Abstract
Recent investigations have increasingly focussed attention on the roles of intracellular vesicle trafficking in the regulation of epithelial polarity and transformation. Rab25, an epithelial-specific member of the Rab family of small GTPases, has been associated with several epithelial cancers. Whereas Rab25 overexpression is associated with ovarian cancer aggressive behaviour, Rab25 expression is decreased in human colon cancers independent of stage. Recent studies of mouse models of intestinal and colonic neoplasia have demonstrated that Rab25 deficiency markedly promotes the development of neoplasia. Some of these effects appear related to alterations in β1-integrin trafficking to the cell surface. These findings all suggest that Rab25 is a tumour suppressor for colonic neoplasia.
Keywords: Rab proteins, colon cancer, vesicle trafficking, Rab11-FIP, integrin, Smad3
Transformation of cancer cells involves alterations in both cell shape and behaviour. Intrinsic to the pathophysiology of epithelial cancers is a loss of polarised epithelial monolayers and adoption of migratory cell behaviours that lead to locally invasive disease and metastasis. This transition in cell behaviour during carcinogenesis requires global changes in cell surface components that maintain the normal polarised phenotype including junctional proteins, integrins and plasma membrane receptors, pumps and channels. Over the past decade, research in a number of cell systems has revealed that directed trafficking of cell surface proteins is not a simple matter of protein synthesis and targeting. Rather, the phenotype of cell surface protein expression reflects a dynamic interplay of de novo delivery, internalisation, targeted degradation and recycling (Mellman and Nelson, 2008). At any given moment, the compendium of cell surface proteins present on normal or abnormal cells is determined by the net effects of all of these trafficking processes. It is therefore now increasingly clear that changes in critical trafficking pathways can induce alterations in cell surface proteins leading to alterations in polarity and susceptibility to further steps that may lead to transformation.
The importance of the loss of polarity in the generation of early neoplasia has received greater scrutiny over the past several years. The establishment of polarity with segregation of apical and basolateral membrane domains is perhaps the most obvious manifestation of polarity. The maintenance of the boundaries between these polarised membrane regions by components of the tight and adherens junctions is critical for control of the polarised epithelial barrier (Mellman and Nelson, 2008). Polarity also sets up critical functions of ligand movement between polarised surfaces (transcytosis) as well as polarised transport of ions and nutrients and polarised signalling through membrane receptors. The appropriate presentation of proteins and their density at the polarised surfaces markedly impacts the physiology of the epithelial monolayer. Loss of junctional components such as E-cadherin or p120 can lead to loss in epithelial polarity and eventual derangement of the epithelial monolayer, a precancerous event (Chen et al, 2003; Balzac et al, 2005; Lioni et al, 2007; Dohn et al, 2009; Smalley-Freed et al, 2010). As individual alterations of particular proteins may not be sufficient to alter general epithelial polarity, multiple derangements are likely required to induce the level of functional polarity loss necessary as an early step in carcinogenesis. Multiple hits are then likely to lead to transformation (loss of contact inhibition), invasion and metastasis.
The Rab proteins comprise the largest family of small GTPases, with over 60 mammalian gene products (Schwartz et al, 2007). Rab proteins associate with distinct membrane compartments within cells and are thought to represent the nidus for the assembly of multiprotein complexes that determine the specificity of vesicle trafficking pathways within cells (Somsel Rodman and Wandinger-Ness, 2000). In particular, the family of Rab11 GTPases, containing Rab11a, Rab11b and Rab25, is involved in the regulation of recycling of internalised membrane proteins (Ullrich et al, 1996; Casanova et al, 1999; Wang et al, 2000) as well as the movement of membrane proteins between polarised surfaces of epithelial cells (Jones et al, 2006). Although Rab11a and Rab11b are ubiquitously expressed, Rab25 is expressed only in epithelial cells (Goldenring et al, 1993). Rab25 contains an unusual WDTAGLE sequence in its GTP-binding domain. This sequence mimics the Q to L mutation seen in Ras and other Rab that confers a loss of GTPase activity (the dominant active phenotype). However, we have demonstrated previously that Rab25 retains an active GTPase activity (Casanova et al, 1999). Although expression of Rab25 is generally low, overexpression studies in MDCK cells indicated that Rab25 was a negative regulator of epithelial cell basolateral-to-apical transcytosis (Casanova et al, 1999; Wang et al, 2000). More recently, investigations by Lencer and colleagues (Tzaban et al, 2009) have demonstrated that Rab25 regulates apical-to-basolateral transcytosis in polarised MDCK cells. These findings suggest that Rab25 is an important regulator of polarised cell surface composition. Although most previous studies have focussed on investigation of model trafficking cargoes expressed in MDCK cells (e.g., polymeric IgA for basolateral-to-apical transcytosis and FcRN for apical-to-basolateral transcytosis of IgG), the endogenous cargoes for Rab25-dependent trafficking have remained generally obscure.
All of the Rab11 family members can interact with classes of Rab11-family interacting proteins (Rab11-FIPs) as well as the actin-based motors Myosin Va and Myosin Vb (Hales et al, 2001; Lapierre et al, 2001; Roland et al, 2009). In vitro data indicate that these proteins can bind to all three Rab11 family members, in the case of Rab11-FIPs, through a conserved amphipathic α-helix domain (Hales et al, 2001). Nevertheless, it is not at all clear whether there are real differences in affinity in vivo. A number of recent studies have implicated Rab11-family effector proteins in the regulation of the polarised cell phenotype. Phosphorylation of Rab11-FIP2 by Par1b/MARK2 appears to regulate the establishment of polarity in MDCK cells (Ducharme et al, 2006). Overexpression of Rab11-FIP2(S227A) inhibits the re-establishment of the polarised monolayer following disruption by calcium chelation. Loss of functional myosin Vb has also been identified recently as the key mutation in neonates with microvillous inclusion disease (MVID) (Erickson et al, 2008; Muller et al, 2008). The intestinal epithelia in MVID patients show a prominent loss of apical microvilli as well as apical membrane inclusions, which lead to devastating neonatal diarrhoea (Iancu et al, 2007). Recent studies have suggested that these losses in apical differentiation are associated with defects in polarity (Muller et al, 2008). Thus, alterations in membrane recycling system functions can lead to remarkable changes in polarised function. Nevertheless, it is important to note that in the case of MDCK cells expressing the Rab11-FIP2(S227A) mutation, once the epithelial polarity is established, the general functional characteristics of the resistance of the monolayer are essentially normal (Ducharme et al, 2006). Thus, single defects can be compensated for through other mechanisms. In addition, whereas defects in the intestinal epithelia of MVID patients are remarkable, kidney epithelia appear unaffected. Thus, different epithelial cells may have greater or lesser abilities to compensate for specific deficits.
A number of recent investigations have implicated Rab proteins in the process of carcinogenesis (recently reviewed in Chia and Tang, 2009). A number of studies have implicated the early endosomal Rab proteins Rab5 and Rab21 in the regulation of tumour cell behaviour (Pellinen et al, 2006, 2008; Torres et al, 2010). However, specific trafficking defects in recycling system function have not been studied extensively. It was therefore of interest in 2004 when Mills and colleagues (Cheng et al, 2004) noted that regions of chromosome 1 amplification associated with ovarian cancer were associated with marked increases in the expression of Rab25. In those investigations, Rab25 overexpression correlated with the aggressiveness of the cancer. The investigators also suggested that Rab25 was overexpressed in breast cancers. Nevertheless, in the past 2 years, it has become clear that Rab25 overexpression in breast cancer is likely less common and that indeed, at least in oestrogen receptor-deficient cancers, expression is actually decreased significantly (Cheng et al, 2006, 2010).
We have investigated the role of Rab25 in colon cancer patients (Nam et al, 2010). In two independent patient cohorts, gene microarray studies demonstrated that Rab25 expression was decreased in colon cancers independent of clinical and pathological staging. Importantly, Rab25 loss was associated with poorer survival. Interestingly, we did not observe any significant changes in the expression of Rab11a, Rab11b, any of the Rab11-FIP proteins or myosin Vb. These results suggested that loss of this epithelial-specific Rab protein was associated with an increased susceptibility to colon carcinogenesis.
To address the impact of loss of Rab25 on colon carcinogenesis, we developed a novel mouse model for genetic deletion of Rab25. Although we did not observe any effects of Rab25 loss in the knockout mouse strains themselves, when these mice were crossed onto mice with established susceptibility to developing intestinal neoplasms, we observed marked acceleration of tumourigenesis. In the case of the adenomatous polyposis coli model ApcMin mice, breeding onto the Rab25 knockout background led to a four-fold increase in polyp number in the intestines and a two-fold increase in the tumour number in the colon (Nam et al, 2010). Interestingly, these polyps retained relatively well-differentiated morphologies of adenomatous polyps and did not show any increase in invasive properties. These results supported a concept that Rab25 promoted polyp initiation.
Although the ApcMin mouse is a good model for polyposis (D’Abaco et al, 1996), it is less suitable to address colon carcinogenesis. We therefore also examined the influence of Rab25 loss on colon tumourigenesis in the Smad3 knockout mouse model (Zhu et al, 1998). Smad3 knockout mice develop colon tumours by 6 months of age. Heterozygous Smad3+/− mice do not develop any pathology. We therefore were surprised to find that >80% of Smad3+/−; Rab25−/− mice demonstrated large invasive colon tumours throughout the colon by 15 weeks of age. Interestingly, we could still demonstrate, although reduced, Smad3 expression in the tumours, and hence the effects appeared to be synergistic. Although it is presently not clear what mechanisms account for the remarkable increase in colon carcinogenesis in this model, it is important to note that the Smad3+/−; Rab25−/− mice may represent a critical new model for colon carcinogenesis. Whereas Smad3−/− mice show a marked overall deficit in weight gain throughout life, the Smad3+/−; Rab25−/− mice grow similarly to wild-type mice. The highly invasive, and perhaps locally metastatic, character of the tumours and their rapid and highly penetrant phenotype makes this model perhaps the most adaptable model for the study of colon carcinogenesis in mice. The size of the tumours developed will likely be amenable to imaging and endoscopy approaches and could be of high utility in preclinical studies of novel colon cancer treatments (Deane et al, 2007).
What alterations in Rab25 knockout mice could be responsible for the increased susceptibility to intestinal and colon neoplasia when a second deficiency is added (either mutant Apc or reduction in Smad3)? Norman and colleagues (Caswell et al, 2007) have previously demonstrated that increases in Rab25 in ovarian cancer cells were associated with aberrations in the cell surface localisation of β1-integrin. We therefore evaluated the expression of β1-integrin in the Rab25-deficient mouse intestine. Whereas in wild-type mice β1-integrin was strongly expressed in the cells of the villus, we observed a decrement in basolateral β1-integrin and an increase in intracellular β1-integrin in the Rab25-deficient mice (Nam et al, 2010). Interestingly, we also observed the highest concentrations of Rab25 expression in the cells in the transition between the crypt and villus regions of the intestinal mucosa. This is the critical region for the upregulation of β1-integrin expression in the mucosa and is also the region implicated in the formation of adenomas in ApcMin mice. Thus, a loss of proper β1-integrin trafficking to the basolateral membranes during maturation of cells from the crypt to the villus may provide the predisposing decrement that, with the addition of Apc loss, could lead to increased polyp formation. Interestingly, in the case of ApcMin mice, we also observed that Rab25 heterozygote mice, which display an intermediate amount of Rab25 expression, showed an intermediate increase in polyp formation (Nam et al, 2010). Combined with the results in ovarian cancer cells, it appears that manipulation of Rab25 levels can lead to aberrations in the trafficking of β1-integrin and perhaps other critical regulators of cell adhesion (Figure 1). Nevertheless, although evidence does exists for the regulation of integrin trafficking in non-polarised cells by Rab11a (Mitchell et al, 2004), the exact mechanism that could account for the alterations of integrin trafficking remain unclear. Norman and colleagues (Caswell et al, 2007) have observed that under certain circumstances, some overlap of internalised β1-integrin and Rab25 can be observed; however, we have not been able to demonstrate colocalisation of β1-integrin with Rab25 in intestinal systems. Norman et al (Caswell et al, 2007) have also shown evidence for a possible direct interaction of Rab25 with β1-integrin. Nevertheless, the effects of Rab25 expression changes on integrins may also reflect en passant effects because of either alteration in the trafficking of another cargo (e.g., the EGF receptor) or perhaps by changing the effective concentration of direct effectors shared with Rab11a (e.g., Rab11-FIPs; Figure 1). Alternatively, the alterations seen with loss of Rab25 could reflect an alteration in the downstream effects mediated by Rab11-FIP1C/RCP. Although we did not observe any alterations in expression of Rab11-FIP1C in human colon cancers, recent investigations in breast cancer have implicated overexpression of Rab11-FIP1C in breast cancers (Zhang et al, 2009). In addition, these studies have suggested that Rab11-FIP1C can interact with and regulate activation of Ras (Figure 1). Similarly, in colon cancers, changes in Rab25 expression may lead to alterations in Ras through changes in competition between Rab25 and Ras for Rab11-FIP1C.
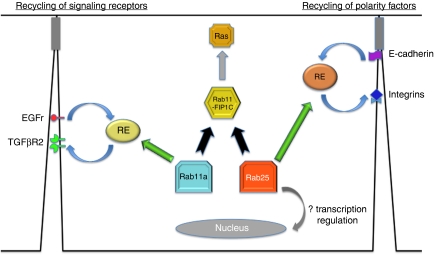
Figure 1.
A scheme for regulation of transformation and cell polarity by Rab25. The figure shows the possible interactions of vesicle recycling through recycling endosomes (RE) of either signalling receptors (on the left) or polarity-related proteins (on the right). Loss of Rab25 in colon cells could lead to either direct dysregulation of these pathways or imbalances in parallel trafficking pathways regulated by Rab11a or by alteration of influences on downstream shared effectors such as Rab11-FIP1C (also known as RCP). Effects of Rab25 on transcription remain unknown, although there is no evidence that Rab25 ever enters the nucleus.
Thus, although it is clear that Rab25 can impact epithelial cell transformation, the exact mechanism remains elusive. It appears that the effects of Rab25 expression depend largely on context. Thus, either overexpression or loss of Rab25 expression can lead to transformation. It seems likely that these findings result from cell-specific roles for Rab25 in the trafficking of specific cargoes. Thus, if, as is documented in MDCK cells (Tzaban et al, 2009), Rab25 is involved in apical-to-basolateral transcytosis, either overexpression or loss of expression could lead to imbalances in the distribution of trafficking cargoes between the apical and basolateral membranes. Imbalances on either end of the spectrum could lead to loss in polarity that predisposes to transformation. Similarly, if trafficking outcomes depend on a competition between Rab25 and Rab11a for Rab11-FIPs (Figure 1), then overexpression or loss of Rab25 expression could have marked effects on the flow through membrane recycling systems of multiple cargoes, including signalling receptors such as the EGF receptor or TGF-β receptors. In considering these rather subtle effects, it is important to note that small defects in the efficiency of dynamic trafficking over an extended period of time may lead to progressive deficits, perhaps contributing to the age-related increase in cancer incidence.
Conclusion
Loss of Rab25 in human colon cancers is associated with poorer patient prognosis. Although Rab25-deficient mice do not develop spontaneous colon tumours, breeding of ApcMin mice onto the Rab25-deficient background induces a marked increase in tumours, and crossing of Smad3+/− mice onto the Rab25 knockout background yields large invasive tumours. These results indicate that Rab25 is an important tumour suppressor in the colon. Given the results in both ovarian and breast cancer studies, it is likely that Rab25 influences the trafficking and recycling of a number of key regulators of polarity and signalling involved in transformation. Thus, either loss of Rab25 or overexpression of Rab25 may lead to cell transformation based on the cell-specific alteration in surface protein delivery.
Acknowledgments
Dr Goldenring is supported by the NIH Grants RO1 DK48370 and R21 AR49311 and a VA Merit Review award.
References
- Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, Small JV, Retta SF (2005) E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci 118(Part 20): 4765–4783 [DOI] [PubMed] [Google Scholar]
- Casanova JE, Wang X, Kumar R, Bhartur SG, Navarre J, Woodrum JE, Altschuler Y, Ray GS, Goldenring JR (1999) Association of Rab25 and Rab11a with the apical recycling system of polarized Madin-Darby canine kidney cells. Mol Biol Cell 10(1): 47–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell PT, Spence HJ, Parsons M, White DP, Clark K, Cheng KW, Mills GB, Humphries MJ, Messent AJ, Anderson KI, McCaffrey MW, Ozanne BW, Norman JC (2007) Rab25 associates with alpha5beta1 integrin to promote invasive migration in 3D microenvironments. Dev Cell 13(4): 496–510 [DOI] [PubMed] [Google Scholar]
- Chen HC, Chu RY, Hsu PN, Hsu PI, Lu JY, Lai KH, Tseng HH, Chou NH, Huang MS, Tseng CJ, Hsiao M (2003) Loss of E-cadherin expression correlates with poor differentiation and invasion into adjacent organs in gastric adenocarcinomas. Cancer Lett 201(1): 97–106 [DOI] [PubMed] [Google Scholar]
- Cheng JM, Ding M, Aribi A, Shah P, Rao K (2006) Loss of RAB25 expression in breast cancer. Int J Cancer 118(12): 2957–2964 [DOI] [PubMed] [Google Scholar]
- Cheng JM, Volk L, Janaki DK, Vyakaranam S, Ran S, Rao KA (2010) Tumor suppressor function of Rab25 in triple-negative breast cancer. Int J Cancer 126(12): 2799–2812 [DOI] [PubMed] [Google Scholar]
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, Liu J, Smith-McCune K, Lu KH, Fishman D, Gray JW, Mills GB (2004) The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med 10(11): 1251–1256 [DOI] [PubMed] [Google Scholar]
- Chia WJ, Tang BL (2009) Emerging roles for Rab family GTPases in human cancer. Biochim Biophys Acta 1795(2): 110–116 [DOI] [PubMed] [Google Scholar]
- D’Abaco GM, Whitehead RH, Burgess AW (1996) Synergy between Apc min and an activated ras mutation is sufficient to induce colon carcinomas. Mol Cell Biol 16(3): 884–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane NG, Manning HC, Foutch AC, Washington MK, Aronow BJ, Bornhop DJ, Coffey RJ (2007) Targeted imaging of colonic tumors in smad3-/- mice discriminates cancer and inflammation. Mol Cancer Res 5(4): 341–349 [DOI] [PubMed] [Google Scholar]
- Dohn MR, Brown MV, Reynolds AB (2009) An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J Cell Biol 184(3): 437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme NA, Hales CM, Lapierre LA, Ham AJ, Oztan A, Apodaca G, Goldenring JR (2006) MARK2/EMK1/Par-1Balpha phosphorylation of Rab11-family interacting protein 2 is necessary for the timely establishment of polarity in Madin-Darby canine kidney cells. Mol Biol Cell 17(8): 3625–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson RP, Larson-Thome K, Valenzuela RK, Whitaker SE, Shub MD (2008) Navajo microvillous inclusion disease is due to a mutation in MYO5B. Am J Med Genet A 146A(24): 3117–3119 [DOI] [PubMed] [Google Scholar]
- Goldenring JR, Shen KR, Vaughan HD, Modlin IM (1993) Identification of a small GTP-binding protein, Rab25, expressed in the gastrointestinal mucosa, kidney and lung. J Biol Chem 268: 18419–18422 [PubMed] [Google Scholar]
- Hales CM, Griner R, Hobdy-Henderson KC, Dorn MC, Hardy D, Kumar R, Navarre J, Chan EKL, Lapierre LA, Goldenring JR (2001) Identification and characterization of a family of Rab11-interacting proteins. J Biol Chem 276: 39067–39075 [DOI] [PubMed] [Google Scholar]
- Iancu TC, Mahajnah M, Manov I, Shaoul R (2007) Microvillous inclusion disease: ultrastructural variability. Ultrastruct Pathol 31(3): 173–188 [DOI] [PubMed] [Google Scholar]
- Jones MC, Caswell PT, Norman JC (2006) Endocytic recycling pathways: emerging regulators of cell migration. Curr Opin Cell Biol 18(5): 549–557 [DOI] [PubMed] [Google Scholar]
- Lapierre LA, Kumar R, Hales CM, Navarre J, Bhartur SG, Burnette JO, Mercer JA, Bahler M, Goldenring JR (2001) Myosin Vb is associated with and regulates plasma membrane recycling systems. Mol Biol Cell 12: 1843–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioni M, Brafford P, Andl C, Rustgi A, El-Deiry W, Herlyn M, Smalley KS (2007) Dysregulation of claudin-7 leads to loss of E-cadherin expression and the increased invasion of esophageal squamous cell carcinoma cells. Am J Pathol 170(2): 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ (2008) Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol 9(11): 833–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell H, Choudhury A, Pagano RE, Leof EB (2004) Ligand-dependent and -independent transforming growth factor-beta receptor recycling regulated by clathrin-mediated endocytosis and Rab11. Mol Biol Cell 15(9): 4166–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T, Hess MW, Schiefermeier N, Pfaller K, Ebner HL, Heinz-Erian P, Ponstingl H, Partsch J, Rollinghoff B, Kohler H, Berger T, Lenhartz H, Schlenck B, Houwen RJ, Taylor CJ, Zoller H, Lechner S, Goulet O, Utermann G, Ruemmele FM, Huber LA, Janecke AR (2008) MYO5B mutations cause microvillus inclusion disease and disrupt epithelial cell polarity. Nat Genet 40(10): 1163–1165 [DOI] [PubMed] [Google Scholar]
- Nam KT, Lee HJ, Smith JJ, Lapierre LA, Kamath VP, Chen X, Aronow BJ, Yeatman TJ, Bhartur SG, Calhoun BC, Condie B, Manley NR, Beauchamp RD, Coffey RJ, Goldenring JR (2010) Loss of Rab25 promotes the development of intestinal neoplasia in mice and is associated with human colorectal adenocarcinomas. J Clin Invest 120(3): 840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen T, Arjonen A, Vuoriluoto K, Kallio K, Fransen JA, Ivaska J (2006) Small GTPase Rab21 regulates cell adhesion and controls endosomal traffic of beta1-integrins. J Cell Biol 173(5): 767–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellinen T, Tuomi S, Arjonen A, Wolf M, Edgren H, Meyer H, Grosse R, Kitzing T, Rantala JK, Kallioniemi O, Fassler R, Kallio M, Ivaska J (2008) Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev Cell 15(3): 371–385 [DOI] [PubMed] [Google Scholar]
- Roland JT, Lapierre LA, Goldenring JR (2009) Alternative splicing in class V myosins determines association with Rab10. J Biol Chem 284(2): 1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz SL, Cao C, Pylypenko O, Rak A, Wandinger-Ness A (2007) Rab GTPases at a glance. J Cell Sci 120(Part 22): 3905–3910 [DOI] [PubMed] [Google Scholar]
- Smalley-Freed WG, Efimov A, Burnett PE, Short SP, Davis MA, Gumucio DL, Washington MK, Coffey RJ, Reynolds AB (2010) p120-catenin is essential for maintenance of barrier function and intestinal homeostasis in mice. J Clin Invest 120(6): 1824–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somsel Rodman J, Wandinger-Ness A (2000) Rab GTPases coordinate endocytosis. J Cell Sci 113(Part 2): 183–192 [DOI] [PubMed] [Google Scholar]
- Torres VA, Mielgo A, Barbero S, Hsiao R, Wilkins JA, Stupack DG (2010) Rab5 mediates caspase-8-promoted cell motility and metastasis. Mol Biol Cell 21(2): 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzaban S, Massol RH, Yen E, Hamman W, Frank SR, Lapierre LA, Hansen SH, Goldenring JR, Blumberg RS, Lencer WI (2009) The recycling and transcytotic pathways for IgG transport by FcRn are distinct and display an inherent polarity. J Cell Biol 185(4): 673–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Reinsch S, Urbe S, Zerial M, Parton RG (1996) Rab11 regulates recycling through the pericentriolar recycling endosome. J Cell Biol 135: 913–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kumar R, Navarre J, Casanova JE, Goldenring JR (2000) Regulation of vesicle trafficking in Madin-Darby canine kidney cells by Rab11a and Rab25. J Biol Chem 275: 29138–29146 [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu X, Datta A, Govindarajan K, Tam WL, Han J, George J, Wong C, Ramnarayanan K, Phua TY, Leong WY, Chan YS, Palanisamy N, Liu ET, Karuturi KM, Lim B, Miller LD (2009) RCP is a human breast cancer-promoting gene with Ras-activating function. J Clin Invest 119(8): 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, Graff JM (1998) Smad3 mutant mice develop metastatic colorectal cancer. Cell 94(6): 703–714 [DOI] [PubMed] [Google Scholar]