Abstract
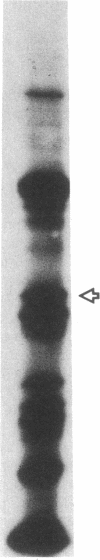
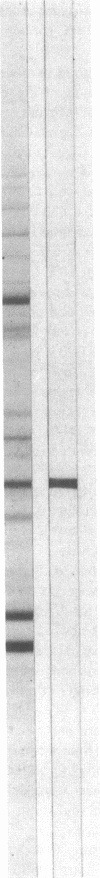
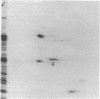
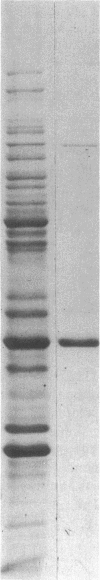
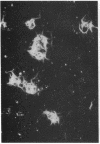
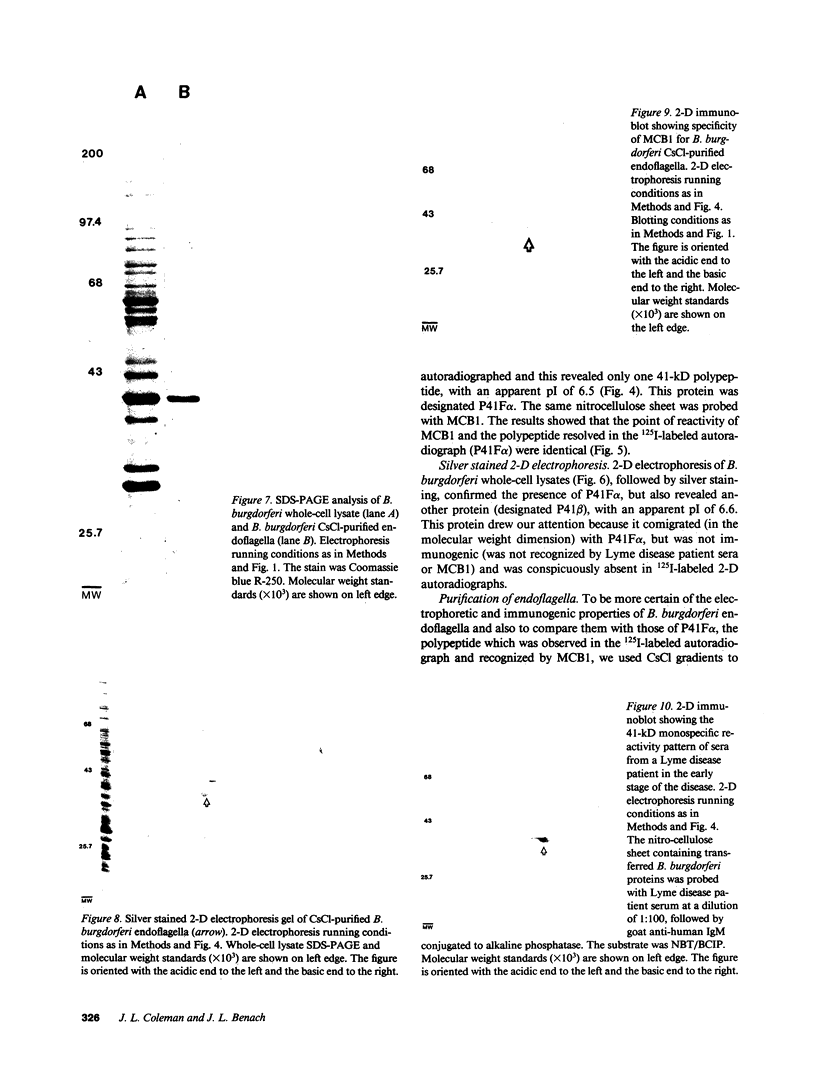
The 41-kD antigen of Borrelia burgdorferi is an immunodominant protein that is recognized early by antibodies in sera from Lyme disease patients and known to be associated with the endoflagella. We identified the 41-kD endoflagellar antigen to be a single polypeptide with an apparent isoelectric point (pI) of 6.5 by two-dimensional (2-D) electrophoresis. This polypeptide, which we designated P41F alpha, was heavily labeled by 125I in 2-D autoradiographs of B. burgdorferi whole-cell lysates and was recognized by a murine monoclonal antibody (MCB1) and human antisera in 2-D immunoblots. NH2-terminal sequence analysis showed 80% homology between P41F alpha and the 33-kD endoflagellar protein of Treponema pallidum. Results of indirect immunofluorescence assays (IFA), Triton X-114 phase partitioning, and agglutination studies suggested a possible surface exposure of the polypeptide. Silver stained 2-D gels also revealed the presence of another 41-kD species, with an apparent pI of 6.6 (designated P41 beta), which was not radioiodinated in 2-D autoradiographs, and was not recognized by MCB1 or human antisera. NH2-terminal sequence analysis of P41 beta revealed no homology with P41F alpha, leading to the conclusion that they are not related.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker-Zander S. A., Lukehart S. A. Antigenic cross-reactivity between Treponema pallidum and other pathogenic members of the family Spirochaetaceae. Infect Immun. 1984 Oct;46(1):116–121. doi: 10.1128/iai.46.1.116-121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Hayes S. F., Heiland R. A., Schrumpf M. E., Tessier S. L. A Borrelia-specific monoclonal antibody binds to a flagellar epitope. Infect Immun. 1986 May;52(2):549–554. doi: 10.1128/iai.52.2.549-554.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour A. G., Tessier S. L., Hayes S. F. Variation in a major surface protein of Lyme disease spirochetes. Infect Immun. 1984 Jul;45(1):94–100. doi: 10.1128/iai.45.1.94-100.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach J. L., Bosler E. M., Hanrahan J. P., Coleman J. L., Habicht G. S., Bast T. F., Cameron D. J., Ziegler J. L., Barbour A. G., Burgdorfer W. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983 Mar 31;308(13):740–742. doi: 10.1056/NEJM198303313081302. [DOI] [PubMed] [Google Scholar]
- Benach J. L., Coleman J. L., Golightly M. G. A murine IgM monoclonal antibody binds an antigenic determinant in outer surface protein A, an immunodominant basic protein of the Lyme disease spirochete. J Immunol. 1988 Jan 1;140(1):265–272. [PubMed] [Google Scholar]
- Benach J. L., Fleit H. B., Habicht G. S., Coleman J. L., Bosler E. M., Lane B. P. Interactions of phagocytes with the Lyme disease spirochete: role of the Fc receptor. J Infect Dis. 1984 Oct;150(4):497–507. doi: 10.1093/infdis/150.4.497. [DOI] [PubMed] [Google Scholar]
- Berger B. W., Kaplan M. H., Rothenberg I. R., Barbour A. G. Isolation and characterization of the Lyme disease spirochete from the skin of patients with erythema chronicum migrans. J Am Acad Dermatol. 1985 Sep;13(3):444–449. doi: 10.1016/s0190-9622(85)70187-9. [DOI] [PubMed] [Google Scholar]
- Bharier M. A., Rittenberg S. C. Chemistry of axial filaments of Treponema zuezerae. J Bacteriol. 1971 Jan;105(1):422–429. doi: 10.1128/jb.105.1.422-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco D. R., Champion C. I., Miller J. N., Lovett M. A. Antigenic and structural characterization of Treponema pallidum (Nichols strain) endoflagella. Infect Immun. 1988 Jan;56(1):168–175. doi: 10.1128/iai.56.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W., Barbour A. G., Hayes S. F., Benach J. L., Grunwaldt E., Davis J. P. Lyme disease-a tick-borne spirochetosis? Science. 1982 Jun 18;216(4552):1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- Coleman J. L., Benach J. L. Isolation of antigenic components from the Lyme disease spirochete: their role in early diagnosis. J Infect Dis. 1987 Apr;155(4):756–765. doi: 10.1093/infdis/155.4.756. [DOI] [PubMed] [Google Scholar]
- Craft J. E., Fischer D. K., Shimamoto G. T., Steere A. C. Antigens of Borrelia burgdorferi recognized during Lyme disease. Appearance of a new immunoglobulin M response and expansion of the immunoglobulin G response late in the illness. J Clin Invest. 1986 Oct;78(4):934–939. doi: 10.1172/JCI112683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch R. N., Sjoblad R. D. Flagellar structure and function in eubacteria. Annu Rev Microbiol. 1980;34:69–108. doi: 10.1146/annurev.mi.34.100180.000441. [DOI] [PubMed] [Google Scholar]
- Duray P. H., Steere A. C. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. doi: 10.1111/j.1749-6632.1988.tb31839.x. [DOI] [PubMed] [Google Scholar]
- Grodzicki R. L., Steere A. C. Comparison of immunoblotting and indirect enzyme-linked immunosorbent assay using different antigen preparations for diagnosing early Lyme disease. J Infect Dis. 1988 Apr;157(4):790–797. doi: 10.1093/infdis/157.4.790. [DOI] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hansen K., Hindersson P., Pedersen N. S. Measurement of antibodies to the Borrelia burgdorferi flagellum improves serodiagnosis in Lyme disease. J Clin Microbiol. 1988 Feb;26(2):338–346. doi: 10.1128/jcm.26.2.338-346.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy P. H., Jr, Fredericks W. R., Nell E. E. Isolation and antigenic characteristics of axial filaments from the Reiter Treponeme. Infect Immun. 1975 Feb;11(2):380–386. doi: 10.1128/iai.11.2.380-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978 Mar;42(1):114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joys T. M. The flagellar filament protein. Can J Microbiol. 1988 Apr;34(4):452–458. doi: 10.1139/m88-078. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum D. M. A compilation of amino acid analyses of proteins. VII. Residues per molecule-5. Anal Biochem. 1975 May 26;66(1):123–150. doi: 10.1016/0003-2697(75)90732-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Johnson R. C. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. J Infect Dis. 1987 Jul;156(1):183–188. doi: 10.1093/infdis/156.1.183. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Schleicher L. R., 3rd, Bergman K. The amino acid composition of Rhizobium meliloti flagellin: occurrence of a single tryptophan residue. Biochem Biophys Res Commun. 1981 Jun;100(4):1634–1641. doi: 10.1016/0006-291x(81)90706-3. [DOI] [PubMed] [Google Scholar]
- Stamm L. V., Hodinka R. L., Wyrick P. B., Bassford P. J., Jr Changes in the cell surface properties of Treponema pallidum that occur during in vitro incubation of freshly extracted organisms. Infect Immun. 1987 Sep;55(9):2255–2261. doi: 10.1128/iai.55.9.2255-2261.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Malawista S. E., Hardin J. A., Ruddy S., Askenase W., Andiman W. A. Erythema chronicum migrans and Lyme arthritis. The enlarging clinical spectrum. Ann Intern Med. 1977 Jun;86(6):685–698. doi: 10.7326/0003-4819-86-6-685. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]