Abstract
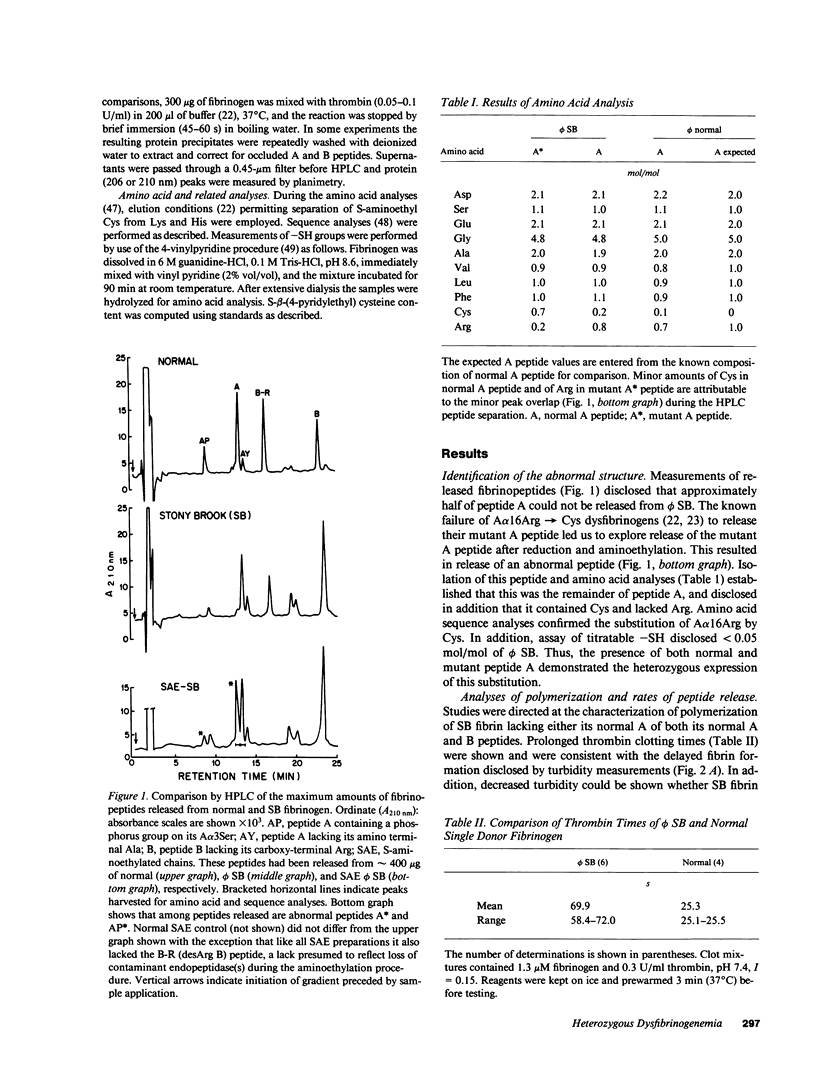
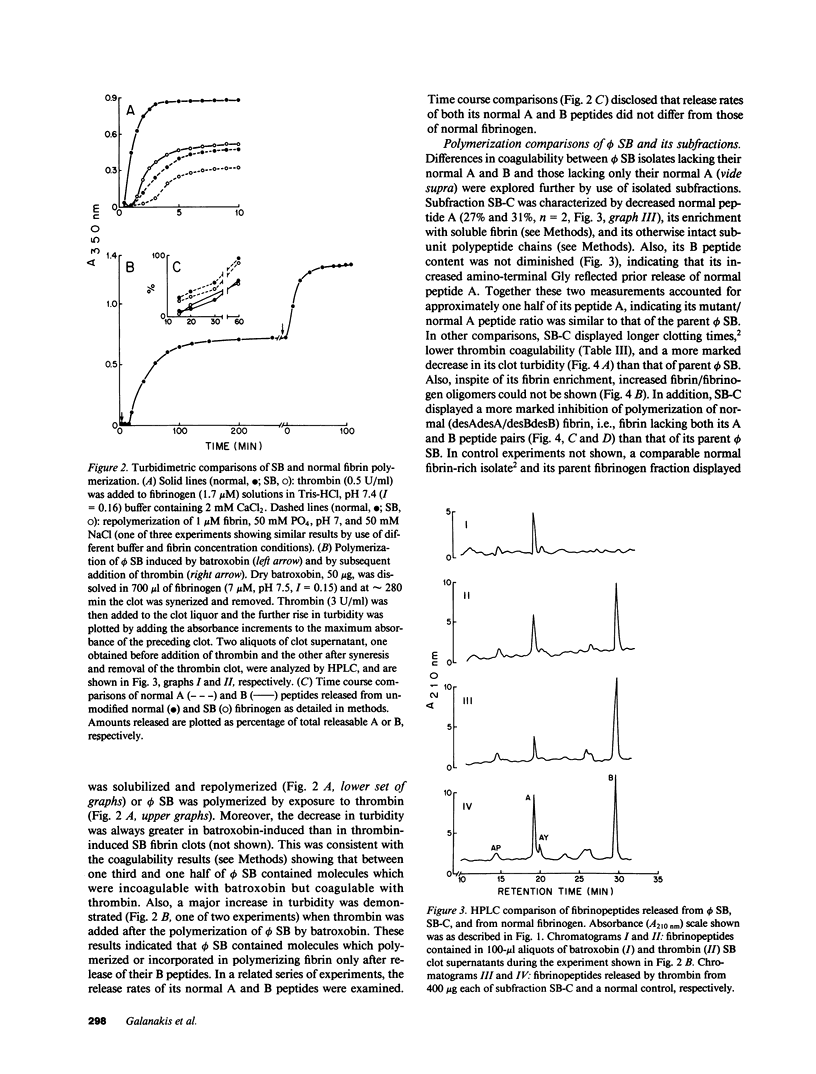
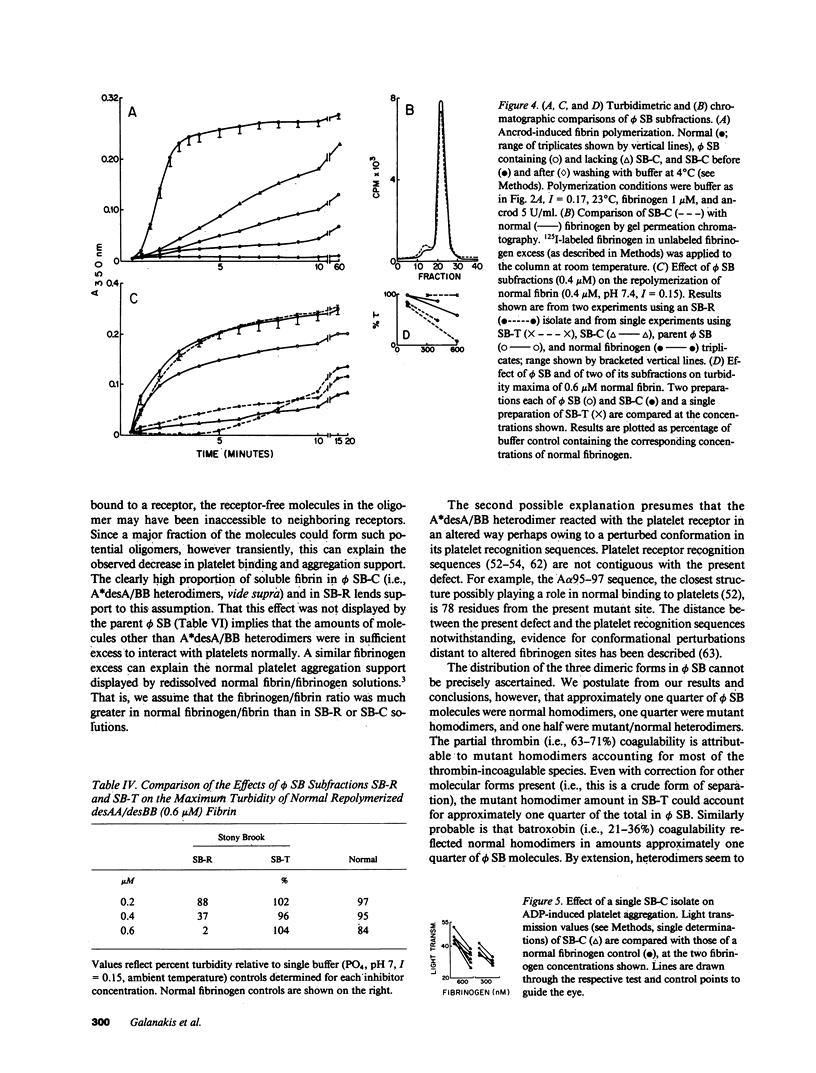
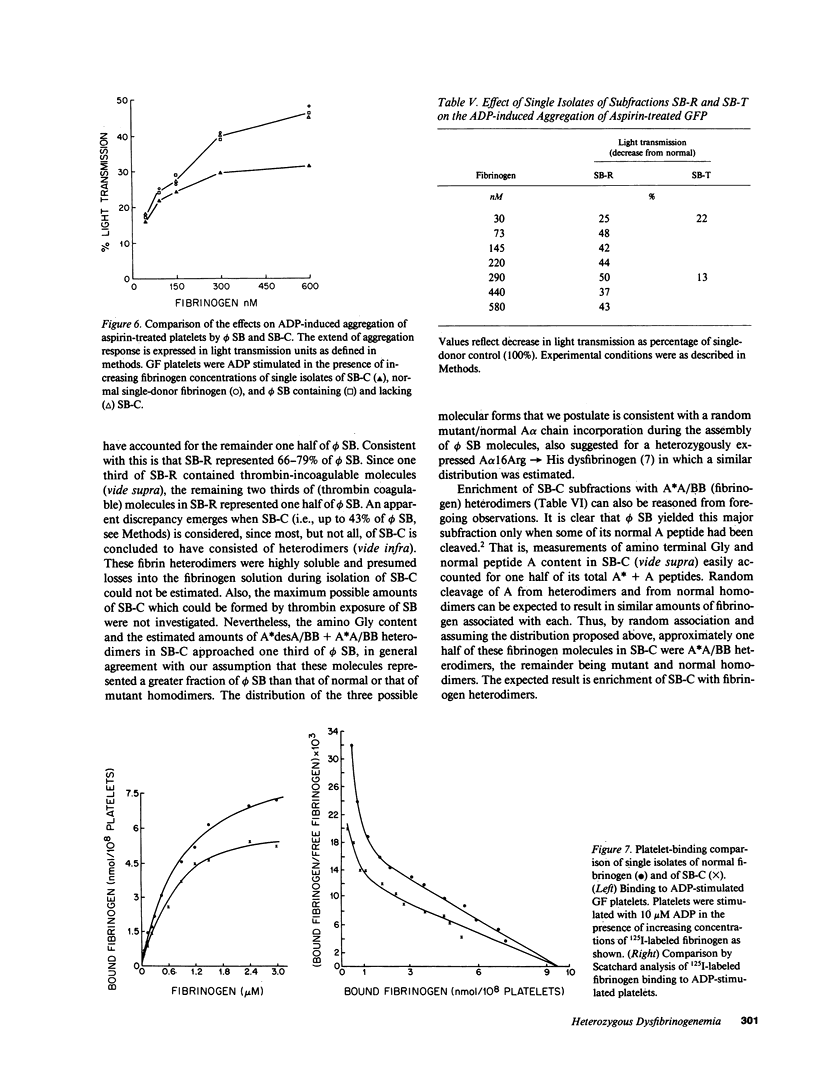
Assessed by high performance liquid chromatographic and amino acid sequence determinations, approximately one half (n = 4) of A peptide in fibrinogen Stony Brook (phi SB) contained the A alpha 16Arg----Cys substitution. To examine its functional behavior, mutant molecule-rich soluble subfractions that partly or fully lacked their normal A peptide were obtained from cryoprecipitates or from incoagulable material, respectively. Such subfractions consistently induced a more pronounced decrease (n = 3) in the turbidity of normal polymerizing fibrin than that induced by normal fibrinogen, by whole phi SB (n = 4) or by fibrinogen from an unrelated homozygous proband. These subfractions also exhibited decreased (12-50% of normal controls, fibrinogen 30-590 nM, n = 5) ADP-induced aggregation support of gel-sieved platelets, a decrease not demonstrable by whole phi SB, by fibrinogen from the homozygous proband, or by enrichment of the latter with normal soluble fibrin. A single isolate displaying diminished platelet aggregation support was 125I-labeled and examined further. It exhibited decreased binding to platelets, and Scatchard analysis indicated decreased binding affinity but normal maximum binding. We infer that phi SB contained heterodimers that exhibited these distinct functional properties when their normal A peptide had been cleaved.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving B. M., Henschen A. H. Fibrinogen giessen I: a congenital homozygously expressed dysfibrinogenemia with A alpha 16 Arg----His substitution. Am J Hematol. 1987 Aug;25(4):479–482. doi: 10.1002/ajh.2830250414. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Hessel B., Hogg D., Therkildsen L. A two-step fibrinogen--fibrin transition in blood coagulation. Nature. 1978 Oct 12;275(5680):501–505. doi: 10.1038/275501a0. [DOI] [PubMed] [Google Scholar]
- Blombäck M., Blombäck B., Mammen E. F., Prasad A. S. Fibrinogen Detroit--a molecular defect in the N-terminal disulphide knot of human fibrinogen? Nature. 1968 Apr 13;218(5137):134–137. doi: 10.1038/218134a0. [DOI] [PubMed] [Google Scholar]
- Budzynski A. Z., Marder V. J., Ménaché D., Guillin M. C. Defect in the gamma polypeptide chain of a congenital abnormal fibrinogen (Paris I). Nature. 1974 Nov 1;252(5478):66–68. doi: 10.1038/252066a0. [DOI] [PubMed] [Google Scholar]
- CLAUSS A. Gerinnungsphysiologische Schnellmethode zur Bestimmung des Fibrinogens. Acta Haematol. 1957 Apr;17(4):237–246. doi: 10.1159/000205234. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Egeberg O. Inherited fibrinogen abnormality causing thrombophilia. Thromb Diath Haemorrh. 1967 Feb 28;17(1-2):176–187. [PubMed] [Google Scholar]
- Friedman M., Krull L. H., Cavins J. F. The chromatographic determination of cystine and cysteine residues in proteins as s-beta-(4-pyridylethyl)cysteine. J Biol Chem. 1970 Aug 10;245(15):3868–3871. [PubMed] [Google Scholar]
- Galanakis D. K. Dysfibrinogenemia. A current perspective. Clin Lab Med. 1984 Jun;4(2):395–418. [PubMed] [Google Scholar]
- Galanakis D. K., Lane B. P., Simon S. R. Albumin modulates lateral assembly of fibrin polymers: evidence of enhanced fine fibril formation and of unique synergism with fibrinogen. Biochemistry. 1987 Apr 21;26(8):2389–2400. doi: 10.1021/bi00382a046. [DOI] [PubMed] [Google Scholar]
- Galanakis D. K., Laurent P., Janoff A. Cigarette smoke contains anticoagulants against fibrin aggregation and factor XIIIa in plasma. Science. 1982 Aug 13;217(4560):642–645. doi: 10.1126/science.6124042. [DOI] [PubMed] [Google Scholar]
- Galanakis D. K., Mosesson M. W., Stathakis N. E. Human fibrinogen heterogeneities: distribution and charge characteristics of chains of A alpha origin. J Lab Clin Med. 1978 Sep;92(3):376–386. [PubMed] [Google Scholar]
- Harfenist E. J., Packham M. A., Mustard J. F. Effects of variant gamma chains and sialic acid content of fibrinogen upon its interactions with ADP-stimulated human and rabbit platelets. Blood. 1984 Dec;64(6):1163–1168. [PubMed] [Google Scholar]
- Harris J. U., Johnson A. J., Merskey C., Wang M. T., Robinson D. Quantitative N-terminal analysis of fibrinogen-fibrin-related antigen [FR antigen] from human plasma. Biochem J. 1979 Dec 1;183(3):623–632. doi: 10.1042/bj1830623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschen A., Edman P. Large scale preparation of S-carboxymethylated chains of human fibrin and fibrinogen and the occurrence of -chain variants. Biochim Biophys Acta. 1972 Apr 15;263(2):351–367. doi: 10.1016/0005-2795(72)90088-8. [DOI] [PubMed] [Google Scholar]
- Henschen A., Kehl M., Deutsch E. Novel structure elucidation strategy for genetically abnormal fibrinogens with incomplete fibrinopeptide release as applied to fibrinogen Schwarzach. Hoppe Seylers Z Physiol Chem. 1983 Dec;364(12):1747–1751. doi: 10.1515/bchm2.1983.364.2.1747. [DOI] [PubMed] [Google Scholar]
- Higgins D. L., Shafer J. A. Fibrinogen Petoskey, a dysfibrinogenemia characterized by replacement of Arg-A alpha 16 by a histidyl residue. Evidence for thrombin-catalyzed hydrolysis at a histidyl residue. J Biol Chem. 1981 Dec 10;256(23):12013–12017. [PubMed] [Google Scholar]
- Kehl M., Lottspeich F., Henschen A. Analysis of human fibrinopeptides by high-performance liquid chromatography. Hoppe Seylers Z Physiol Chem. 1981 Dec;362(12):1661–1664. [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Hawiger J. Localization of a site interacting with human platelet receptor on carboxy-terminal segment of human fibrinogen gamma chain. Biochem Biophys Res Commun. 1982 Jul 16;107(1):181–187. doi: 10.1016/0006-291x(82)91686-2. [DOI] [PubMed] [Google Scholar]
- Lam S. C., Plow E. F., Smith M. A., Andrieux A., Ryckwaert J. J., Marguerie G., Ginsberg M. H. Evidence that arginyl-glycyl-aspartate peptides and fibrinogen gamma chain peptides share a common binding site on platelets. J Biol Chem. 1987 Jan 25;262(3):947–950. [PubMed] [Google Scholar]
- Landis W. J., Waugh D. F. Interactions of bovine fibrinogen and thrombin. Early events in the development of clot structure. Arch Biochem Biophys. 1975 Jun;168(2):498–511. doi: 10.1016/0003-9861(75)90280-5. [DOI] [PubMed] [Google Scholar]
- Liu C. Y., Koehn J. A., Morgan F. J. Characterization of fibrinogen New York 1. A dysfunctional fibrinogen with a deletion of B beta(9-72) corresponding exactly to exon 2 of the gene. J Biol Chem. 1985 Apr 10;260(7):4390–4396. [PubMed] [Google Scholar]
- Miyata T., Terukina S., Matsuda M., Kasamatsu A., Takeda Y., Murakami T., Iwanaga S. Fibrinogens Kawaguchi and Osaka: an amino acid substitution of A alpha arginine-16 to cysteine which forms an extra interchain disulfide bridge between the two A alpha chains. J Biochem. 1987 Jul;102(1):93–101. doi: 10.1093/oxfordjournals.jbchem.a122046. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., DiOrio J. P., Müller M. F., Shainoff J. R., Siebenlist K. R., Amrani D. L., Homandberg G. A., Soria J., Soria C., Samama M. Studies on the ultrastructure of fibrin lacking fibrinopeptide B (beta-fibrin). Blood. 1987 Apr;69(4):1073–1081. [PubMed] [Google Scholar]
- Mosesson M. W., Sherry S. The preparation and properties of human fibrinogen of relatively high solubility. Biochemistry. 1966 Sep;5(9):2829–2835. doi: 10.1021/bi00873a008. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I. Evidence for interaction between platelet fibrinogen receptors. Blood. 1982 Oct;60(4):973–978. [PubMed] [Google Scholar]
- Peerschke E. I., Francis C. W., Marder V. J. Fibrinogen binding to human blood platelets: effect of gamma chain carboxyterminal structure and length. Blood. 1986 Feb;67(2):385–390. [PubMed] [Google Scholar]
- Peerschke E. I., Galanakis D. K. Binding of fibrinogen to ADP-treated platelets. Comparison of plasma fibrinogen fractions and early plasmic fibrinogen derivatives. J Lab Clin Med. 1983 Mar;101(3):453–460. [PubMed] [Google Scholar]
- Peerschke E. I., Galanakis D. K. The synthetic RGDS peptide inhibits the binding of fibrinogen lacking intact alpha chain carboxyterminal sequences to human blood platelets. Blood. 1987 Mar;69(3):950–952. [PubMed] [Google Scholar]
- Peerschke E. I., Zucker M. B., Grant R. A., Egan J. J., Johnson M. M. Correlation between fibrinogen binding to human platelets and platelet aggregability. Blood. 1980 May;55(5):841–847. [PubMed] [Google Scholar]
- Plow E. F., Edgington T. S. Surface markers of fibrinogen and its physiologic derivatives revealed by antibody probes. Semin Thromb Hemost. 1982 Jan;8(1):36–56. doi: 10.1055/s-2007-1005041. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., MENZIE C. A new method for the determination of fibrinogen in small samples of plasma. J Lab Clin Med. 1951 Feb;37(2):316–320. [PubMed] [Google Scholar]
- Ratnoff O. D., Forman W. B. Criteria for the differentiation of dysfibrinogenemic states. Semin Hematol. 1976 Apr;13(2):141–157. [PubMed] [Google Scholar]
- Reber P., Furlan M., Beck E. A., Finazzi G., Buelli M., Barbui T. Fibrinogen Bergamo I (A alpha 16Arg----Cys): susceptibility towards thrombin following aminoethylation, methylation or carboxamidomethylation of cysteine residues. Thromb Haemost. 1985 Aug 30;54(2):390–393. [PubMed] [Google Scholar]
- Reber P., Furlan M., Henschen A., Kaudewitz H., Barbui T., Hilgard P., Nenci G. G., Berrettini M., Beck E. A. Three abnormal fibrinogen variants with the same amino acid substitution (gamma 275 Arg----His): fibrinogens Bergamo II, Essen and Perugia. Thromb Haemost. 1986 Dec 15;56(3):401–406. [PubMed] [Google Scholar]
- Reber P., Furlan M., Rupp C., Kehl M., Henschen A., Mannucci P. M., Beck E. A. Characterization of fibrinogen Milano I: amino acid exchange gamma 330 Asp----Val impairs fibrin polymerization. Blood. 1986 Jun;67(6):1751–1756. [PubMed] [Google Scholar]
- Shainoff J. R., Dardik B. N. Fibrinopeptide B in fibrin assembly and metabolism: physiologic significance in delayed release of the peptide. Ann N Y Acad Sci. 1983 Jun 27;408:254–268. doi: 10.1111/j.1749-6632.1983.tb23249.x. [DOI] [PubMed] [Google Scholar]
- Siebenlist K. R., Prchal J. T., Mosesson M. W. Fibrinogen Birmingham: a heterozygous dysfibrinogenemia (A alpha 16 Arg----His) containing heterodimeric molecules. Blood. 1988 Mar;71(3):613–618. [PubMed] [Google Scholar]
- Soria J., Soria C., Samama M., Poirot E., Kling C. Fibrinogen Troyes--fibrinogen Metz. Two new cases of congenital dysfibrinogenemia. Thromb Diath Haemorrh. 1972 Jul 31;27(3):619–633. [PubMed] [Google Scholar]
- Soria J., Soria C., Samama M., Tabori S., Kehl M., Henschen A., Nieuwenhuizen W., Rimon A., Tatarski I. Fibrinogen Haifa: fibrinogen variant with absence of protective effect of calcium on plasmin degradation of gamma chains. Thromb Haemost. 1987 Jun 3;57(3):310–313. [PubMed] [Google Scholar]
- Thorsen L. I., Brosstad F., Solum N. O., Stormorken H. Increased binding to ADP-stimulated platelets and aggregation effect of the dysfibrinogen Oslo I as compared with normal fibrinogen. Scand J Haematol. 1986 Feb;36(2):203–210. doi: 10.1111/j.1600-0609.1986.tb00829.x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wolfenstein-Todel C., Mosesson M. W. Carboxy-terminal amino acid sequence of a human fibrinogen gamma-chain variant (gamma'). Biochemistry. 1981 Oct 13;20(21):6146–6149. doi: 10.1021/bi00524a036. [DOI] [PubMed] [Google Scholar]
- Yoshida N., Ota K., Moroi M., Matsuda M. An apparently higher molecular weight gamma-chain variant in a new congenital abnormal fibrinogen Tochigi characterized by the replacement of gamma arginine-275 by cysteine. Blood. 1988 Feb;71(2):480–487. [PubMed] [Google Scholar]
- Yoshida N., Terukina S., Okuma M., Moroi M., Aoki N., Matsuda M. Characterization of an apparently lower molecular weight gamma-chain variant in fibrinogen Kyoto I. The replacement of gamma-asparagine 308 by lysine which causes accelerated cleavage of fragment D1 by plasmin and the generation of a new plasmin cleavage site. J Biol Chem. 1988 Sep 25;263(27):13848–13856. [PubMed] [Google Scholar]


