to stabilisation after surgery and medication in only 1 out of 4 patients (25%) after 10 years of follow-up (Hansmann et al, 2004). Based on these results, the authors advised high-dose tamoxifen and sulindac as the primary treatment for FAP-related desmoid tumours. Recently, another retrospective study reported effective hormonal therapy for desmoid tumours (De Camargo et al, 2010). Our study showed no significant differences in PFS rates between NSAIDs and hormonal treatment including a combination of both medicines. The PFS was about 50% at 5 years of follow-up. However, patients in our study received various doses of hormonal agents. Possibly, hormonal treatment at higher doses would have led to significant better outcomes. Based on personal experience from our authors (H.G.), the optimal dose of tamoxifen is 40 mg 4 times a day, and for toremifene 60 mg 4 times a day. Based on this and previous studies, treatment with NSAIDs and/or hormonal agents seems to be the best option for large and/or progressive desmoids.
Recently, several studies reported successful treatment of desmoids with pegylated liposomal doxorubicin, with acceptable side effects (Gega et al, 2006; Bertagnolli et al, 2008; Constantinidou et al, 2009; De Camargo et al, 2010). All four patients in our study who reached stabilisation or regression after chemotherapy were treated with doxorubicin. Our findings and those of others suggest that (pegylated liposomal) doxorubicin-based chemotherapeutic regimens are effective for patients with progressive, symptomatic desmoid tumours. Inspite of previous promising reports (Heinrich et al, 2006; Wcisio et al, 2007), imatinib treatment had no evident positive effects in our patients. Long-term effects of targeted therapies are yet to be evaluated. Recently, a study to evaluate imatinib in desmoid tumours was initiated (NCT01137916).
In the past, radiotherapy alone or in combination with surgery was shown to be effective in sporadic, mainly extra-abdominal desmoid tumours (Ballo et al, 1999; Nuyttens et al, 2000; Lev et al, 2007; Guadagnolo et al, 2008). Radiotherapy enabled surgery in one of our patients, but in other patients disease progression after radiotherapy was observed. Recently, an EORTC study (EORTC-62991, EORTC-22998, and NCT00030680) was performed to evaluate moderate dose radiotherapy for inoperable desmoid tumours. Results of this study are not yet available. According to the American National Comprehensive Cancer Network guidelines, radiotherapy should be considered only in desmoid tumours located at the extremities (http://www.nccn.org). Embolisation showed not to be a reliable option for desmoid treatment.
In the current study, we evaluated the effectiveness of long-term treatment of desmoid tumours in FAP patients. Nationwide data, both from university hospitals as well as local hospitals were included, thus avoiding potential confounding by patient selection. However, because of the retrospective study design, we were not able to gain information about the selection of patients for certain treatment modalities. Nevertheless, this is a complete and informative series on desmoid treatment to date. For future studies, a prospective, randomised study design would be a more robust approach to this research question.
For clinical practice, we recommend surgery for patients with extra-abdominal and abdominal wall desmoid tumours, unless major surgical defects are expected. For patients with stable intra-abdominal desmoid tumours, both a wait-and-see strategy as well as pharmacological treatment are appropriate. Cytotoxic chemotherapy may be effective in patients with progressively growing desmoids. In the case of severe complications, including ileus, perforations, and abscesses, surgery is indicated.
In conclusion, desmoid disease is a heterogeneous disease entity, with various treatment modalities. Clustering of desmoid patients in some specialised referral centres will benefit treatment and follow-up, and enables further research into this controversial topic.
Acknowledgments
We are grateful to Sara L Herd for checking spelling and grammar.
Footnotes
The authors declare no conflict of interest.
References
- Arvanitis ML, Jagelman DG, Fazio VW, Lavery IC, McGannon E (1990) Mortality in patients with familial adenomatous polyposis. Dis Colon Rectum 33: 639–642 [DOI] [PubMed] [Google Scholar]
- Ballo MT, Zagars GK, Pollack A, Pisters PW, Pollack RA (1999) Desmoid tumor: prognostic factors and outcome after surgery, radiation therapy, or combined surgery and radiation therapy. J Clin Onc 17: 158–167 [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, Morgan JA, Fletcher CDM, Raut CP, Dileo P, Gill RR, Demetri GD, George S (2008) Multimodality treatment of mesenteric desmoid tumours. Eur J Cancer 44: 2404–2410 [DOI] [PubMed] [Google Scholar]
- Bertario L, Russo A, Sala P, Eboli M, Giarola M, Varesco L, Pierotti MA, Radice P, Hereditary Colorectal Tumours Registry (2001) Genotype and phenotype factors as determinants of desmoid tumors in patients with familial adenomatous polyposis. Int J Cancer 95: 102–107 [DOI] [PubMed] [Google Scholar]
- Bonvalot S, Eldweny H, Haddad V, Rimareix F, Missenard G, Oberlin O, Vanel D, Terrier P, Blay JY, Le Cesne A, Le Pechoux C (2008) Extra-abdominal primary fibromatosis: aggressive management could be avoided in a subgroup of patients. EJSO 34: 462–468 [DOI] [PubMed] [Google Scholar]
- Casali PG, Blay JY, ESMO/CONTICANET/EUROBONET Consensus Panel of experts (2010) Soft tissue sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 21(Suppl 5): v198–v203 [DOI] [PubMed] [Google Scholar]
- Clark SK, Neale KF, Landgrebe JC, Phillips RK (1999) Desmoid tumours complicating familial adenomatous polyposis. Br J Surg 86: 1185–1189 [DOI] [PubMed] [Google Scholar]
- Constantinidou A, Jones RL, Scurr M, Al-Muderis O, Judson I (2009) Pegylated liposomal doxorubicin, an effective, well-tolerated treatment for refractory aggressive fibromatosis. Eur J Cancer 45: 2930–2934 [DOI] [PubMed] [Google Scholar]
- De Camargo VP, Keohan ML, D’Adamo DR, Antonescu CR, Brennan MF, Singer S, Ahn LS, Maki RG (2010) Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 116: 2258–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallen T, Wilson M, Morlan B, Lindor NM (2006) Desmoid tumors-a characterization of patients seen at Mayo Clinic 1976–1999. Fam Cancer 5: 191–194 [DOI] [PubMed] [Google Scholar]
- Fiore M, Rimareix F, Mariani L, Domont J, Collini P, Le Pechoux C, Casali PG, Le Cesne A, Gronchi A, Bonvalot S (2009) Desmoid-type fibromatosis: a front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 16: 2587–2593 [DOI] [PubMed] [Google Scholar]
- Gega M, Yanagi H, Yoshikawa R, Noda M, Ikeuchi H, Tsukamoto K, Oshima T, Fujiwara Y, Gondo N, Tamura K, Utsunomiya J, Hashimoto-Tamaoki T, Yamamura T (2006) Successful chemotherapeutic modality of doxorubicin plus dacarbazine for the treatment of desmoid tumors in association with familial adenomatous polyposis. J Clin Oncol 24: 102–105 [DOI] [PubMed] [Google Scholar]
- Goldblum J, Fletcher JA (2002) Desmoid-type fibromatoses. In World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone, Fletcher CDM, Unni KK, Mertens F (eds) pp 83–84. IARC Press: Lyon [Google Scholar]
- Guadagnolo BA, Zagars GK, Ballo MT (2008) Long-term outcomes for desmoid tumors treated with radiation therapy. Int J Radiat Oncol Biol Phys 71: 441–447 [DOI] [PubMed] [Google Scholar]
- Hansmann A, Adolph C, Vogel T, Unger A, Moeslein G (2004) High-dose tamoxifen and sulindac as first-line treatment for desmoid tumors. Cancer 100: 612–620 [DOI] [PubMed] [Google Scholar]
- Heinrich MC, McArthur GA, Demetri GD, Joensuu H, Bono P, Herrmann R, Hirte H, Cresta S, Koslin DB, Corless CL, Dirnhofer S, van Oosterom AT, Nikolova Z, Dimitrijevic S, Fletcher JA (2006) Clinical and molecular studies of the effect of imatinib on advanced aggressive fibromatosis (desmoid tumor). J Clin Oncol 24: 1195–1203 [DOI] [PubMed] [Google Scholar]
- Heiskanen I, Järvinen HJ (1996) Occurrence of desmoid tumours in familial adenomatous polyposis and results of treatment. Int J Colorect Dis 11: 157–162 [DOI] [PubMed] [Google Scholar]
- Janinis J, Patriki M, Vini L, Aravantinos G, Whelan JS (2003) The pharmacological treatment of aggressive fibromatosis: a systematic review. Ann Oncol 14: 181–190 [DOI] [PubMed] [Google Scholar]
- Latchford AR, Sturt NJH, Neale K, Rogers PA, Phillips RKS (2006) A 10-year review of surgery for desmoid disease associated with familial adenomatous polyposis. Br J Surg 93: 1258–1264 [DOI] [PubMed] [Google Scholar]
- Lev D, Kotilingam D, Wei C, Ballo MT, Zagars GK, Pisters PW, Lazar AA, Patel SR, Benjamin RS, Pollock RE (2007) Optimizing treatment of desmoid tumors. J Clin Oncol 25: 1785–1791 [DOI] [PubMed] [Google Scholar]
- Lynch HT, Lynch JF, Lynch PM, Attard T (2008) Hereditary colorectal cancer syndromes: molecular genetics, genetic counseling, diagnosis and management. Fam Cancer 7: 27–39 [DOI] [PubMed] [Google Scholar]
- Melis M, Zager JS, Sondak VK (2008) Multimodality management of desmoid tumors: how important is a negative surgical margin? J Surg Oncol 98: 594–602 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis MH, Casparie M, Mathus-Vliegen E, Dekkers OM, Vasen HFA (2010) A nation-wide study comparing sporadic and familial adenomatous polyposis (FAP) related desmoid-type fibromatoses. Int J Cancer, Doi: 10.1002/ijc.25664 [DOI] [PubMed]
- Nuyttens JJ, Rust PF, Thomas CR, Turrisi III AT (2000) Surgery versus radiation therapy for patients with aggressive fibromatosis or desmoid tumors. A comparative review of 22 articles. Cancer 88: 1517–1523 [PubMed] [Google Scholar]
- Rodriguez-Bigas MA, Mahoney MC, Karakousis CP, Petrelli NJ (1994) Desmoid tumors in patients with familial adenomatous polyposis. Cancer 74: 1270–1274 [DOI] [PubMed] [Google Scholar]
- Sleijfer S (2009) Management of aggressive fibromatosis: can we unravel the maze of treatment options? Eur J Cancer 45: 2928–2929 [DOI] [PubMed] [Google Scholar]
- Stoeckle E, Coindre JM, Longy M, Bui Nguyen Binh M, Kantor G, Kind M, Tunon de Lara C, Avril A, Bonichon F, Nguyen Bui B (2009) A critical analysis of treatment strategies in desmoid tumours: a review of a series of 106 cases. Eur J Surg Oncol 35: 129–134 [DOI] [PubMed] [Google Scholar]
- Tolan S, Shanks JH, Loh MY, Taylor B, Wylie JP (2007) Fibromatosis: benign by name but not necessarily by nature. Clin Oncol 19: 319–326 [DOI] [PubMed] [Google Scholar]
- Wcisio G, Szarlej-Wcisio K, Szczylic C (2007) Control of aggressive fibromatosis by treatment with imatinib mesylate. A case-report and review of the literature. J Cancer Res Clin Oncol 133: 533–538 [DOI] [PMC free article] [PubMed] [Google Scholar]