Abstract
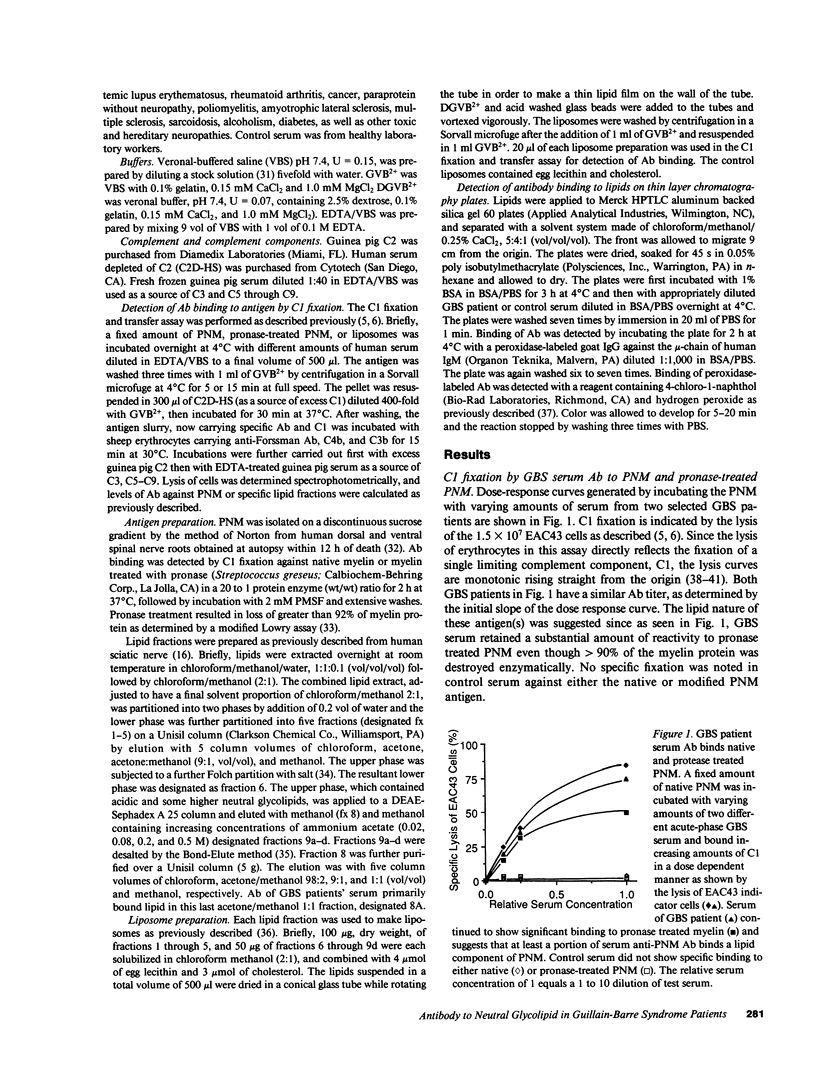
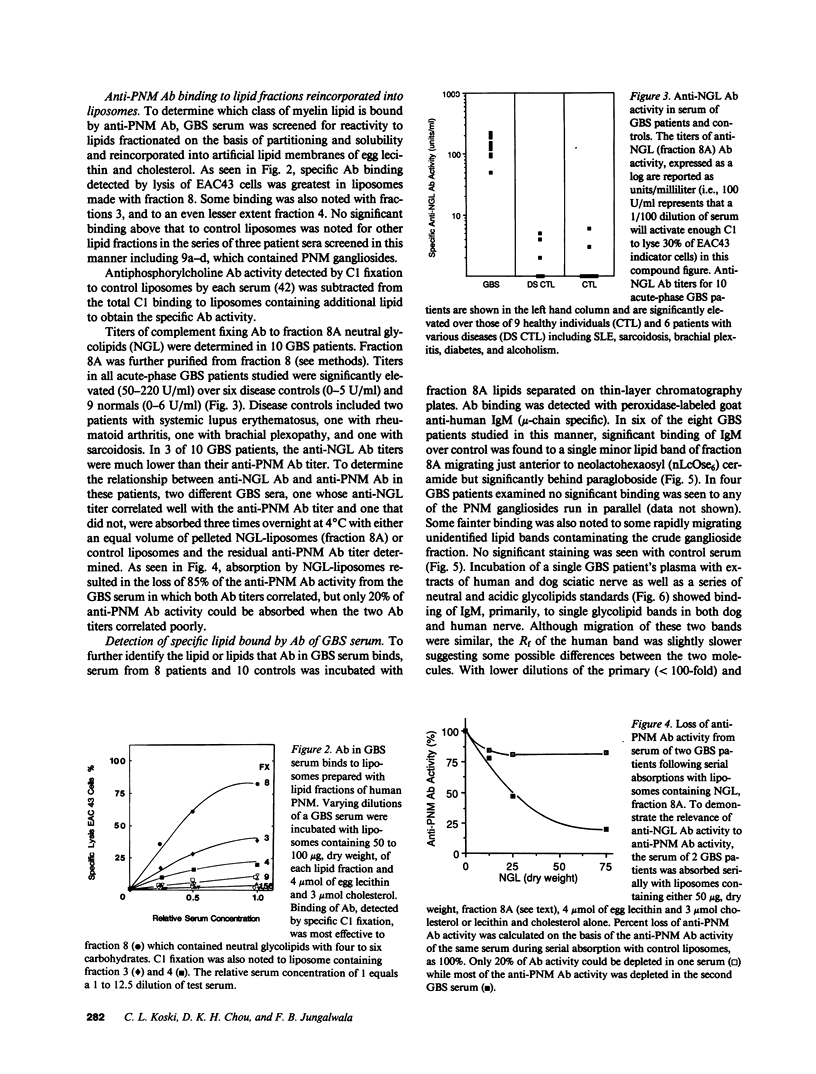
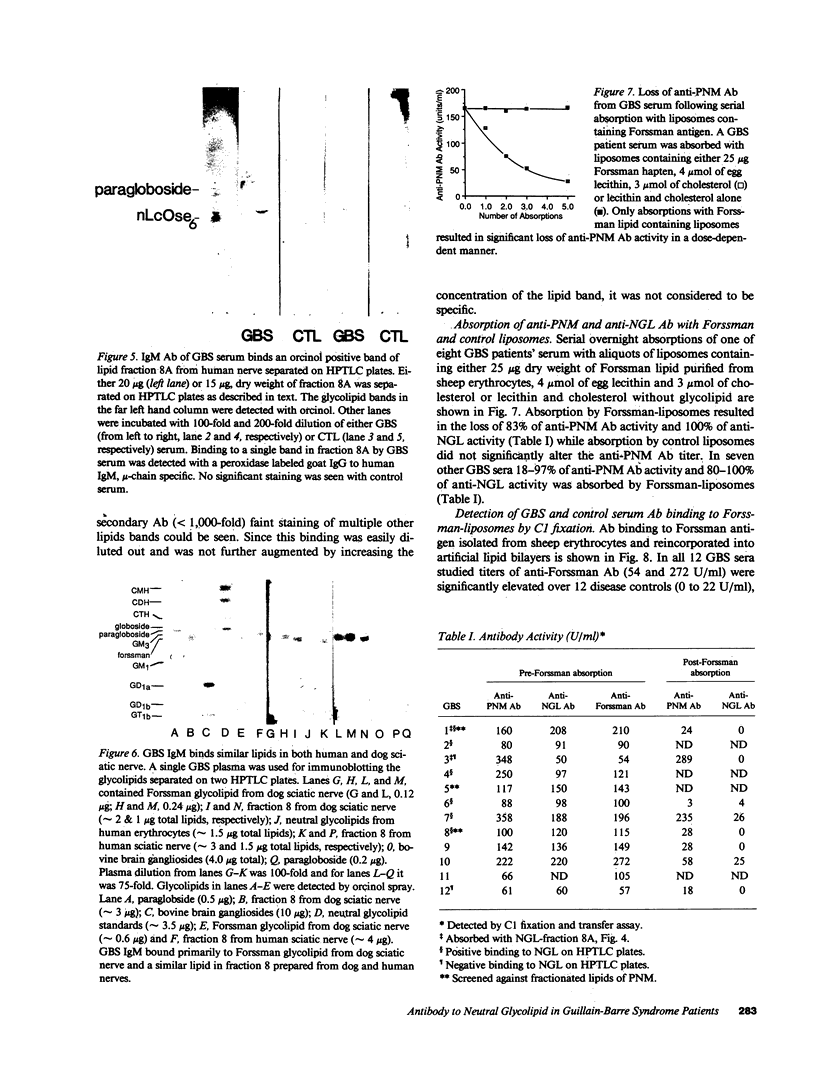
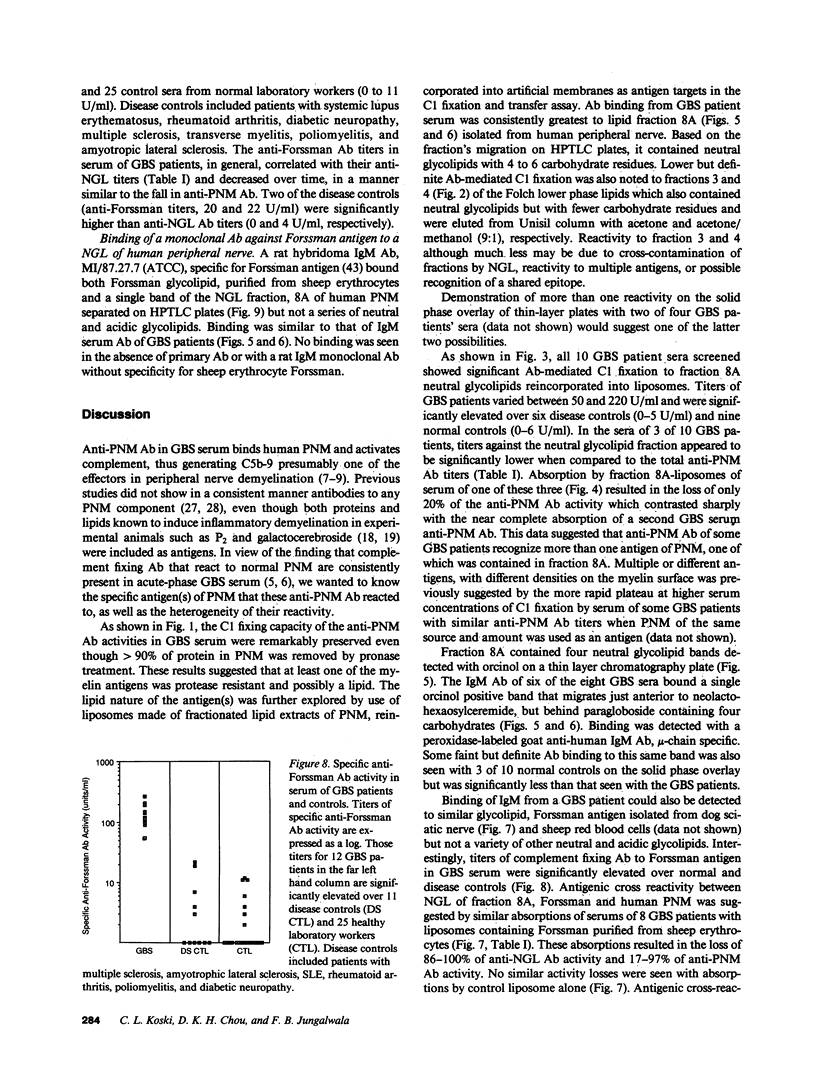
During acute-phase illness, serum of patients with Guillain-Barre syndrome (GBS) contain complement-fixing antibodies (Ab) to peripheral nerve myelin (PNM). We investigated PNM lipids as putative antigens for these Ab since GBS serum retained significant reactivity to PNM treated with protease. Ab binding to specific lipids was studied with a C1 fixation and transfer (C1FT) assay using fractions of PNM lipid reincorporated into liposomes as antigen targets or to lipids on HPTLC plates with peroxidase-labeled goat Ab to human IgM. Reactivity was detected to a neutral glycolipid (NGL) of human PNM with a similar number of carbohydrates residues to that of Forssman hapten (Forss). Anti-NGL Ab titers in GBS patients (50-220 U/ml) were significantly elevated over disease and normal controls (0-5 and 0-6 U/ml). We studied possible antigenic cross-reactivity of these Ab with Forss by first quantitating Ab activity with C1FT assay and liposomes containing Forss. All 12 GBS sera tested showed titers (54-272 U/ml) significantly elevated over 11 disease controls (0-22 U/ml) and 25 normal controls (0-11 U/ml). GBS serum Ab reacted with Forss isolated from dog nerve or sheep erythrocytes on HPTLC plates. Further, absorption of 80-100% of anti-NGL Ab activity and 17-97% of anti-PNM Ab activity from eight GBS patient serums was accomplished with liposomes containing Forss but not with control liposomes. In seven GBS patients anti-NGL Ab activity represented only a portion of anti-PNM Ab activity. These results suggest that a glycolipid with antigenic cross-reactivity to Forssman hapten may be responsible for some of the anti-PNM Ab activity in GBS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Joseph K. C., Wistar R. Influence of membrane composition on the interaction of a human monoclonal "anti-Forssman" immunoglobulin with liposomes. Biochemistry. 1974 Nov 5;13(23):4818–4824. doi: 10.1021/bi00720a021. [DOI] [PubMed] [Google Scholar]
- Alving C. R. Natural antibodies against phospholipids and liposomes in humans. Biochem Soc Trans. 1984 Apr;12(2):342–344. doi: 10.1042/bst0120342. [DOI] [PubMed] [Google Scholar]
- Armati-Gulson P. J., Lisak R. P., Kuchmy D., Pollard J. 51Cr release cytotoxicity radioimmunoassay to detect immune cytotoxic reactions to rat Schwann cells in vitro. Neurosci Lett. 1983 Mar 14;35(3):321–326. doi: 10.1016/0304-3940(83)90338-5. [DOI] [PubMed] [Google Scholar]
- Baker J. R., Bullock G. R., Butler K. D., Williamson I. H., White A. M. An ultrastructural analysis of the vascular damage in the lethal and sublethal Forssman reaction in the guinea-pig. Br J Exp Pathol. 1985 Dec;66(6):709–718. [PMC free article] [PubMed] [Google Scholar]
- Borsos T., Colten H. R., Spalter J. S., Rogentine N., Rapp H. J. The C'la fixation and transfer test: examples of its applicability to the detection and enumeration of antigens and antibodies at cell surfaces. J Immunol. 1968 Sep;101(3):392–398. [PubMed] [Google Scholar]
- Brostoff S. W., Levit S., Powers J. M. Induction of experimental allergic neuritis with a peptide from myelin P2 basic protein. Nature. 1977 Aug 25;268(5622):752–753. doi: 10.1038/268752a0. [DOI] [PubMed] [Google Scholar]
- Chou D. K., Ilyas A. A., Evans J. E., Costello C., Quarles R. H., Jungalwala F. B. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J Biol Chem. 1986 Sep 5;261(25):11717–11725. [PubMed] [Google Scholar]
- Chou D. K., Schwarting G. A., Evans J. E., Jungalwala F. B. Sulfoglucuronyl-neolacto series of glycolipids in peripheral nerves reacting with HNK-1 antibody. J Neurochem. 1987 Sep;49(3):865–873. doi: 10.1111/j.1471-4159.1987.tb00974.x. [DOI] [PubMed] [Google Scholar]
- Criteria for diagnosis of Guillain-Barré syndrome. Ann Neurol. 1978 Jun;3(6):565–566. doi: 10.1002/ana.410030628. [DOI] [PubMed] [Google Scholar]
- Dellagi K., Brouet J. C., Danon F. Cross-idiotypic antigens among monoclonal immunoglobulin M from patients with Waldenström's macroglobulinemia and polyneuropathy. J Clin Invest. 1979 Nov;64(5):1530–1534. doi: 10.1172/JCI109612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M., Buyse M., Buyse G., Gorce F. The action of Guillain-Barre syndrome serum on myelin. A tissue culture and electron microscopic analysis. J Neurol Sci. 1971 May;13(1):67–83. doi: 10.1016/0022-510x(71)90207-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Feasby T. E., Hahn A. F., Gilbert J. J. Passive transfer studies in Guillain-Barré polyneuropathy. Neurology. 1982 Oct;32(10):1159–1167. doi: 10.1212/wnl.32.10.1151-a. [DOI] [PubMed] [Google Scholar]
- Hakomori S., Wang S. M., Young W. W., Jr Isoantigenic expression of Forssman glycolipid in human gastric and colonic mucosa: its possible identity with "A-like antigen" in human cancer. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3023–3027. doi: 10.1073/pnas.74.7.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung H. P., Schwenke C., Bitter-Suermann D., Toyka K. V. Guillain-Barré syndrome: activated complement components C3a and C5a in CSF. Neurology. 1987 Jun;37(6):1006–1009. doi: 10.1212/wnl.37.6.1006. [DOI] [PubMed] [Google Scholar]
- Hays A. P., Latov N., Takatsu M., Sherman W. H. Experimental demyelination of nerve induced by serum of patients with neuropathy and an anti-MAG IgM M-protein. Neurology. 1987 Feb;37(2):242–256. doi: 10.1212/wnl.37.2.242. [DOI] [PubMed] [Google Scholar]
- Ilyas A. A., Quarles R. H., MacIntosh T. D., Dobersen M. J., Trapp B. D., Dalakas M. C., Brady R. O. IgM in a human neuropathy related to paraproteinemia binds to a carbohydrate determinant in the myelin-associated glycoprotein and to a ganglioside. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1225–1229. doi: 10.1073/pnas.81.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilyas A. A., Willison H. J., Quarles R. H., Jungalwala F. B., Cornblath D. R., Trapp B. D., Griffin D. E., Griffin J. W., McKhann G. M. Serum antibodies to gangliosides in Guillain-Barré syndrome. Ann Neurol. 1988 May;23(5):440–447. doi: 10.1002/ana.410230503. [DOI] [PubMed] [Google Scholar]
- Julien J., Vital C., Vallat J. M., Lagueny A., Deminiere C., Darriet D. Polyneuropathy in Waldenström's macroglobulinemia. Deposition of M component on myelin sheaths. Arch Neurol. 1978 Jul;35(7):423–425. doi: 10.1001/archneur.1978.00500310025005. [DOI] [PubMed] [Google Scholar]
- Kano K., Milgrom F. Heterophile antigens and antibodies in medicine. Curr Top Microbiol Immunol. 1977;77:43–69. doi: 10.1007/978-3-642-66740-4_2. [DOI] [PubMed] [Google Scholar]
- Koski C. L., Gratz E., Sutherland J., Mayer R. F. Clinical correlation with anti-peripheral-nerve myelin antibodies in Guillain-Barré syndrome. Ann Neurol. 1986 Jun;19(6):573–577. doi: 10.1002/ana.410190609. [DOI] [PubMed] [Google Scholar]
- Koski C. L. Guillain-Barré syndrome. Neurol Clin. 1984 May;2(2):355–366. [PubMed] [Google Scholar]
- Koski C. L., Humphrey R., Shin M. L. Anti-peripheral myelin antibody in patients with demyelinating neuropathy: quantitative and kinetic determination of serum antibody by complement component 1 fixation. Proc Natl Acad Sci U S A. 1985 Feb;82(3):905–909. doi: 10.1073/pnas.82.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski C. L., Sanders M. E., Swoveland P. T., Lawley T. J., Shin M. L., Frank M. M., Joiner K. A. Activation of terminal components of complement in patients with Guillain-Barré syndrome and other demyelinating neuropathies. J Clin Invest. 1987 Nov;80(5):1492–1497. doi: 10.1172/JCI113231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latov N., Sherman W. H., Nemni R., Galassi G., Shyong J. S., Penn A. S., Chess L., Olarte M. R., Rowland L. P., Osserman E. F. Plasma-cell dyscrasia and peripheral neuropathy with a monoclonal antibody to peripheral-nerve myelin. N Engl J Med. 1980 Sep 11;303(11):618–621. doi: 10.1056/NEJM198009113031105. [DOI] [PubMed] [Google Scholar]
- Levine P. Blood group and tissue genetic markers in familial adenocarcinoma: potential specific immunotherapy. Semin Oncol. 1978 Mar;5(1):25–34. [PubMed] [Google Scholar]
- MELNICK S. C. Thirty-eight cases of the Guillain-Barre syndrome: an immunological study. Br Med J. 1963 Feb 9;1(5327):368–373. doi: 10.1136/bmj.1.5327.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Nobile-Orazio E., Hays A. P., Latov N., Perman G., Golier J., Shy M. E., Freddo L. Specificity of mouse and human monoclonal antibodies to myelin-associated glycoprotein. Neurology. 1984 Oct;34(10):1336–1342. doi: 10.1212/wnl.34.10.1336. [DOI] [PubMed] [Google Scholar]
- Norton W. T. Isolation of myelin from nerve tissue. Methods Enzymol. 1974;31:435–444. doi: 10.1016/0076-6879(74)31049-x. [DOI] [PubMed] [Google Scholar]
- Nowinski R., Berglund C., Lane J., Lostrom M., Bernstein I., Young W., Hakomori S. I., Hill L., Cooney M. Human monoclonal antibody against Forssman antigen. Science. 1980 Oct 31;210(4469):537–539. doi: 10.1126/science.7423202. [DOI] [PubMed] [Google Scholar]
- Osterman P. O., Fagius J., Lundemo G., Pihlstedt P., Pirskanen R., Sidén A., Säfwenberg J. Beneficial effects of plasma exchange in acute inflammatory polyradiculoneuropathy. Lancet. 1984 Dec 8;2(8415):1296–1299. doi: 10.1016/s0140-6736(84)90819-5. [DOI] [PubMed] [Google Scholar]
- Propp R. P., Means E., Deibel R., Sherer G., Barron K. Waldenström's macroglobulinemia and neuropathy. Deposition of M-component on myelin sheaths. Neurology. 1975 Oct;25(10):980–988. doi: 10.1212/wnl.25.10.980. [DOI] [PubMed] [Google Scholar]
- Ramm L. E., Whitlow M. B., Mayer M. M. Transmembrane channel formation by complement: functional analysis of the number of C5b6, C7, C8, and C9 molecules required for a single channel. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4751–4755. doi: 10.1073/pnas.79.15.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami A. M., Burns J. B., Eccleston P. A., Manning M. C., Lisak R. P., Silberberg D. H. Search for antibodies to galactocerebroside in the serum and cerebrospinal fluid in human demyelinating disorders. Ann Neurol. 1987 Sep;22(3):381–383. doi: 10.1002/ana.410220316. [DOI] [PubMed] [Google Scholar]
- Saida T., Saida K., Dorfman S. H., Silberberg D. H., Sumner A. J., Manning M. C., Lisak R. P., Brown M. J. Experimental allergic neuritis induced by sensitization with galactocerebroside. Science. 1979 Jun 8;204(4397):1103–1106. doi: 10.1126/science.451555. [DOI] [PubMed] [Google Scholar]
- Saida T., Saida K., Lisak R. P., Brown M. J., Silberberg D. H., Asbury A. K. In vivo demyelinating activity of sera from patients with Guillain-Barré syndrome. Ann Neurol. 1982 Jan;11(1):69–75. doi: 10.1002/ana.410110112. [DOI] [PubMed] [Google Scholar]
- Saida T., Saida K., Silberberg D. H. Demyelination produced by experimental allergic neuritis serum and anti-galactocerebroside antiserum in CNS cultures. An ultrastructural study. Acta Neuropathol. 1979 Oct;48(1):19–25. doi: 10.1007/BF00691786. [DOI] [PubMed] [Google Scholar]
- Sanders M. E., Koski C. L., Robbins D., Shin M. L., Frank M. M., Joiner K. A. Activated terminal complement in cerebrospinal fluid in Guillain-Barré syndrome and multiple sclerosis. J Immunol. 1986 Jun 15;136(12):4456–4459. [PubMed] [Google Scholar]
- Schluesener H. J., Sobel R. A., Linington C., Weiner H. L. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J Immunol. 1987 Dec 15;139(12):4016–4021. [PubMed] [Google Scholar]
- Seil F. J., Kies M. W., Bacon M. L. A comparison of demyelinating and myelination-inhibiting factor induction by whole peripheral nerve tissue and P2 protein. Brain Res. 1981 Apr 6;210(1-2):441–448. doi: 10.1016/0006-8993(81)90924-0. [DOI] [PubMed] [Google Scholar]
- Shin M. L., Paznekas W. A., Abramovitz A. S., Mayer M. M. On the mechanism of membrane damage by C: exposure of hydrophobic sites on activated C proteins. J Immunol. 1977 Oct;119(4):1358–1364. [PubMed] [Google Scholar]
- Springer T., Galfrè G., Secher D. S., Milstein C. Monoclonal xenogeneic antibodies to murine cell surface antigens: identification of novel leukocyte differentiation antigens. Eur J Immunol. 1978 Aug;8(8):539–551. doi: 10.1002/eji.1830080802. [DOI] [PubMed] [Google Scholar]
- Steck A. J., Murray N., Justafre J. C., Meier C., Toyka K. V., Heininger K., Stoll G. Passive transfer studies in demyelinating neuropathy with IgM monoclonal antibodies to myelin-associated glycoprotein. J Neurol Neurosurg Psychiatry. 1985 Sep;48(9):927–929. doi: 10.1136/jnnp.48.9.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi N., Yokosawa N., Narita M., Mitsuyama T., Makita A. Expression of Forssman antigen synthesis and degradation in human lung cancer. J Natl Cancer Inst. 1981 Sep;67(3):577–583. [PubMed] [Google Scholar]
- Williams M. A., McCluer R. H. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J Neurochem. 1980 Jul;35(1):266–269. doi: 10.1111/j.1471-4159.1980.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Young W. W., Jr, Hakomori S. I., Levine P. Characterization of anti-Forssman (anti-Fs) antibodies in human sera: their specificity and possible changes in patients with cancer. J Immunol. 1979 Jul;123(1):92–96. [PubMed] [Google Scholar]