Abstract
Background:
α-Tocopherol ether-linked acetic acid (α-TEA) is a promising agent for cancer prevention/therapy based on its antitumour actions in a variety of cancers.
Methods:
Human breast cancer cells, MCF-7 and HCC-1954, were used to study the effect of α-TEA using Annexin V/PI staining, western blot analyses, and siRNA knockdown techniques.
Results:
α-Tocopherol ether-linked acetic acid suppressed constitutively active basal levels of pAKT, pERK, pmTOR, and their downstream targets, as well as induced both cell types to undergo apoptosis. Phosphoinositide 3-kinase (PI3K) inhibitor wortmannin suppressed pAKT, pERK, pmTOR, and their downstream targets, indicating PI3K to be a common upstream mediator. In addition, α-TEA induced increased levels of pIRS-1 (Ser-307), a phosphorylation site correlated with insulin receptor substrate-1 (IRS-1) inactivation, and decreased levels of total IRS-1. Small interfering RNA (siRNA) knockdown of JNK blocked the impact of α-TEA on pIRS-1 and total IRS-1 and impeded its ability to downregulate the phosphorylated status of AKT, ERK, and mTOR. Combinations of α-TEA+MEK or mTOR inhibitor acted cooperatively to induce apoptosis and reduce basal levels of pERK and pmTOR. Importantly, inhibition of MEK and mTOR resulted in increased levels of pAKT and IRS-1, and α-TEA blocked them.
Conclusions:
Downregulation of IRS-1/PI3K pathways via JNK are critical for α-TEA and α-TEA+MEK or mTOR inhibitor-induced apoptosis in human MCF-7 and HCC-1954 breast cancer cells.
Keywords: α-TEA, Akt, breast cancer, ERK, IRS-1, mTOR
The PI3K, AKT, ERK, and mTOR prosurvival mediators are important therapeutic targets, as they are constitutively activated in many cancers and contribute to cancer progression by promoting cellular proliferation and inhibiting cell death signalling pathways (Falasca, 2010). Phosphoinositide 3-kinase (PI3K) is activated at the cell membrane by tyrosine kinase growth factor receptors, such as members of the epidermal growth factor receptor family (EGFR and Her-2), and by the insulin-like growth factor-1 receptor (IGFR), as well as its downstream signalling substrate IRS-1 (insulin receptor substrate-1; Schlessinger, 2000). Phosphoinositide 3-kinase promotes cancer cell survival by activation of downstream mediators AKT and Ras, the latter leading to ERK activation (McCubrey et al, 2007). AKT exerts its survival role via a diverse array of substrates, which control key cellular processes, including apoptosis, cell cycle progression, transcription, and translation (Chang et al, 2003). A major downstream substrate of AKT is the serine/threonine kinase mTOR. AKT can directly phosphorylate mTOR at ser-2448 and activate it, as well as cause indirect activation of mTOR by phosphorylating and inactivating TSC2 (tuberous sclerosis complex 2, also called tuberin). The raptor–mTOR complex signals to its downstream effectors S6 kinase/ribosomal protein S6 (p70S6K) and the eIF4E-binding protein (p4E-BP1) to control transcription and translation, which selectively regulates multiple proteins that control cell cycle and apoptosis (Gibbons et al, 2009). Additionally, AKT can directly regulate apoptosis by phosphorylating and inactivating proapoptotic proteins such as Bad and caspase-9 (Datta et al, 1997; Cardone et al, 1998; Mabuchi et al, 2002). Extracellular signal-regulated kinase (ERK) exerts its antiapoptotic effects by phosphorylating and inactivating Bad (Mabuchi et al, 2002). As with most intracellular signalling cascades, cross-talk and negative and positive feedback loops complicate final signalling outcomes.
For example, the mTOR substrate p70S6K can ultimately diminish prosurvival signalling via PI3K/AKT by catalysing an inhibitory phosphorylation site on insulin receptor substrate-1, an upstream mediator of PI3K (Wan et al, 2007). Likewise, ERK can diminish prosurvival signalling by PI3K/AKT via p70S6K (Jiang et al, 2009). Therefore, although ERK and mTOR showed potential as anticancer targets, inhibitors of ERK or mTOR alone are limited in clinical application because of the mitigation of these negative feedback loops essential for controlling AKT activity (Sun et al, 2005). Thus, this more in-depth understanding of signalling pathways suggests that ERK or mTOR inhibitors need to be combined with agents that can circumvent the loss of negative feedback controls on AKT and/or effectively block AKT activity.
α-Tocopherol ether-linked acetic acid (α-TEA) is a promising agent for cancer prevention/therapy based on its antitumour actions reported in several in vitro and in vivo studies on a variety of cancers, including human oestrogen-responsive and nonresponsive breast cancers (Anderson et al, 2002; Lawson et al, 2004; Shun et al, 2004; Hahn et al, 2006; Yu et al, 2006; Jia et al, 2008a, 2008b; Wang et al, 2008; Hahn et al, 2009; Shun et al, 2010). These previous studies showed that α-TEA induces apoptosis in human breast cancer cells via activation of proapoptotic extrinsic death receptor Fas and DR5 as well as activation of a JNK/p73/Noxa pathway, leading to activation of caspase-8 and mitochondrial-dependent apoptosis (Shun et al, 2004; Wang et al, 2008; Yu et al, 2010). In this study, we demonstrate that α-TEA induces apoptosis not only by activation of pro-apoptotic pathways but also by targeting IRS-1/PI3K growth/survival pathways to suppress multiple prosurvival factors via c-Jun N-terminal kinase (JNK). Lessons learned from this study are that α-TEA in combination with ERK or mTOR inhibitors is an effective anticancer strategy and lays the groundwork for a mechanism-based combinational therapeutic strategy for employing ERK and mTOR inhibitors more effectively in the clinic.
Materials and methods
Chemicals
α-Tocopherol ether-linked acetic acid (FW=488.8) was prepared in-house as described previously (Lawson et al, 2004). Map kinase kinase (MEK) inhibitor (U01260) and PI3K inhibitor (wortmannin) were purchased from Cell Signaling Technology (Beverly, MA, USA). Mammalian target of rapamycin (mTOR) inhibitor (rapamycin) and JNK inhibitor (SP600125) were purchased from Calbiochem (La Jolla, CA, USA).
Cell culture
The oestrogen receptor-negative human breast cancer cells, HCC-1954, were obtained from the American Type Culture Collection (Manassas, VA, USA). The oestrogen-responsive human breast cancer cells, MCF-7, were originally provided by Dr Suzanne Fuqua (Baylor College of Medicine, Houston, TX, USA). The MCF-7 cells were cultured as previously described (Wang et al, 2008). The HCC-1954 cells were cultured in RPMI media with 10% FBS. For experiments, FBS was reduced to 2% and cells were allowed to attach overnight before treatments. α-Tocopherol ether-linked acetic acid (40 mM) dissolved in ethanol served as stock solution. Equivalent final concentrations of ethanol (0.05–0.1%) for concentration of α-TEA used served as vehicle controls (VEH).
Western blot analyses
Whole-cell protein lysates and western blot analyses were conducted as described previously (Jia et al, 2008a, 2008b). Proteins at 20–50 μg per lane were separated by SDS–PAGE and transferred to nitrocellulose (Optitran BA-S supported nitrocellulose; Schleicher and Schuell, Keene, NH, USA). Antibodies to the following proteins were used: poly (ADP-ribose) polymerase (PARP), phospho-JNK (pJNK thr-183/tyr-185), phospho-ERK (pERK thr-202/tyr-204), total ERK and phospho-caspase-9 (pcaspase-9 ser-196) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Phospho-AKT (pAKT ser-473), pAKT (ser-308), total AKT, phospho-GSK (pGSK α/β ser-21/9), total GSK α/β, phospho-Bad (pBad ser-136), phospho-Bad (pBad ser-112), total Bad, total casapse-9, phospho-p90RSK (pp90RSK ser-380), total p90RSK, phospho-mTOR (pmTOR ser-2448), total mTOR, phospho-p70S6K (pp70S6K thr-389), total p70S6K, phospho-4E-BP1 (p4E-BP1 thr-37/46), total 4E-BP1, phospho-IRS (pIRS ser-307), total IRS, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology).
Small interfering RNA (siRNA) transfection
A scrambled RNA duplex that does not target any known genes was used as a nonspecific negative control for RNAi (referred to as control siRNA) (Ambion, Austin, TX, USA). Transfection of siRNAs to AKT1, AKT2, JNK1/2, or control (Ambion) was performed in 100 mm2 cell culture dishes at a density of 2 × 106 cells per dish using Lipofectamine-2000 (Invitrogen, Carlsbad, CA, USA) and siRNA duplex, resulting in a final siRNA concentration of 30 nM following the company's instructions. At 1 day after transfection, the cells were re-cultured in 100 mm2 dishes at 2 × 106 cells per dish and incubated for 1 day followed by treatments.
Quantification of apoptosis
Apoptosis was quantified by Annexin V-FITC/propidium iodide (PI) assay following the manufacturer's instructions. The Annexin V-FITC/PI assay measures the amount of phosphatidylserine on the outer surface of the plasma membrane (a biochemical alteration unique to membranes of apoptotic cells) and the amount of PI, a dye that does not cross the plasma membrane of viable cells but readily enters dead cells or cells in the late stages of apoptosis and binds DNA. Fluorescence was measured using fluorescence activated cell sorter (FACS) analyses with a FACSCalibur flow cytometer, and data were analysed using CellQuest software (BD Biosciences, San Jose, CA, USA). Cells displaying phosphatidylserine on their surface (i.e., positive for annexin-V fluorescence) were considered to be apoptotic.
Statistical analysis
Apoptosis data were analysed using a one-way analysis of variance (ANOVA) followed by Tukey's test for comparison of more than two treatments or a two-tailed Student's t-test for comparison between two treatments to determine statistical differences. Differences were considered statistically significant at P<0.05.
Results
α-TEA induces apoptosis in HCC-1954 and MCF-7 human breast cancer cells
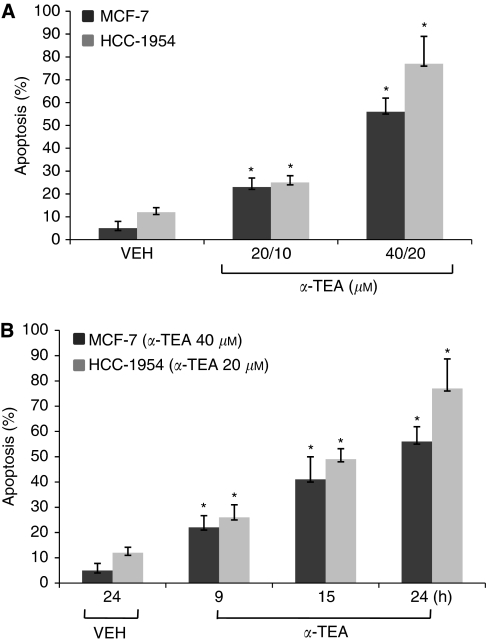
Human breast cancer cells, MCF-7 and HCC-1954, were treated with α-TEA at different doses (Figure 1A) or for different periods of time (Figure 1B). Apoptosis was quantified by FACS analyses of cells labelled with FITC-Annexin V. Treatment of MCF-7 cells with α-TEA at 20 or 40 μM and HCC-1954 cells at 10 or 20 μM for 24 h induced both cell types to undergo apoptosis in a dose-dependent manner, with HCC-1954 cells exhibiting greater sensitivity than MCF-7 cells (Figure 1A). Treatment of the MCF-7 and HCC-1954 cells with α-TEA at 40 and 20 μM, respectively, for 9, 15, and 24 h induced apoptosis in a time-dependent manner (Figure 1B). These data show that α-TEA is a potent apoptotic inducer of both ER-responsive and nonresponsive human breast cancer cells.
Figure 1.
α-Tocopherol ether-linked acetic acid (α-TEA) induces apoptosis in human breast cancer cells in a dose- and time-dependent manner. (A) MCF-7 and HCC-1954 cells were treated with different concentrations of α-TEA for 24 h and the proapoptotic property of α-TEA was evaluated by FACS/Annexin V assay. (B) MCF-7 and HCC-1954 cells were treated with 40 and 20 μM α-TEA, respectively, for different times, and the proapoptotic property of α-TEA was evaluated by FACS/Annexin V assay. Data are the mean±s.d. of different independent experiments. *Significantly different from VEH (P<0.05).
α-TEA reduces high basal levels of phosphorylated AKT, ERK1/2, and mTOR
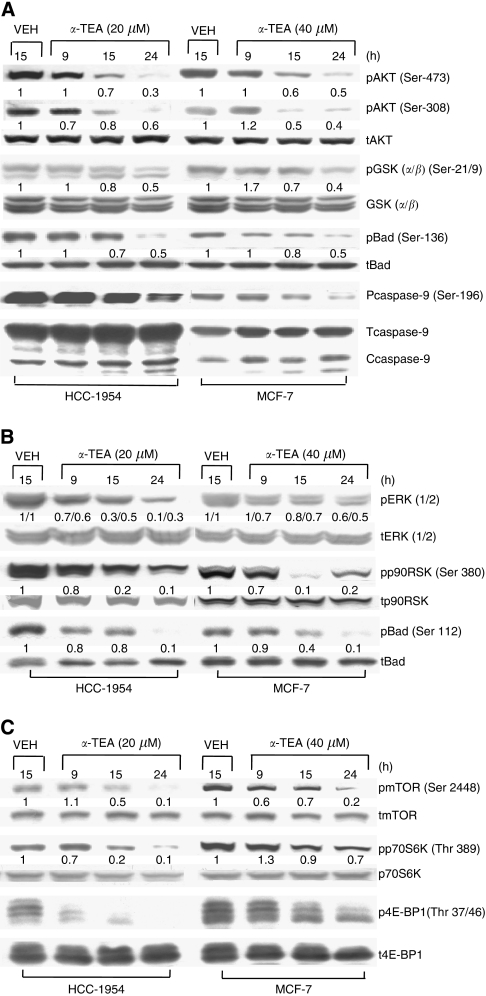
Vehicle-treated HCC-1954 and MCF-7 cells express high levels of active AKT (pAKT) and phosphorylated downstream substrates GSK (pGSK), Bad (pBad), and caspase 9 (pcaspase-9). Treatment of HCC-1954 (20 μM) and MCF-7 cells (40 μM) with α-TEA for 9, 15, and 24 h: (1) reduces levels of pAKT (both Ser-473 and Ser-308) as well as its downstream substrates pGSK (α/β) (Ser-21/9), pBad (Ser-136), and pcaspase-9 (Ser-196) and increases cleaved caspase 9 in a time-dependent manner (Figure 2A), (2) downregulates pERK1/2 and its substrates pp90SK (Ser-380) and pBad (Ser-112) (Figure 2B), and (3) downregulates pmTOR (Ser-2448) and its substrates pp70S6K (Thr-389) and p4E-BP1 (Thr-37/46) (Figure 2C). These data show that α-TEA suppresses the active forms of AKT, ERK, mTOR, and their downstream substrates, resulting in activation of proapoptotic mediators Bad and caspase-9.
Figure 2.
α-Tocopherol ether-linked acetic acid (α-TEA) suppresses AKT, ERK, mTOR, as well as their downstream targets. HCC-1954 and MCF-7 cells were treated with 20 and 40 μM α-TEA, respectively, for 9, 15, and 24 h. (A) Protein levels of pAKT (Ser-473 and Ser-308), pGSK (α/β) (Ser 21/9), pBad (Ser-136), pcaspase-9 (Ser-196), as well as levels of total AKT, GSK, Bad, and caspase-9 were determined by western blot analyses. (B) Protein levels of pERK1/2, pp90RSK (Ser-380), and pBad (Ser-112), as well as total ERK, p90RSK, and Bad were determined by western blot analyses. (C) Protein levels of pmTOR (Ser-2448), pp70S6K (Thr-389), and p4E-BP1 (Thr-37/46), and levels of total mTOR, p70S6K, and 4E-BP1 were determined by western blot analyses. Data are representative of two separate experiments.
PI3K inhibitor (wortmannin) suppresses pAKT, pERK1/2, and pmTOR in MCF-7 cells
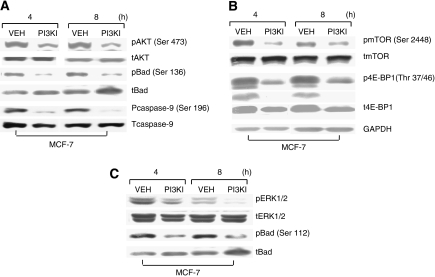
To better understand how the basal levels of pAKT, pERK, and pmTOR are regulated, MCF-7 cells were treated with 1 μM wortmannin (PI3KI) for 4 and 8 h. Wortmannin reduced the phosphorylated forms of: (1) AKT and its substrates pBad (Ser-136) and pcaspase-9 (Ser-196) (Figure 3A), (2) ERK1/2 and its substrate pBad (Ser-112) (Figure 3B), and pmTOR (Ser-2448) and its substrate p4E-BP1 (Thr 37/46) (Figure 3C), indicating that PI3K is a major contributor to the basal levels of AKT, ERK, and mTOR.
Figure 3.
Extracellular signal-regulated kinase (ERK) and mTOR are downstream targets of PI3K. MCF-7 cells were treated with 1 μM PI3K inhibitor wortmannin for 4 and 8 h. (A) Protein levels of pAKT (Ser-473), pBad (Ser-136), and pcaspase-9 (Ser-196), and levels of total AKT, Bad, and caspase-9 were determined by western blot analyses. (B) Protein levels of pERK1/2 and pBad (Ser-112) and total levels of ERK1/2 and Bad were determined by western blot analyses. (C) Protein levels of pmTOR (Ser-2448) and p4E-BP1 (Thr-37/46), and total levels of mTOR and 4E-BP1 were determined by western blot analyses. Data are representative of two separate experiments.
α-TEA induces increased levels of phospho-IRS-1 (Ser-307) and decreased levels of total IRS-1 via JNK
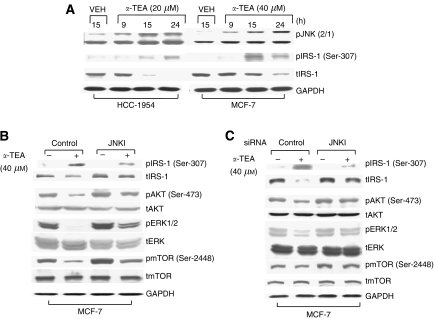
To identify a common upstream factor that might account for how α-TEA suppresses pAKT, pERK, and pmTOR in unison, the effect of α-TEA on phospho-IRS-1 (pIRS-1 Ser-307) was examined. Ser-307 is a phosphorylation site regulated by JNK for inactivation of IRS (Mamay et al, 2003) via reduction of total IRS protein levels (Hiratani et al, 2005). As α-TEA has been reported to induce a prolonged activation of JNK (Shun et al, 2004; Yu et al, 2006; Jia et al, 2008a, 2008b; Wang et al, 2008), IRS-1 may be a promising upstream target for α-TEA-mediated events. The HCC-1954 and MCF7 cells were treated with 20 and 40 μM α-TEA, respectively, for 9, 15, and 24 h. α-Tocopherol ether-linked acetic acid induced increased levels of pJNK2/1, increased levels of pIRS-1 (ser-307), and reduced levels of total IRS-1 in both cell lines (Figure 4A). Knockdown of JNK using a chemical inhibitor SP600125 (JNKI) (Figure 4B) or siRNA (Figure 4C) blocked the ability of α-TEA to increase pIRS-1 (Ser-307) and decrease levels of total IRS-1 protein, and suppressed the ability of α-TEA to reduce pAKT (Ser-473), pERK1/2, and pmTOR (Ser-2448). These data suggest that downregulation of IRS-1 is involved in the ability of α-TEA to suppress AKT, ERK, and mTOR signalling and demonstrated that JNK is involved in this event via phosphorylation of IRS-1 at Ser-307, leading to its degradation.
Figure 4.
α-Tocopherol ether-linked acetic acid (α-TEA) downregulates AKT, ERK, and mTOR via JNK-mediated decrease in IRS protein expression. (A) HCC-1954 and MCF-7 cells were treated with 20 and 40 μM α-TEA, respectively, for 9, 15, and 24 h. Western blot analyses were performed to detect phospho-JNK2/1, pIRS-1 (Ser-307), and total IRS, with GAPDH serving as loading control. (B) MCF-7 cells were treated with 10 μM JNK inhibitor SP600125 (JNKI)+40 μM α-TEA for 18 h. Protein levels of pAKT (Ser-473), pERK1/2, pmTOR (Ser-2448), pIRS-1 (Ser-307), and total levels of AKT, ERK1/2, mTOR, and IRS-1 were determined by western blot analyses, with GAPDH serving as lane control. (C) MCF-7 cells transfected with siRNA to JNK or control siRNA were treated with 40 μM α-TEA for 18 h. Protein levels of pAKT (Ser-473), pERK1/2, pmTOR (Ser-2448), pIRS-1 (Ser-307), and levels of total AKT, ERK, mTOR, and IRS were determined by western blot analyses, with GAPDH as lane control. Data are representative of two separate experiments.
Inhibitors of PI3K, MEK/ERK, and mTOR as well as siRNA to AKT1 and AKT2 cooperated with α-TEA to induce apoptosis
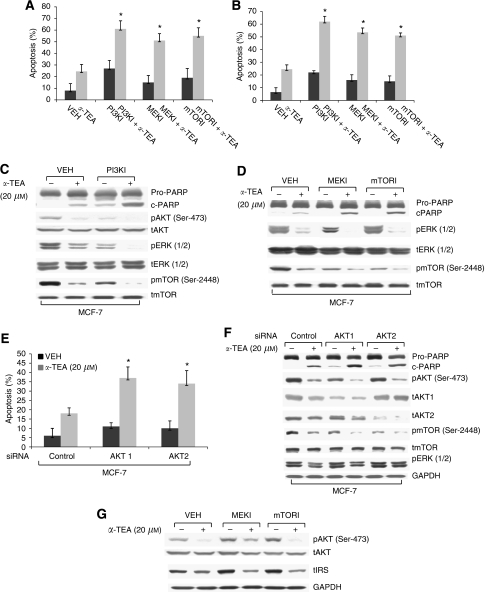
To determine the effects of inhibition of members of the PI3K pathway on α-TEA-induced apoptosis, HCC-1954 and MCF-7 cells were cultured with 10 and 20 μM α-TEA, respectively, plus 1 μM PI3K inhibitor (PI3KI) wortmannin, 10 μM MEK inhibitor (MEKI) U01260, and 50 nM mTOR inhibitor (mTORI) rapamycin for 18 h. Single treatments with α-TEA or inhibitors induced low levels of apoptosis, whereas combinations of α-TEA plus each of the three inhibitors individually significantly enhanced induction of apoptosis in both cell types in comparison with single treatments (Figure 5A and B). Both α-TEA and PI3K inhibitors reduced levels of pAKT (Ser-473), pERK1/2, and pmTOR (Ser-2448), and the combination of α-TEA+PI3K inhibitor acted cooperatively to enhance PARP cleavage and to reduce levels of pAKT (Ser-473), pERK1/2, and pmTOR (Ser-2448) when compared with individual treatments or control (Figure 5C). Both MEK and mTOR inhibitors enhanced α-TEA-induced PARP cleavage and cooperatively enhanced α-TEA inhibition of pERK1/2 and pmTOR, respectively (Figure 5D). Combinations of α-TEA+AKT1 or 2 siRNA acted cooperatively to induce apoptosis (Figure 6E) and inhibit pAKT (Ser-473) and total AKT1 and AKT2, respectively, as well as pmTOR (Ser-2448), but had no effect on basal levels of pERK1/2, showing that the ERK signalling pathway is independent of AKT signalling (Figure 5F). Taken together, data in Figure 5 show that α-TEA can downregulate the basal levels of active AKT, ERK1/2, and mTOR, and can act cooperatively with either chemical- or genetic-based inhibitors of these prosurvival mediators to enhance breast cancer cell death.
Figure 5.
Inhibitors of PI3K, MEK/ERK, mTOR, as well as siRNAs to AKT1 and AKT2 enhance α-TEA-induced apoptosis. (A and B) HCC-1954 and MCF-7 cells were treated with 1 μM PI3K inhibitor wortmannin (PI3KI), 10 μM MEK inhibitor U01260 (MEKI), or 50 nM mTOR inhibitor rapamycin (mTORI) plus 10 and 20 μM of α-TEA for 18 h, respectively. Apoptosis was determined by Annexin V/PI staining. (C and D) Protein levels of cleaved PARP, pAKT (Ser-473), pERK1/2, and pmTOR (Ser-2448) as well as levels of total AKT, ERK1/2, and mTOR were determined by western blot analyses in MCF-7 cells treated with the three inhibitors plus α-TEA. (E) MCF-7 cells transfected with siRNAs to AKT1, AKT2, or control was treated with 20 μM α-TEA for 18 h. Apoptosis was determined by Annexin V/PI staining. (F) Protein levels of cleaved PARP, pAKT (Ser-473), pmTOR (Ser-2448), pERK1/2, and levels of total AKT1, AKT2, and mTOR were determined by western blot analyses. Levels of GAPDH served as lane controls. (G) MCF-7 cells treated with 10 μM MEK inhibitor U01260 (MEKI) or 50 nM mTOR inhibitor rapamycin (mTORI) plus 20 μM of α-TEA for 18 h. Protein levels of pAKT (Ser-473), AKT, IRS-1, and GAPDH were determined by western blot analyses. Data in (C, D, F, and G) are representative of two separate experiments. Data in (A, B, and E) are presented as mean±s.d. of three independent experiments. *Significantly different from control (P<0.05).
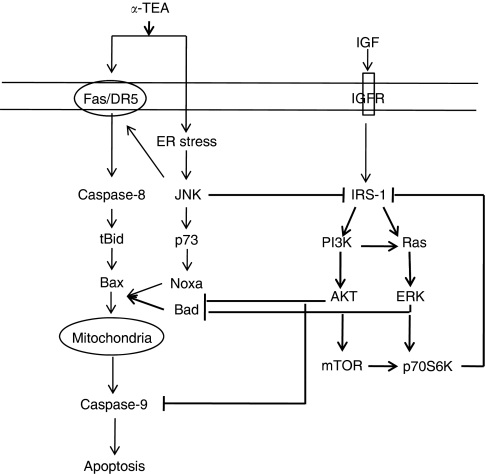
Figure 6.
Schematic diagram depicting signalling pathways that are involved in α-TEA alone and α-TEA+MEK or mTOR inhibitor-induced apoptosis. Schematic diagram depicts cross-talk between survival and death signalling pathways in α-TEA alone and α-TEA+MEK or mTOR inhibitor-induced apoptosis in human breast cancer cells. Constitutively active insulin receptor substrate-1 (IRS-1) activates AKT and ERK via phosphorylations mediated by PI3K and Ras, respectively, leading to subsequent phosphorylation events that inhibit proapoptotic mediators Bad and caspase 9, and activate prosurvival mTOR. Beneficial antitumour actions by inhibitors of mTOR (rapamycin) and ERK (MEK inhibitor) are compromised by elimination of mTOR or ERK-regulated p70S6K negative loop inhibiting IRS-1 signalling. The loss of this negative feedback loop results in continued activation of AKT signalling and inhibition of apoptosis by inactivation of Bad and caspase 9. α-Tocopherol ether-linked acetic acid (α-TEA) induces death receptor-mediated apoptosis (depicted by non-bolded signalling pathways described in references Shun et al, 2004; Yu et al, 2006; Jia et al, 2008a, 2008b; Wang et al, 2008) with involvement of prolonged activation of JNK. Our data show that JNK is activated by endoplasmic reticulum (ER) stress (Tiwary et al, 2010). Active JNK blocks IRS-1 signalling, thereby downregulating downstream survival signalling mediators, resulting in the activation of proapoptotic mediators Bad and caspase 9, leading to promoting mitochondria-dependent apoptosis. Combinations of suboptimal levels of α-TEA plus mTOR or MEK inhibitors act synergistically to induce apoptosis by reduction of AKT- and ERK-mediated inhibition of proapoptotic factors Bad and caspase 9.
α-TEA blocked the ability of MEK and mTOR inhibitors to induce increased levels of pAkt and IRS
It has been reported that MEK and mTOR inhibitors induce increased levels of phospho-Akt via negative feedback regulation of IRS (Wan et al, 2007; Jiang et al, 2009). As expected, MEK and mTOR inhibitors induced increased levels of pAKT and IRS-1, an outcome that limits their clinical anticancer efficacy; importantly, co-treatment with α-TEA was able to block this counterproductive increase in these potent prosurvival mediators (Figure 5G).
Based on these data, we hypothesise a signalling pathway depicting cross-talk between survival and death signalling pathways in α-TEA alone and α-TEA+MEK or mTOR inhibitor-induced apoptosis in human breast cancer cells (Figure 6). In the untreated cells, IGF/IGFR via downstream mediator IRS-1 regulates two distinct signalling pathways (PI3K and Ras) that contribute to basal levels of activated AKT, ERK, and mTOR. Activated AKT and ERK1/2 phosphorylate proapoptotic mediators Bad and caspase-9, thereby inhibiting their proapoptotic actions and thus enhancing cancer cell survival. Both ERK and mTOR trigger a negative feedback loop via their substrate p70S6K that downregulates IRS-1 signalling. Previous studies (non-bolded signalling pathway) have shown that α-TEA induces apoptosis via upregulation of cell surface death receptor-mediated caspase-8 and mitochondrial-dependent pathways (Shun et al, 2004; Yu et al, 2006; Jia et al, 2008b; Tiwary et al, 2010). Previous studies have also identified JNK as a key player in α-TEA-induced apoptosis (Shun et al, 2004; Yu et al, 2006; Jia et al, 2008a, 2008b; Wang et al, 2008). In addition to signalling apoptosis via p73/Noxa (Wang et al, 2008) and a Fas/JNK positive feedback loop (Jia et al, 2008a, 2008b), we report here that JNK suppresses PI3K prosurvival signalling pathways via phosphorylation (inactivation and degradation) of IRS-1. As IRS-1 has an apical role in activation of AKT, ERK, and mTOR survival signalling pathways via PI3K, elimination of IRS-1 deprives the cells of these signalling mediators as well as restores proapoptotic activity by Bad and caspase-9 via their dephosphorylation, leading to cell death by apoptosis. In summary, the results reported in this study help clarify why α-TEA is such a potent apoptotic agent in human breast cancer cell lines, and suggest potential utility for α-TEA in combination therapy with ERK and mTOR inhibitors.
Discussion
These studies demonstrate, for the first time, that α-TEA suppresses AKT- and ERK-mediated antiapoptotic events, namely, inhibitory phosphorylation of two proapoptotic factors Bad and caspase-9. Additionally, α-TEA suppression of AKT leads to decreases in mTOR activity, as measured by reduction in downstream mediators p4E-BP1 and p70S6K. Functional knockdown assessments indicate that α-TEA-mediated activation of JNK has a critical role in these events via downregulation of IRS-1. The following sequence of events are proposed for α-TEA-mediated apoptosis: α-TEA inhibits PI3K via JNK-mediated phosphorylation of IRS-1 at ser-307, resulting in inactivation of AKT/mTOR and Ras/ERK, which act cooperatively in α-TEA-induced mitochondrial-dependent proapoptotic events via activation of Bad and caspase-9. Especially noteworthy is that these studies showed that the harmful outcome of drug inhibition of either ERK or mTOR, namely, activation of AKT, could be prevented by combination treatment with α-TEA. In summary, data show that α-TEA suppression of IRS-1 not only has an important role in α-TEA-induced apoptosis but can also suppress activation of AKT induced by ERK and mTOR inhibitors, suggesting that α-TEA might improve clinical outcomes of treatment with ERK or mTOR inhibitors.
The pleiotropic effects of PI3K/AKT signalling on inhibition of apoptosis have been reported to be a major mechanism for drug resistance (McCubrey et al, 2007; Gibbons et al, 2009; Falasca, 2010). Therefore, identification of agents that can both block survival and induce death signalling pathways should aid development of strategies to sensitise drug-resistant breast cancer cells. α-Tocopherol ether-linked acetic acid has been reported to suppress AKT in prostate and ovarian cancer cells and that suppression of AKT contributes to α-TEA-induced apoptosis (Yu et al, 2006; Jia et al, 2008a, 2008b; Shun et al, 2010). In prostate cancer cells, α-TEA suppression of AKT causes activation of Fox-1, a proapoptotic transcription factor capable of triggering apoptosis via upregulation of Bim (Jia et al, 2008a, 2008b). In ovarian cancer cells, α-TEA suppresses c-FLIP and survivin protein expression via AKT-mediated events (Shun et al, 2010). In this study we report that α-TEA suppression of AKT via targeting IRS-1/PI3K leads to activation of proapoptotic Bad and caspase-9 in human breast cancer cells.
Bad is a proapoptotic member of the Bcl-2 family, which belongs to the BH3-only protein family comprising Bad, Bik, Bmf, Hrk, Noxa, tBid, Bim, and Puma (Kim et al, 2006). Bcl-2 associated death (Bad) promoter, Bcl-2 antagonist of cell death) is a proapoptotic factor that promotes apoptosis via forming heterodimers with prosurvival proteins Bcl-2 and Bcl-xL, preventing them from binding with Bax (Yang et al, 1995). Phosphorylation of Bad at Ser-112 and Ser-136 disrupts its association with Bcl-2 or Bcl-xl, promoting cell survival (Datta et al, 2000; Hayakawa et al, 2000). Phosphorylation of Bad at ser-112 and ser-136 has been proposed to be mediated by ERK/p90RSK1 and PI3K/AKT pathways, respectively (Hayakawa et al, 2000). Thus, the phosphorylation status of Bad at these critical serine residues serves as a determinant of either cell death or survival (Downward, 1999). Data showing that α-TEA reduces phosphorylation of Bad at both ser-112 and ser-136 sites suggest that the ability of α-TEA to reduce AKT and ERK activities contributes to restoration of Bad's proapoptotic function. Furthermore, data showing that inhibition of PI3K with wortmannin suppressed phosphorylation of Bad at both ser-112 and ser-136 supports a role for PI3K in regulating both AKT and ERK/p90RSK1 in these cells. Previous data from our lab showed that α-TEA induces mitochondria-dependent apoptosis via activation of Bax, a critical step in triggering mitochondria-dependent apoptosis (Yu et al, 2006; Jia et al, 2008b; Yu et al, 2010). Two other BH3-only proapoptotic Bcl-2 members, caspase-8-mediated tBid (active form of Bid) and p73-mediated Noxa, have been shown to be upregulated by α-TEA (Yu et al, 2006; Jia et al, 2008b; Wang et al, 2008; Yu et al, 2010). In this study, for the first time, we report that α-TEA induces Bad activation via inhibiting AKT and ERK. These data demonstrate that α-TEA triggers mitochondria-dependent apoptosis via targeting different Bcl-2-associated death promoters, namely, Bid, Noxa, and Bad, in cancer cell types.
Caspase-9, a mitochondria-mediated initiation caspase, is directly activated by Apaf-1 and cytochrome c and triggers activation of execution caspases 3, 6, and 7, leading to DNA fragmentation and cell death (Li et al, 1997). It has been reported that caspase-9 activity is regulated by phosphorylation (Cardone et al, 1998). AKT phosphorylates caspase-9 at Ser-196, leading to inactivation of caspase-9 (Cardone et al, 1998). Therefore, caspase-9 is another target for AKT to prevent cells from undergoing apoptosis. Thus, α-TEA suppression of AKT phosphorylation of caspase-9 at ser-196 contributes to α-TEA-induced mitochondria-dependent apoptosis.
Mammalian target of rapamycin is a downstream mediator of PI3K/AKT signalling, regulating proliferation, survival, mobility, and angiogenesis via targeting p70S6 kinase (p70S6K) and 4E-BP1 in breast cancers that exhibit constitutively activated PI3K/AKT signalling (Bjornsti and Houghton, 2004). Accumulating evidence suggests that PI3K/AKT/mTOR promote breast cancer cell survival and resistance to chemotherapeutics such as trastuzumab (a blocking antibody to Her-2) and tamoxifen (Hynes and Dey, 2009; Ghayad et al, 2010). The mTOR inhibitors rapamycin and rapamycin analogues (CCI-779, RAD001, and AP23573) have exhibited impressive growth inhibitory effects against a broad range of human cancers, including breast cancer, in preclinical and early clinical studies (Chan, 2004; Vignot et al, 2005). In this study, we demonstrate that α-TEA functions as an mTOR inhibitor, capable of suppressing mTOR by decreasing constitutively activated mTOR (phosphorylated status of mTOR) and its downstream mediators p70S6K and 4E-BP1. In addition, our data show that α-TEA not only enhances rapamycin suppression of mTOR and induction of apoptosis, but also suppresses rapamycin-mediated feedback activation of AKT, providing a rationale for developing a combination regimen of mTOR+α-TEA for breast cancer treatment.
Insulin receptor substrate-1 is an adaptor protein important for the insulin receptor and IGF-1 receptor signal transduction to downstream targets, including PI3K (Surmacz, 2000; Valentinis and Baserga, 2001). It has important roles in maintaining insulin sensitivity in adipocytes and cell growth in cancer cells (Hartley and Cooper, 2002). Its activity is positively and negatively regulated via its phosphorylation at different sites by not only ligand-activated cell surface receptors but also by different intracellular Ser/Thr protein kinases, including mTOR, ERK, protein kinase C, and AMP-activated protein kinase, as well as JNK (De Fea and Roth, 1997; Ozes et al, 2001; Rui et al, 2001; Horike et al, 2003; Hiratani et al, 2005; Mingo-Sion et al, 2005). Insulin receptor substrate-1 Ser-307 lies near the phospho-tyrosine binding domain in IRS-1 and confers an inhibitory effect on both insulin and IGF-1 signalling (Greene et al, 2003). Activation of JNK has been established as a stress-mediated inducer of insulin resistance in diabetic animal models via phosphorylation of IRS-1 at Ser-307, leading to inactivation of IRS-1 by interfering with the interaction of the insulin receptor and IRS-1 and promoting IRS-1 degradation (Mamay et al, 2003). An inhibitory effect of JNK on IRS-1 activity via phosphorylation at ser-307 in human breast cancer cells has also been reported in Taxol treatments (Mamay et al, 2003). In this study we report that α-TEA functions as an IRS-1 suppressor in human breast cancer cells via JNK-dependent phosphorylation of IRS-1 at ser-307. Thus, α-TEA-mediated phosphorylation of IRS-1 at ser-307 is correlated with downregulation of total protein levels of IRS-1, and both α-TEA-mediated phosphorylation of IRS-1 and downregulation of total protein level of IRS-1 are JNK dependent, suggesting that α-TEA downregulation of total protein level of IRS-1 may be subsequent to phosphorylation of IRS-1 at ser-307, as phosphorylation of IRS-1 at ser-307 has been reported to decrease IRS-1 stability (Greene et al, 2003). Mammalian target of rapamycin and ERK have been reported to negatively regulate IRS-1 via their downstream mediator p70S6K (Wan et al, 2007; Jiang et al, 2009), providing a negative feedback mechanism for turning off activation of AKT (Sun et al, 2005). Thus, inhibitors of MEK/ERK and mTOR produce a counterproductive increase in pAKT via loss of the negative feedback control of IRS-1 levels, which limits their clinic applications. It is highly significant that α-TEA functions as an inhibitor of not only ERK and mTOR, but also of AKT. It is also highly significant that α-TEA in combination with ERK and mTOR inhibitors blocks inhibitor-induced increases in pAKT. The potent anticancer actions by α-TEA appear to be because of its ability to inhibit IRS-1/PI3K via activation of JNK.
c-Jun N-terminal kinase, a proapoptotic mediator, has been reported to be activated by α-TEA and involved in α-TEA-induced Fas and Fas L protein expression in prostate cancer (Jia et al, 2008b), as well as p73/Noxa protein expression (Wang et al, 2008) and CHOP/DR5 protein expression (Tiwary et al, 2010) in breast cancer cells. In this study we report that JNK suppressed pro-survival PI3K and its downstream mediators via targeting IRS-1. These data demonstrate that JNK has a critical pleiotropic role in α-TEA-induced apoptosis via both proapoptotic and antisurvival mechanisms. Mechanisms involved in the initiation of JNK activation in response to α-TEA are not fully understood. However, our recent data show that JNK is at least partially activated by endoplasmic reticulum (ER) stress in α-TEA-induced apoptosis (Tiwary et al, 2010). Following ER stress, Ire1 is released from GRP78 in ER, leading to activation of ASK1 via TRAF2 (Xu et al, 2005). Apoptosis signal-regulating kinase 1 (ASK1) has been shown to be critical in ER stress-mediated apoptosis and activation of JNK (Nishitoh et al, 2002). Thus, ER stress-mediated ASK1 is a possible upstream mediator for JNK activation.
In summary, the ability of α-TEA to target PI3K-mediated prosurvival factors via JNK-mediated inhibition of IRS-1 not only has a role in enhancing α-TEA-induced apoptosis by inhibition of AKT, ERK, mTOR, and their downstream mediators, but also suggests a novel means for preventing procancer impact of AKT activation induced by ERK and mTOR inhibitors.
Acknowledgments
This work was supported by grants from the Public Health Service/National Institutes of Health (CA59739), the Clayton Foundation for Research, the National Institute of Environmental Health Sciences (ES007784), and the National Institute of Environmental Health Sciences Toxicology Training Grant T32 ES07247.
Footnotes
The Clayton Foundation for Research holds patents on α-TEA. The authors declare no other conflict of interest.
References
- Anderson K, Lawson KA, Simmons-Menchaca M, Sun L, Sanders BG, Kline K (2002) α-TEA plus cisplatin reduces human cisplatin-resistant ovarian cancer cell tumor burden and metastasis. Exp Biol Med 229: 1169–1176 [DOI] [PubMed] [Google Scholar]
- Bjornsti MA, Houghton PJ (2004) The mTOR pathway: a target for cancer therapy. Nat Rev Cancer 4: 335–348 [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318–1321 [DOI] [PubMed] [Google Scholar]
- Chan S (2004) Targeting the mammalian target of rapamycin (mTOR): a new approach to treating cancer. Br J Cancer 91: 1420–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Lee JT, Navolanic PM, Steelman LS, Shelton JG, Blalock WL, Franklin RA, McCubrey JA (2003) Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia 17: 590–603 [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241 [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME (2000) 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6: 41–51 [PubMed] [Google Scholar]
- De Fea K, Roth RA (1997) Protein kinase C modulation of insulin receptor substrate-1 tyrosine phosphorylation requires serine 612. Biochemistry 36: 12939–12947 [DOI] [PubMed] [Google Scholar]
- Downward J (1999) How BAD phosphorylation is good for survival. Nat Cell Biol 1: E33–E35 [DOI] [PubMed] [Google Scholar]
- Falasca M (2010) PI3K/Akt signaling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des 16: 1410–1416 [DOI] [PubMed] [Google Scholar]
- Ghayad SE, Vendrell JA, Larbi SB, Dumontet C, Bieche I, Cohen PA (2010) Endocrine resistance associated with activated ErbB system in breast cancer cells is reversed by inhibiting MAPK or PI3K/Akt signaling pathways. Int J Cancer 126: 545–562 [DOI] [PubMed] [Google Scholar]
- Gibbons JJ, Abraham RT, Yu K (2009) Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol 36(Suppl 3): S3–S17 [DOI] [PubMed] [Google Scholar]
- Greene MW, Sakaue H, Wang L, Alessi DR, Roth RA (2003) Modulation of insulin-stimulated degradation of human insulin receptor substrate-1 by serine 312 phosphorylation. Biol Chem 278: 8199–8211 [DOI] [PubMed] [Google Scholar]
- Hahn T, Fried LH, Hurley LH, Akporiaye ET (2009) Orally active α-tocopheryloxyacetic acid suppresses tumor growth and multiplicity of spontaneous murine breast cancer. Mol Cancer Ther 8: 1570–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn T, Szabo L, Gold M, Ramanathapuram L, Hurley LH, Akporiaye ET (2006) Dietary administration of the proapoptotic vitamin E analogue α-tocopheryloxyacetic acid inhibits metastatic murine breast cancer. Cancer Res 66: 9374–9378 [DOI] [PubMed] [Google Scholar]
- Hartley D, Cooper GM (2002) Role of mTOR in the degradation of IRS-1: regulation of PP2A activity. J Cell Biochem 85: 304–314 [DOI] [PubMed] [Google Scholar]
- Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, Adachi K, Tasaka K, Kanzaki T, Murata Y (2000) Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res 60: 5988–5994 [PubMed] [Google Scholar]
- Hiratani K, Haruta T, Tani A, Kawahara J, Usui I, Kobayashi M (2005) Roles of mTOR and JNK in serine phosphorylation, translocation, and degradation of IRS-1. Biochem Biophys Res Commun 335: 836–842 [DOI] [PubMed] [Google Scholar]
- Horike N, Takemori H, Katoh Y, Doi J, Min L, Asano T, Sun XJ, Yamamoto H, Kasayama S, Muraoka M, Nonaka Y, Okamoto M (2003) Adipose-specific expression, phosphorylation of Ser794 in insulin receptor substrate-1, and activation in diabetic animals of salt-inducible kinase-2. J Biol Chem 278: 18440–18447 [DOI] [PubMed] [Google Scholar]
- Hynes NE, Dey JH (2009) PI3K inhibition overcomes trastuzumab resistance: blockade of ErbB2/ErbB3 is not always enough. Cancer Cell 15: 353–355 [DOI] [PubMed] [Google Scholar]
- Jia L, Yu W, Wang P, Sanders BG, Kline K (2008a) In vivo and in vitro studies of anticancer actions of α-TEA for human prostate cancer cells. Prostate 68: 849–860 [DOI] [PubMed] [Google Scholar]
- Jia L, Yu W, Wang P, Li J, Sanders BG, Kline K (2008b) Critical roles for JNK, c-Jun, and Fas/FasL-signaling in vitamin E analog-induced apoptosis in human prostate cancer cells. Prostate 68: 427–441 [DOI] [PubMed] [Google Scholar]
- Jiang X, Sinnett-Smith J, Rozengurt E (2009) Carbachol induces p70S6K1 activation through an ERK-dependent but Akt-independent pathway in human colonic epithelial cells. Biochem Biophys Res Commun 387: 521–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH (2006) Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8: 1348–1358 [DOI] [PubMed] [Google Scholar]
- Lawson KA, Anderson K, Simmons-Menchaca M, Atkinson J, Sun L, Sanders BG, Kline K (2004) Comparison of vitamin E derivatives α-TEA and VES in reduction of mouse mammary tumor burden and metastasis. Exp Biol Med 229: 954–963 [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X (1997) Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489 [DOI] [PubMed] [Google Scholar]
- Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, Adachi K, Takahashi K, Arimoto-Ishida E, Nakatsuji Y, Tasaka K, Murata Y (2002) Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem 277: 33490–33500 [DOI] [PubMed] [Google Scholar]
- Mamay CL, Mingo-Sion AM, Wolf DM, Molina MD, Van Den Berg CL (2003) An inhibitory function for JNK in the regulation of IGF-I signaling in breast cancer. Oncogene 22(4): 602–614 [DOI] [PubMed] [Google Scholar]
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M, Tafuri A, Stivala F, Libra M, Basecke J, Evangelisti C, Martelli AM, Franklin RA (2007) Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta 1773: 1263–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingo-Sion AM, Ferguson HA, Koller E, Reyland ME, Van Den Berg CL (2005) PKC delta and mTOR interact to regulate stress and IGF-I induced IRS-1 Ser312 phosphorylation in breast cancer cells. Breast Cancer Res Treat 91: 259–269 [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H. (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB (2001) A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA 98: 4640–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Aguirre V, Kim JK, Shulman GI, Lee A, Corbould A, Dunaif A, White MF (2001) Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107: 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103: 211–225 [DOI] [PubMed] [Google Scholar]
- Shun MC, Yu W, Gapor A, Parsons R, Atkinson J, Sanders BG, Kline K (2004) Pro-apoptotic mechanisms of action of a novel vitamin E analog (α-TEA) and a naturally occurring form of vitamin E (δ-tocotrienol) in MDA-MB-435 human breast cancer cells. Nutr Cancer 48: 95–105 [DOI] [PubMed] [Google Scholar]
- Shun MC, Yu W, Park SK, Sanders BG, Kline K (2010) Downregulation of epidermal growth factor receptor expression contributes to alpha-TEA's proapoptotic effects in human ovarian cancer cell lines. J Oncol 2010: 824571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun SY, Rosenberg LM, Wang X, Zhou Z, Yue P, Fu H, Khuri FR (2005) Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition. Cancer Res 65: 7052–7058 [DOI] [PubMed] [Google Scholar]
- Surmacz E (2000) Function of the IGF-I receptor in breast cancer. J Mammary Gland Biol Neoplasia 5: 95–105 [DOI] [PubMed] [Google Scholar]
- Tiwary R, Yu W, Li J, Park SK, Sanders BG, Kline K (2010) Role of endoplasmic reticulum stress in alpha-TEA mediated TRAIL/DR5 death receptor dependent apoptosis. PLoS One 5(7): e11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinis B, Baserga R (2001) IGF-I receptor signaling in transformation and differentiation. Mol Pathol 54: 133–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignot S, Faivre S, Aguirre D, Raymond E (2005) mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol 16: 525–537 [DOI] [PubMed] [Google Scholar]
- Wan X, Harkavy B, Shen N, Grohar P, Helman LJ (2007) Rapamycin induces feedback activation of Akt signaling through an IGF-1R-dependent mechanism. Oncogene 26: 1932–1940 [DOI] [PubMed] [Google Scholar]
- Wang P, Yu W, Hu Z, Jia L, Iyer VR, Sanders BG, Kline K (2008) Involvement of JNK/p73/NOXA in vitamin E analog-induced apoptosis of human breast cancer cells. Mol Carcinogenesis 7: 436–445 [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115: 2656–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Tiwary R, Li J, Park SK, Jia L, Xiong A, Simmons-Menchaca M, Sanders BG, Kline K (2010) α-TEA induces apoptosis of human breast cancer cells via activation of TRAIL/DR5 death receptor pathway. Mol Carcinog 49: 964–973 [DOI] [PubMed] [Google Scholar]
- Yu W, Shun MC, Anderson K, Chen H, Sanders BG, Kline K (2006) alpha-TEA inhibits survival and enhances death pathways in cisplatin sensitive and resistant human ovarian cancer cells. Apoptosis 11: 1813–1823 [DOI] [PubMed] [Google Scholar]