Abstract
Using the pH-sensitive dye 2,7-bis(carboxyethyl)-5(6)-carboxy-fluorescein and a continuously perfused subconfluent hepatocyte monolayer cell culture system, we studied rat hepatocyte intracellular pH (pHi) regulation in the presence (+HCO3-) and absence (-HCO3-) of bicarbonate. Baseline pHi was higher (7.28 +/- 09) in +HCO3- than in -HCO3- (7.16 +/- 0.14). Blocking Na+/H+ exchange with amiloride had no effect on pHi in +HCO3- but caused reversible 0.1-0.2-U acidification in -HCO3- or in +HCO3- after preincubation in the anion transport inhibitor 4,4'-diisothiocyano-2,2'-disulfonic acid stilbene (DIDS). Acute Na+ replacement in +HCO3- alos caused acidification which was amiloride independent but DIDS inhibitible. The recovery of pHi from an intracellular acid load (maximum H+ efflux rate) was 50% higher in +HCO3- than in -HCO3-. Amiloride inhibited H+ effluxmax by 75% in -HCO3- but by only 27% in +HCO3-. The amiloride-independent pHi recovery in +HCO3- was inhibited 50-63% by DIDS and 79% by Na+ replacement but was unaffected by depletion of intracellular Cl-, suggesting that Cl-/HCO3- exchange is not involved. Depolarization of hepatocytes (raising external K+ from 5 to 25 mM) caused reversible 0.05-0.1-U alkalinization, which, however, was neither Na+ nor HCO3- dependent, nor DIDS inhibitible, findings consistent with electroneutral HCO3- transport. We conclude that Na+-HCO3- cotransport, in addition to Na+/H+ exchange, is an important regulator of pHi in rat hepatocytes.
Full text
PDF
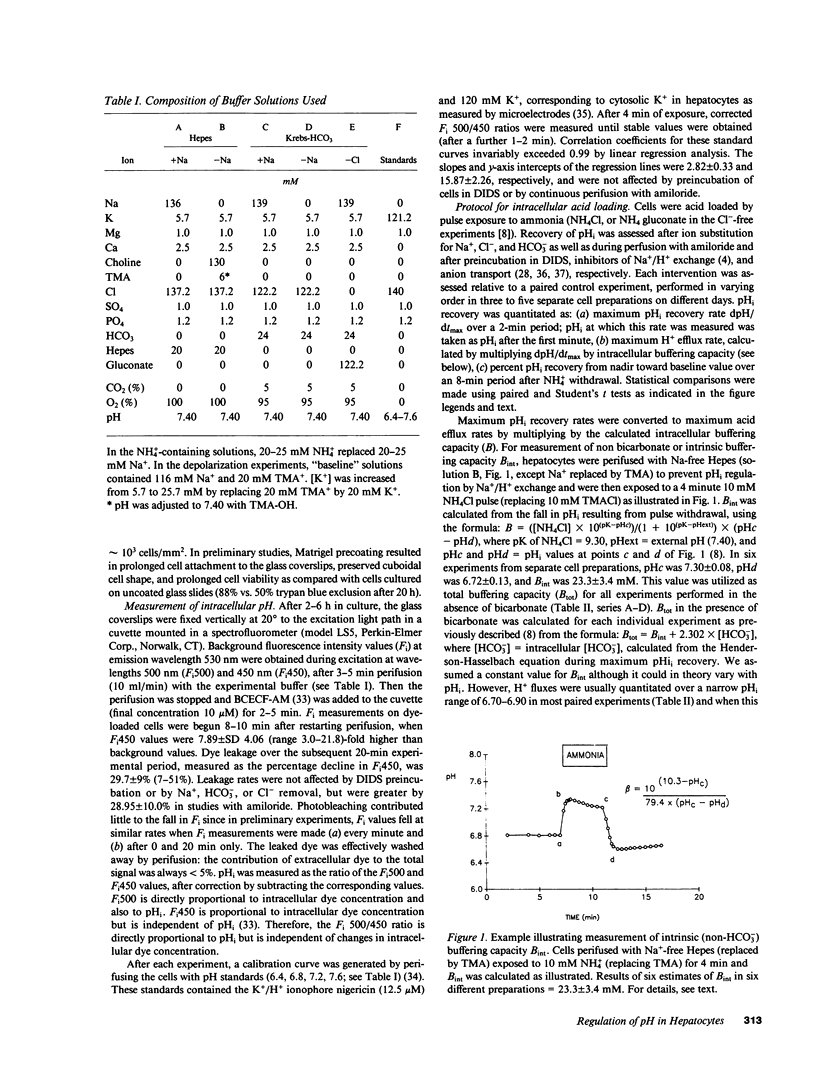
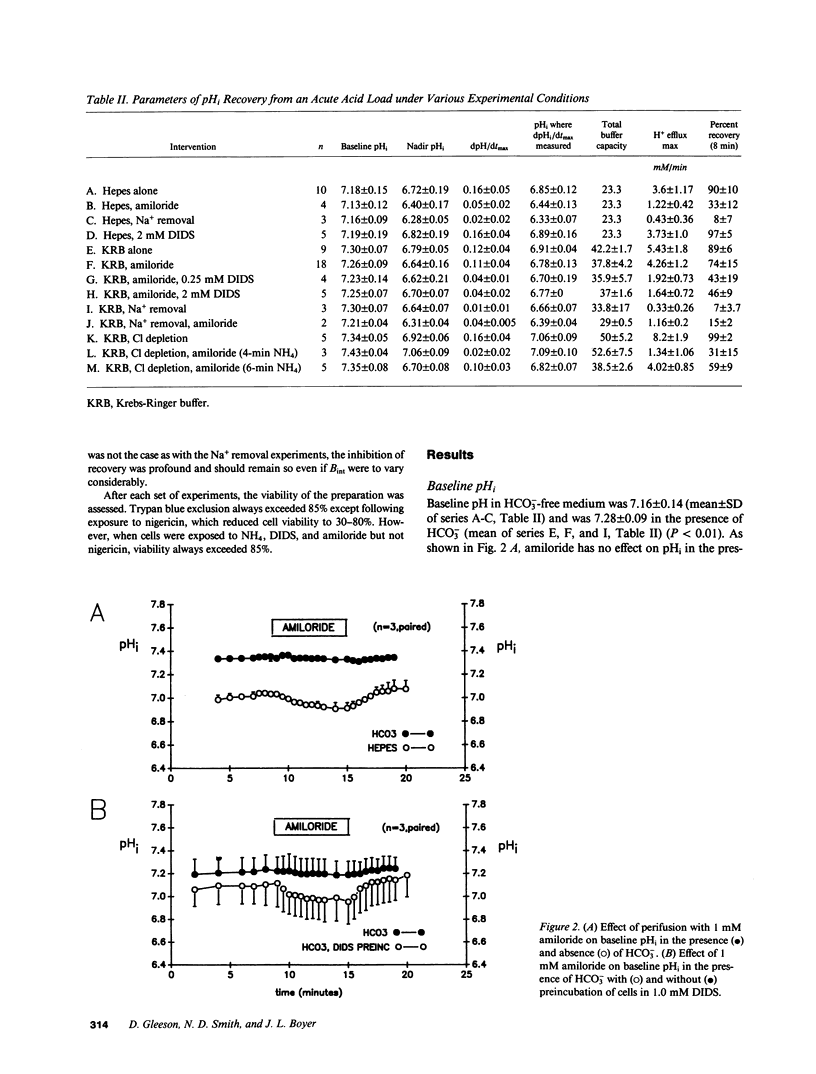







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aickin C. C. Intracellular pH regulation by vertebrate muscle. Annu Rev Physiol. 1986;48:349–361. doi: 10.1146/annurev.ph.48.030186.002025. [DOI] [PubMed] [Google Scholar]
- Alpern R. J. Mechanism of basolateral membrane H+/OH-/HCO-3 transport in the rat proximal convoluted tubule. A sodium-coupled electrogenic process. J Gen Physiol. 1985 Nov;86(5):613–636. doi: 10.1085/jgp.86.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias I. M., Forgac M. The sinusoidal domain of the plasma membrane of rat hepatocytes contains an amiloride-sensitive Na+/H+ antiport. J Biol Chem. 1984 May 10;259(9):5406–5408. [PubMed] [Google Scholar]
- Atkinson D. E., Bourke E. Metabolic aspects of the regulation of systemic pH. Am J Physiol. 1987 Jun;252(6 Pt 2):F947–F956. doi: 10.1152/ajprenal.1987.252.6.F947. [DOI] [PubMed] [Google Scholar]
- Biagi B. A., Sohtell M. Electrophysiology of basolateral bicarbonate transport in the rabbit proximal tubule. Am J Physiol. 1986 Feb;250(2 Pt 2):F267–F272. doi: 10.1152/ajprenal.1986.250.2.F267. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., McCormick W. C., Roos A. pH regulation in barnacle muscle fibers: dependence on extracellular sodium and bicarbonate. Am J Physiol. 1981 Jan;240(1):C80–C89. doi: 10.1152/ajpcell.1981.240.1.C80. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cassel D., Whiteley B., Zhuang Y. X., Glaser L. Mitogen-independent activation of Na+/H+ exchange in human epidermoid carcinoma A431 cells: regulation by medium osmolarity. J Cell Physiol. 1985 Feb;122(2):178–186. doi: 10.1002/jcp.1041220203. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Rottenberg H., Glynn P., Yamane T., Brown T. R., Shulman R. G. P nuclear magnetic resonance studies of isolated rat liver cells. Nature. 1978 Jun 15;273(5663):554–556. doi: 10.1038/273554a0. [DOI] [PubMed] [Google Scholar]
- Fitz J. G., Scharschmidt B. F. Regulation of transmembrane electrical potential gradient in rat hepatocytes in situ. Am J Physiol. 1987 Jan;252(1 Pt 1):G56–G64. doi: 10.1152/ajpgi.1987.252.1.G56. [DOI] [PubMed] [Google Scholar]
- Ganz M. B., Boyarsky G., Boron W. F., Sterzel R. B. Effects of angiotensin II and vasopressin on intracellular pH of glomerular mesangial cells. Am J Physiol. 1988 Jun;254(6 Pt 2):F787–F794. doi: 10.1152/ajprenal.1988.254.6.F787. [DOI] [PubMed] [Google Scholar]
- Gautam A., Ng O. C., Boyer J. L. Isolated rat hepatocyte couplets in short-term culture: structural characteristics and plasma membrane reorganization. Hepatology. 1987 Mar-Apr;7(2):216–223. doi: 10.1002/hep.1840070203. [DOI] [PubMed] [Google Scholar]
- Graf J., Gautam A., Boyer J. L. Isolated rat hepatocyte couplets: a primary secretory unit for electrophysiologic studies of bile secretory function. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6516–6520. doi: 10.1073/pnas.81.20.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf J., Henderson R. M., Krumpholz B., Boyer J. L. Cell membrane and transepithelial voltages and resistances in isolated rat hepatocyte couplets. J Membr Biol. 1987;95(3):241–254. doi: 10.1007/BF01869486. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Cohen S., Goetz J. D., Rothstein A. Na+/H+ exchange in volume regulation and cytoplasmic pH homeostasis in lymphocytes. Fed Proc. 1985 Jun;44(9):2508–2512. [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., London R., Boulpaep E. L., Giebisch G. Chloride transport across the basolateral cell membrane of the Necturus proximal tubule: dependence on bicarbonate and sodium. J Membr Biol. 1983;71(3):227–240. doi: 10.1007/BF01875464. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Graf J., Boyer J. L. Na-H exchange regulates intracellular pH in isolated rat hepatocyte couplets. Am J Physiol. 1987 Jan;252(1 Pt 1):G109–G113. doi: 10.1152/ajpgi.1987.252.1.G109. [DOI] [PubMed] [Google Scholar]
- Henderson R. M., Krumpholz B., Boyer J. L., Graf J. Effect of intracellular pH on potassium conductance in liver. Pflugers Arch. 1988 Aug;412(3):334–335. doi: 10.1007/BF00582518. [DOI] [PubMed] [Google Scholar]
- Jentsch T. J., Keller S. K., Koch M., Wiederholt M. Evidence for coupled transport of bicarbonate and sodium in cultured bovine corneal endothelial cells. J Membr Biol. 1984;81(3):189–204. doi: 10.1007/BF01868713. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Schron C. M., Seifter J., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. II. Evidence for Cl-HCO3 exchange and mechanism of coupling. Am J Physiol. 1985 Aug;249(2 Pt 1):G236–G245. doi: 10.1152/ajpgi.1985.249.2.G236. [DOI] [PubMed] [Google Scholar]
- Koch K. S., Leffert H. L. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979 Sep;18(1):153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- Kurtz I., Golchini K. Na+-independent Cl(-)-HCO-3- exchange in Madin-Darby canine kidney cells. Role in intracellular pH regulation. J Biol Chem. 1987 Apr 5;262(10):4516–4520. [PubMed] [Google Scholar]
- L'Allemain G., Paris S., Pouysségur J. Role of a Na+-dependent Cl-/HCO3- exchange in regulation of intracellular pH in fibroblasts. J Biol Chem. 1985 Apr 25;260(8):4877–4883. [PubMed] [Google Scholar]
- Ladoux A., Krawice I., Cragoe E. J., Jr, Abita J. P., Frelin C. Properties of the Na+-dependent Cl-/HCO3- exchange system in U937 human leukemic cells. Eur J Biochem. 1987 Dec 30;170(1-2):43–49. doi: 10.1111/j.1432-1033.1987.tb13665.x. [DOI] [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985 Jun;56(6):773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- Meier P. J., Knickelbein R., Moseley R. H., Dobbins J. W., Boyer J. L. Evidence for carrier-mediated chloride/bicarbonate exchange in canalicular rat liver plasma membrane vesicles. J Clin Invest. 1985 Apr;75(4):1256–1263. doi: 10.1172/JCI111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Moseley R. H., Meier P. J., Aronson P. S., Boyer J. L. Na-H exchange in rat liver basolateral but not canalicular plasma membrane vesicles. Am J Physiol. 1986 Jan;250(1 Pt 1):G35–G43. doi: 10.1152/ajpgi.1986.250.1.G35. [DOI] [PubMed] [Google Scholar]
- Nord E. P., Brown S. E., Crandall E. D. Cl-/HCO3- exchange modulates intracellular pH in rat type II alveolar epithelial cells. J Biol Chem. 1988 Apr 25;263(12):5599–5606. [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Demarest J. R., Machen T. E. Na-H and Cl-HCO3 exchange in rabbit oxyntic cells using fluorescence microscopy. Am J Physiol. 1987 Jul;253(1 Pt 1):C30–C36. doi: 10.1152/ajpcell.1987.253.1.C30. [DOI] [PubMed] [Google Scholar]
- Paradiso A. M., Tsien R. Y., Machen T. E. Na+-H+ exchange in gastric glands as measured with a cytoplasmic-trapped, fluorescent pH indicator. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7436–7440. doi: 10.1073/pnas.81.23.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock A. S. Intracellular pH of hepatocytes in primary monolayer culture. Am J Physiol. 1984 May;246(5 Pt 2):F738–F744. doi: 10.1152/ajprenal.1984.246.5.F738. [DOI] [PubMed] [Google Scholar]
- Renner E. L., Lake J. R., Scharschmidt B. F., Zimmerli B., Meier P. J. Rat hepatocytes exhibit basolateral Na+/HCO3- cotransport. J Clin Invest. 1989 Apr;83(4):1225–1235. doi: 10.1172/JCI114005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg P., Glaser L., Schlesinger P., Cassel D. Activation of Na+/H+ exchange by epidermal growth factor elevates intracellular pH in A431 cells. J Biol Chem. 1983 Oct 25;258(20):12644–12653. [PubMed] [Google Scholar]
- Sasaki S., Yoshiyama N. Interaction of chloride and bicarbonate transport across the basolateral membrane of rabbit proximal straight tubule. Evidence for sodium coupled chloride/bicarbonate exchange. J Clin Invest. 1988 Apr;81(4):1004–1011. doi: 10.1172/JCI113410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T., Hoshi T. Role of Na+/H+ antiport in intracellular pH regulation by rabbit enterocytes. Biochim Biophys Acta. 1987 Jul 23;901(2):265–272. doi: 10.1016/0005-2736(87)90123-4. [DOI] [PubMed] [Google Scholar]
- Simchowitz L., Roos A. Regulation of intracellular pH in human neutrophils. J Gen Physiol. 1985 Mar;85(3):443–470. doi: 10.1085/jgp.85.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M., Grassi S. M., Aronson P. S. Stoichiometry of Na+-HCO-3 cotransport in basolateral membrane vesicles isolated from rabbit renal cortex. J Clin Invest. 1987 Apr;79(4):1276–1280. doi: 10.1172/JCI112948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The role of bicarbonate, chloride and sodium ions in the regulation of intracellular pH in snail neurones. J Physiol. 1977 Dec;273(1):317–338. doi: 10.1113/jphysiol.1977.sp012096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tønnessen T. I., Ludt J., Sandvig K., Olsnes S. Bicarbonate/chloride antiport in Vero cells: I. Evidence for both sodium-linked and sodium-independent exchange. J Cell Physiol. 1987 Aug;132(2):183–191. doi: 10.1002/jcp.1041320202. [DOI] [PubMed] [Google Scholar]
- Vaughan-Jones R. D. Chloride activity and its control in skeletal and cardiac muscle. Philos Trans R Soc Lond B Biol Sci. 1982 Dec 1;299(1097):537–548. doi: 10.1098/rstb.1982.0150. [DOI] [PubMed] [Google Scholar]
- Walsh P. J. Ionic requirements for intracellular pH regulation in rainbow trout hepatocytes. Am J Physiol. 1986 Jan;250(1 Pt 2):R24–R29. doi: 10.1152/ajpregu.1986.250.1.R24. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Burckhardt B. C., Frömter E. Rheogenic sodium-bicarbonate cotransport in the peritubular cell membrane of rat renal proximal tubule. Pflugers Arch. 1985 Dec;405(4):360–366. doi: 10.1007/BF00595689. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Seifter J. L. Intracellular pH regulation in rabbit renal medullary collecting duct cells. Role of chloride-bicarbonate exchange. J Clin Invest. 1986 May;77(5):1682–1688. doi: 10.1172/JCI112486. [DOI] [PMC free article] [PubMed] [Google Scholar]



