Human immunodeficiency syndrome with loss of DCs, monocytes, and T reg cells; preservation of Langerhans cells; associated loss of BM multilymphoid progenitors; and overproduction of Flt3 ligand.
Abstract
Congenital or acquired cellular deficiencies in humans have the potential to reveal much about normal hematopoiesis and immune function. We show that a recently described syndrome of monocytopenia, B and NK lymphoid deficiency additionally includes the near absence of dendritic cells. Four subjects showed severe depletion of the peripheral blood HLA-DR+ lineage− compartment, with virtually no CD123+ or CD11c+ dendritic cells (DCs) and very few CD14+ or CD16+ monocytes. The only remaining HLA-DR+ lineage− cells were circulating CD34+ progenitor cells. Dermal CD14+ and CD1a+ DC were also absent, consistent with their dependence on blood-derived precursors. In contrast, epidermal Langerhans cells and tissue macrophages were largely preserved. Combined loss of peripheral DCs, monocytes, and B and NK lymphocytes was mirrored in the bone marrow by complete absence of multilymphoid progenitors and depletion of granulocyte-macrophage progenitors. Depletion of the HLA-DR+ peripheral blood compartment was associated with elevated serum fms-like tyrosine kinase ligand and reduced circulating CD4+CD25hiFoxP3+ T cells, supporting a role for DC in T reg cell homeostasis.
DCs are BM-derived cells present in lymphoid and nearly all nonlymphoid tissues. Their principal function is the induction and control of immunity (Steinman and Banchereau, 2007). Recent studies in mice have also highlighted the role of DCs in maintaining tolerance. Constitutive depletion of DCs results in autoimmunity (Ohnmacht et al., 2009), and there appears to be a direct homeostatic balance between the frequency of DCs and regulatory T (T reg) cells (Darrasse-Jèze et al., 2009).
Significant progress has been made in understanding the early events in DC ontogeny. Studies of hematopoiesis have previously documented that DCs can arise from both myeloid and lymphoid progenitors (Merad and Manz, 2009), but the myeloid origin of DCs has been the focus of much attention in recent years, with elucidation of the common origin but subsequent separation of monocyte and DC lineages in mice (Liu et al., 2009; Geissmann et al., 2010).
Adoptive transfer and KO experiments indicate that LN DCs and major subsets of migratory tissue DCs are derived from committed precursors, with the important exception of Langerhans cells (LCs), which are self-renewing (Merad and Manz, 2009). Monocytes contribute to inflammatory DCs in many sites, replace LCs after injury, and are also regarded as the precursors of tissue macrophages (Ginhoux et al., 2006; Geissmann et al., 2010). Several genes have been shown to control DC differentiation in mice, many of which also influence the differentiation of other myeloid or lymphoid lineages (Merad and Manz, 2009; Geissmann et al., 2010).
In humans, it has not been possible to probe the distal relationships between monocytes and DCs or to prove the independence of LCs from BM-derived precursors in the steady state. Hematopoietic stem cell transplantation allows some inferences to be drawn from kinetic differences in the replacement of tissue DCs, LCs, and macrophages (Collin et al., 2006; Haniffa et al., 2009), but these data fall significantly short of the steady-state models that have been so effective in mice. New human models, in which there is spontaneous deficiency of blood DCs or monocytes, are required to test the relationships between DC precursors and their progeny.
To shed light on these issues, we searched for human subjects with DC deficiency. This was found in association with a combined monocyte, B and NK lymphoid defect and clinical features including disseminated nontuberculous mycobacterial infection, papilloma virus infection, and pulmonary alveolar proteinosis. The clinical manifestations of this novel syndrome have recently been characterized in a seminal study (Vinh et al., 2010). In this report, we demonstrate an accompanying profound deficit of blood and tissue DCs and explore the associated ontological and immunoregulatory features of the syndrome.
RESULTS AND DISCUSSION
Combined DC, monocyte, B and NK lymphoid deficiency in humans
The syndrome of autosomal dominant and sporadic monocytopenia has recently been described in a study of 18 individuals (Vinh et al., 2010). The primary features include immunodeficiency with increased susceptibility to mycobacterial infection, papilloma virus infection, and pulmonary alveolar proteinosis. 10 out of 18 patients developed hematological or other malignancies, and 4 had evidence of autoimmune phenomena. A genetic basis for this disorder is strongly implicated by an autosomal-dominant pattern of inheritance. Although heterogeneous clinical features are seen, these often arise even within one pedigree in genetic immunodeficiency disorders, and do not exclude a single gene etiology (Casanova and Abel, 2007).
We identified four subjects with this syndrome. Subject 1 presented at the age of 12 with disseminated bacillus Calmette-Guerin infection after routine vaccination and is included in the published case series (Vinh et al., 2010). Subject 2 was diagnosed at the age of 27 with spontaneous Mycobacterium kansasii infection. Subject 3 developed respiratory failure at age 21 caused by pulmonary alveolar proteinosis. Subject 4 presented with recurrent erythema nodosum and papilloma virus infection at age 23. Subjects 1, 2, and 4 had no affected relatives and are likely sporadic cases, whereas subject 3 reported two generations of ancestors dying of early respiratory death or malignancy, in keeping with autosomal dominant inheritance. Full clinical details are given in Table S1.
Automated peripheral blood counts indicated monocyte deficiency and variable lymphopenia, without significant granulocytopenia, anemia, or thrombocytopenia (Table S2). Historical blood counts demonstrated that monocytopenia had existed for at least 3 yr in subject 1, >4 yr in subject 2, at least 12 yr in subject 3, and a minimum of 10 yr in subject 4 (unpublished data).
We hypothesized that an associated deficit of DCs could contribute to the marked immunodeficiency of this syndrome, as the induction of immunity by DCs is assumed to be as critical as monocyte-macrophage effector function in maintaining immunocompetence. Furthermore, murine studies have argued persuasively that monocytes and DCs arise via separate lineages from a common precursor (Merad and Manz, 2009; Geissmann et al., 2010). This pathway has not been mapped in humans and we reasoned that more detailed analysis of monocyte and DC lineages in this syndrome might be informative.
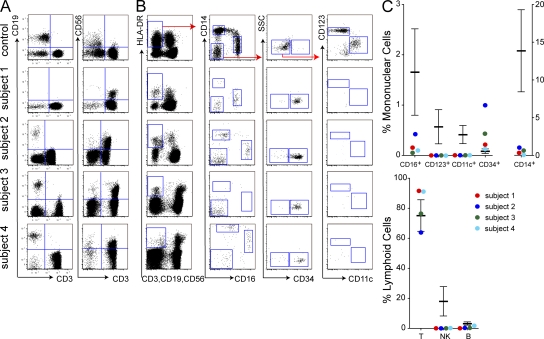
Peripheral blood flow cytometry showed severe depletion of the HLA-DR+ lineage− compartment (Fig. 1). Quantitatively, CD14+ monocytes were as low as 0.1% and no greater than 5.5% of median normal values. CD16+ monocytes were more variable, ranging from 0.2 to 24.2% of normal median values. CD123+ plasmacytoid DCs and CD11c+ myeloid DCs were undetectable in all subjects. CD11c+ cells may normally be subdivided into CD141+ and CD1c+ fractions, but neither was detectable (unpublished data). The only remaining HLA-DR+ lineage− cells were CD34+ progenitor cells, which were relatively expanded. As described previously, CD19+ B cells and CD56+ NK cells were depleted, but CD3+ T cells were in the normal range. Hereafter, we refer to this combined deficit of DC, monocyte, B and NK lymphoid cells as DCML deficiency.
Figure 1.
Deficiency of peripheral blood DCs, monocytes, and lymphoid cells. Flow cytometry of PBMCs and proportion of lymphoid, monocyte, and DC fractions in the subjects relative to controls. Plots show equivalent numbers of total cells analyzed (75,000 for DCs; 65,000 for lymphocytes). Error bars indicate mean ± SD from a population of normal individuals (n = 11 for lymphoid cells; n = 28 for HLA-DR+ lineage− cells). One of at least two independent results is shown for subjects 1–4. (A) CD3+ T cells, CD19+ B cells, and CD56+ NK cells. (B) HLA-DR+ lineage− (CD3/19/56) cells further divided into CD14+ and CD16+ monocyte fractions and double-negative CD14−CD16− cells, which comprise CD34+ progenitor cells, CD123+ PDCs, and CD11c+ myeloid DC fractions. (C) Quantitative analysis of monocyte/DC fractions, expressed as percentage of mononuclear cells, and lymphoid cells, expressed as percentage of cells in the lymphoid SSC/FSC gate, compared with healthy controls.
Functional defects indicating susceptibility to mycobacterial disease
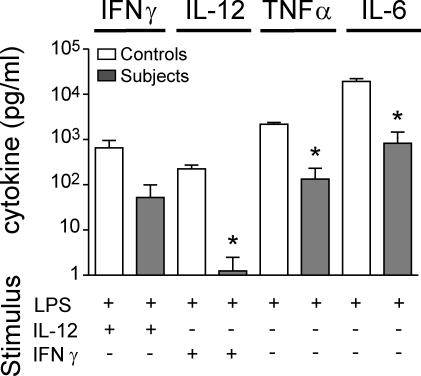
To test the functional consequence of these cellular deficiencies, we analyzed the integrity of the IL-12/IFN-γ axis in vitro by measuring IL-12 and IFN-γ production in response to LPS, complemented in turn with IFN-γ or IL-12. There was severe impairment of IL-12 and a reduction of IFN-γ production with LPS challenge, implying disruption of the IL-12/IFN-γ axis (Fig. 2).
Figure 2.
Dysfunction of the IL-12–IFN-γ axis. Cytokines released into the supernatant when whole blood was stimulated in vitro with LPS, IL-12, or IFN-γ as indicated. A paired “travel control” was run with each subject, and each subject was analyzed at least twice. The graph shows the mean ± SD for subjects (n = 4) compared with controls (n = 4) from one experiment. *, P < 0.05 compared with adjacent control (paired Student’s t test).
Defects of IL-12/IFN-γ in these assays correlate well with in vivo susceptibility to mycobacterial infection and have been confirmed repeatedly in several genetic disorders affecting cytokine production or signal transduction in the IL-12 or IFN-γ pathways, collectively known as Mendelian susceptibility to mycobacterial disease (MSMD; Casanova and Abel, 2007). One feature of DCML deficiency that distinguishes it from classical MSMD is the impaired production of the monokines TNF and IL-6 in response to LPS alone. We surmise that this directly reflects monocyte depletion, which does not occur in classical MSMD. Residual production of TNF and IL-6 in this whole-blood assay is most likely caused by direct activation of neutrophils by LPS.
Deficiency of peripheral migratory myeloid DCs with sparing of LCs and tissue macrophages
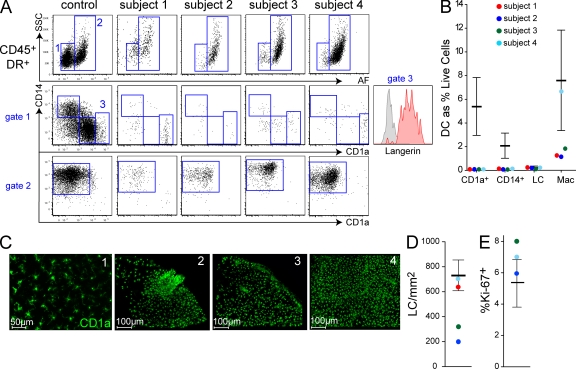
Having observed almost complete loss of DCs and monocytes from the blood, we took macroscopically healthy skin to determine if tissue DCs were similarly affected, using a flow cytometric assay as previously described (Haniffa et al., 2009). The CD45+ HLA-DR+ non-autofluorescent compartment, which typically contains dermal DCs in healthy individuals, was depleted (Fig. 3 A, gate 1), and CD14+ and CD1a+ DCs were virtually absent (Fig. 3 B). This suggests that myeloid tissue DCs share a common origin with circulating HLA-DR+ lineage− cells, although we are unable to dissect the relationship between tissue DCs and either blood DCs or monocytes because both were depleted.
Figure 3.
Depletion of tissue DCs, but preservation of LCs and macrophages. (A) Analysis of dermal DC populations by flow cytometry of collagenase-digested dermis. Plots show equivalent number of total cells analyzed (40,000). Gated CD45+ HLA-DR+ cells normally comprise two fractions separable by autofluorescence (AF) and side scatter (SSC). AF− SSClow cells (gate 1) contain CD14+ DCs, CD1a+ DCs, and occasional CD1ahigh langerin+ migrating LCs (gate 3); AF+ SSChigh cells are macrophages (gate 2). CD1ahigh cells express langerin (subject 1, inset). Subjects 1 and 3 were analyzed twice, independently; subjects 2 and 4 were analyzed once. (B) Dermal DCs, LCs, and macrophage counts relative to normal controls (n = 22; mean ± SD). (C) Whole-mount immunofluorescence staining for CD1a of epidermal sheets showing representative fields of each subject. (D) Total LC counts averaged from entire low-power image relative to controls (n = 12; mean ± SD). (E) Proportion of Ki-67+ LCs in whole-mount epidermal sheets relative to controls (n = 3).
CD1ahigh langerin+ cells, which represent migratory LCs passing through the dermis (Fig. 3 A, gate 3; Angel et al., 2006), remained detectable. CD45+ HLA-DR+ autofluorescent dermal macrophages were also present (Fig. 3 A, gate 2), but were variably depleted compared with normal (Fig. 3 B). Examination of intact epidermal sheets by whole-mount microscopy confirmed the presence of an intact LC network in three of four subjects (Fig. 3, C and D). LC density was reduced in two subjects, with patchy disruption of the network in one (subject 2). This could not be attributed to a decreased proliferative index because the proportion of CD1a+ LCs coexpressing Ki-67 was the same or greater than controls (Fig. 3 E and Fig. S1 A). The preservation of dermal macrophages did not appear to reflect local proliferation because no Ki-67+ S/G2/M phase cells are detectable in this population (Fig. S1 B). Although we took care to biopsy macroscopically healthy skin, prior cutaneous inflammation or ultraviolet exposure may have contributed to lower LC density, particularly the patchy depletion seen in subject 2.
The survival of LCs in the absence of monocytes or blood DCs is predicted by murine experiments showing local self-renewal (Merad and Manz, 2009). Although monocyte deficiency was not absolute, subjects 1, 3, and 4 had very severe monocytopenia with as few as 0.1% CD14+ and 0.2% CD16+ monocytes, compared with healthy controls. Blood DCs were even further depleted. In contrast, their LC networks remained intact and populated at 50–100% of normal density. These results therefore show the persistence of human LCs independent of blood monocytes and DCs. The complete absence of dermal DCs argues against the view that human LCs derive from dermal-based CD14+ precursors in the steady state (Larregina et al., 2001).
We were initially surprised to observe dermal macrophages in the near absence of monocytes. However, CD68+ tissue macrophages were also found in BM and alveolar spaces (Fig. S2) and inflammatory macrophages have been previously documented in this syndrome (Vinh et al., 2010). Similar to LCs, these populations of macrophages appear to persist independently of blood monocytes.
Historical blood counts show that monocytopenia had persisted for more than a decade in at least two of the subjects. Either these surviving populations of macrophages have half-lives of a decade or more, or are derived from nonmonocyte precursors. Recent evidence indicates that dermal and alveolar macrophages can indeed be extremely long-lived and might persist in the absence of circulating precursors (Murphy et al., 2008; Haniffa et al., 2009). Although monocytes were severely depleted, the circulating CD34+ cell is a potential alternative precursor. Trafficking hematopoietic stem cells have been shown to differentiate directly into antigen-presenting cells in the mouse (Massberg et al., 2007). The surprising demonstration of alveolar macrophages in lungs affected by pulmonary alveolar proteinosis strongly suggests that a qualitative rather than quantitative macrophage defect is responsible for this pathology.
Complete loss of the multilymphoid progenitor (MLP) with depletion of granulocyte macrophage progenitors in BM
DCML deficiency occurs in the context of normal erythroid, granulocyte, and platelet production, leading us to ask whether this disorder might be associated with a specific progenitor defect. It has previously been demonstrated that DC potential resides in both myeloid and lymphoid compartments in humans (Galy et al., 1995; Chicha et al., 2004). In particular, we were intrigued by the recent description in humans of MLP; cells capable of forming DCs, macrophages, and lymphoid cells but lacking erythroid, granulocyte, or megakaryocyte potential (Doulatov et al., 2010). The combination of cellular deficiency in DCML suggested that this population might be specifically deleted.
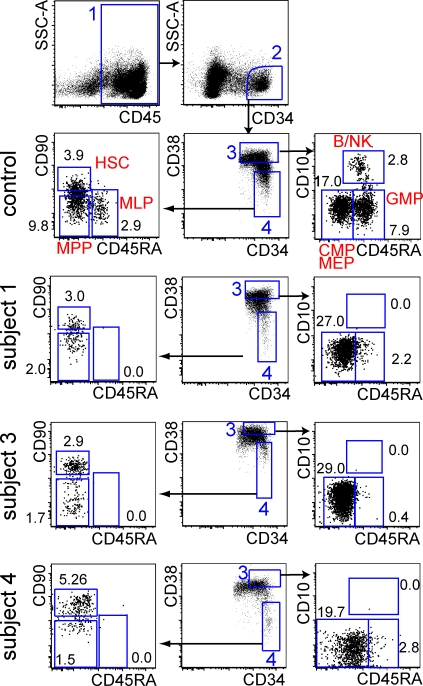
BM of subjects 1, 3, and 4 was available for flow cytometric analysis. We characterized CD34+ progenitors, including the CD38−CD90−/loCD45RA+ MLP and CD38+CD10−CD45RA+ granulocyte macrophage progenitor (GMP; Doulatov et al., 2010). In all subjects, routine histological, cytological, and cytogenetic examination of BM showed normal hematopoiesis (Table S1 and Fig. S3). Despite this, flow cytometry revealed a complete absence of MLP, with 72–95% depletion of GMP. An immediate progeny of the MLP, the more restricted CD38+ CD10+ B/NK precursor, was also completely absent (Fig. 4).
Figure 4.
Depletion of specific compartments of CD34+ BM cells. Flow cytometric analysis of CD34+ progenitor compartments in BM according to a recent protocol (Doulatov et al., 2010). Control (performed 7 times); subjects 1, 3, and 4, as indicated (performed twice). Total BM mononuclear cells are shown. The minimum number of CD34+ cells analyzed was 4,500. Numbers show the percentage of CD34+ cells in each gate. Gate 1 contains CD45+ cells; gate 2 contains CD34+ cells; gate 3 contains committed CD38+ fraction; gate 4 contains a primitive CD38− fraction. All definitions according to Doulatov et al., 2010. HSC, hematopoietic stem cell; MPP, multipotent progenitor; B/NK, committed B/NK precursor; CMP, common myeloid progenitor; MEP megakaryocyte/erythroid progenitor. In this analysis, it was not possible to separate Flt-3+ CMP from Flt-3− MEP because Flt3 was not expressed by the subjects’ CD34+ cells; thus, CMP and MEP were combined.
This analysis proves that DCML deficiency arises as a result of a defect in the BM and identifies a deficiency of both the MLP and GMP. Although sufficient GMPs are preserved to maintain granulopoiesis, these progenitors appear unable to support monocyte and DC development. These findings suggest that MLP at least contributes to the normal homeostasis of DCs and monocytes and are consistent with models of hematopoiesis in which DCs and monocytes may arise from both lymphoid (MLP) and myeloid (GMP) origins (Galy et al., 1995; Chicha et al., 2004; Doulatov et al., 2010).
DC deficiency is associated with elevated fms-like tyrosine kinase ligand
There are multiple lines of evidence that fms-like tyrosine kinase (Flt3) ligand (Flt3L) acts on both BM progenitors and peripheral DC precursors to maintain DC homeostasis and that numbers of DCs and level of Flt3L are inversely correlated (McKenna et al., 2000; Tussiwand et al., 2005; Waskow et al., 2008; Hochweller et al., 2009). Furthermore, constitutive depletion of DCs in mice via CD11c-DTA transgene is associated with approximately threefold elevation of Flt3L and a myeloproliferative syndrome (Birnberg et al., 2008).
The coregulation of DCs and T reg cells has been highlighted by several recent in vivo studies. Depletion of T reg cells increases DC proliferation by a Flt3L-dependent mechanism (Kim et al., 2007; Liu et al., 2009). Conversely, maneuvers to increase or deplete DCs by manipulation of Flt3L concentration led to parallel changes in T reg cell frequency and protective or autoimmune sequelae (Birnberg et al., 2008; Swee et al., 2009). This has led to a model of coordinated DC/T reg cell homeostasis (Darrasse-Jèze et al., 2009).
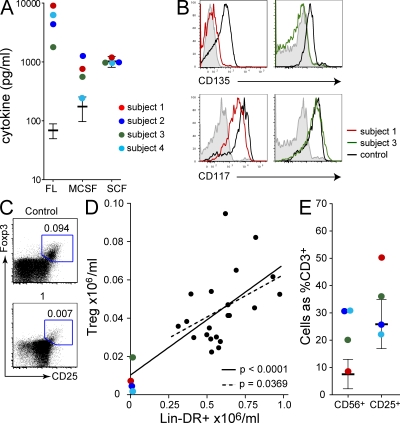
The observation of spontaneous DC deficiency gave us a unique opportunity to examine human DC/Flt3L and DC/T reg cell coregulation in vivo. The models developed in mice predict that DC deficiency should lead to elevated Flt3L in association with a loss of T reg cells. In subjects with DCML deficiency, we found massive elevation of Flt3L up to 100-fold that of controls (Fig. 5 A). M-CSF was also elevated in three of four subjects. In contrast, stem cell factor (SCF), another early acting hematopoietic growth factor, was not elevated (Fig. 5 A). We were also unable to detect surface Flt3/CD135 on any compartment of CD34+ stem cells, whereas SCF-R/CD117 expression was preserved (Fig. 5 B). Down-regulation of Flt-3 by receptor internalization in the presence of high levels of ligand has previously been directly observed in mice (Tussiwand et al., 2005).
Figure 5.
Elevation of Flt3L with T reg cell deficiency. (A) Serum Flt3L, M-CSF, and SCF concentrations measured by ELISA. Each subject was analyzed once. (B) Expression of Flt-3/CD135 and SCF-R/CD117 on BM CD34+ cells of subjects 1 and 3 compared with healthy controls and isotype (filled histogram). Each subject was analyzed twice. (C) Representative example of T reg cell analysis from subject versus normal control. Number indicates absolute count ×106 /ml. (D) Absolute number of CD4+CD25hiFoxP3+ T reg cells compared with HLA-DR+ lineage− (CD3, 19, 20, 56) cells in subjects 1–4, as indicated, and controls (n = 21). Each subject was analyzed once. (E) Proportion of CD56+ and CD25+ CD3+ T cells in subjects 1–4 as indicated relative to controls.
DC deficiency is associated with depletion of T reg cells
Working initially with 21 healthy volunteers, we were surprised to find a significant positive correlation between the absolute frequency of HLA-DR+ lineage− cells (DCs, monocytes, and CD34+ cells) and CD4+CD25hiFoxP3+ T reg cells in peripheral blood. It appears that interindividual variation is sufficient to demonstrate this relationship in humans. As predicted, severe DC deficiency was associated with depletion of T reg cells (Fig. 5, C and D). Plotting the four subjects together with normal blood profiles increased the significance of the relationship without changing the slope of the regression (Fig. 5 D). The subjects had a 53–88% reduction in T reg cell count compared with the normal median value of 0.041 × 106 per ml.
Isolating specific DCs or HLA-DR+ populations did not increase the strength of the association with T reg cells. This is not unexpected, as the model argues that homeostatic proliferation occurs in an MHC class II–dependent fashion.
The ratio of CD4+ to CD8+ T cells was not significantly perturbed, but we noted an increase in the proportion of activated CD56+ CD3+ T cells and CD25+ CD3+ T cells in the subjects (Fig. 5 E). A similar phenomenon has been observed when mice are rendered T reg cell deficient directly or via DC depletion (Kim et al., 2007; Birnberg et al., 2008; Darrasse-Jèze et al., 2009; Ohnmacht et al., 2009). There was no significant association between T reg cell and B cell frequency in healthy individuals (unpublished data). Intriguingly, approximately one quarter of patients with DCML deficiency, including subject 4, have autoimmune syndromes (Vinh et al., 2010). T reg cell depletion as a consequence of DC deficiency may contribute to this phenomenon.
Finally, we considered whether the loss of NK cells might be related to lack of IL-15–bearing DCs, as recently described in mice (Guimond et al., 2010). In contrast to this model, in which there is diminished proliferation of mature NK cells, we have shown a severe deficit of NK precursors in BM, suggesting a more proximal defect. In addition, there was no significant relationship between the frequency of NK cells and HLA-DR+lineage− peripheral blood DCs in healthy volunteers (unpublished data).
The genetic basis of DCML deficiency
The genetic basis of DCML deficiency remains unsolved. We have established that a BM defect is present with the absence of MLP and depletion of GMP. It is also known that the defect is expressed in the hematopoietic compartment because the phenotypes of subjects 1 and 3 were corrected by hematopoietic stem cell transplantation (Fig. S4).
Flt3, Flt3L, and several downstream signaling or transcription factors have been shown to be essential for DC development in mice. Flt3 signaling is critically dependent on STAT3 (Schiavoni et al., 2002), which in turn activates transcription of differentiation factors PU.1 and Flt3 itself; IRF8 and MCSF-R are also downstream targets (Laouar et al., 2003; Carotta et al., 2010).
Although high Flt3L can down-regulate Flt3 expression (Tussiwand et al., 2005) it was important to exclude mutation of this gene as the cause of DCML deficiency. However, mRNA was detectable in sorted peripheral blood CD34+ cells by Q-PCR at 40.1% of normal (Fig. S5), and mutation of the Flt3 gene in germ-line DNA was excluded by sequencing. STAT3 and PU.1 mRNA were also detectable in peripheral blood CD34+ at 76.5 and 46.9% of normal, respectively. No mutation was detected in the PU.1 gene. Interestingly, the expression of IRF8, a key regulator of DC differentiation, was substantially lower than controls (7.8%) and M-CSFR was undetectable (threshold 0.8%). These findings extend previous results obtained using granulocyte mRNA, and identify low IRF8 and M-CSFR expression as key components of the molecular signature of DCML deficiency. We hypothesize that the defect must involve upstream regulators of these genes. Structural defects of IRF8 have been excluded by sequencing (unpublished data). Mutation of M-CSFR remains a formal possibility, but is unlikely given that murine models primarily show defects of macrophage lineages and LCs with only minor DC deficiency (Ginhoux et al., 2009).
Murine KO mice with specific defects in DC development frequently show expanded progenitor compartments and increased granulopoiesis, driven by elevated Flt3L (Schiavoni et al., 2002; Laouar et al., 2003; Birnberg et al., 2008). In contrast, we observed specific cellular defects in DCML deficiency with no proliferative response to Flt3L. This implies the existence of an early block in differentiation that prevents the expansion of progenitors by Flt3L, in addition to the disruption of committed DC, monocyte, and lymphoid lineages. Future studies aim to discover gene defects acting in this manner.
This report shows that DC deficiency is an integral part of the recently described syndrome of autosomal and sporadic monocytopenia for which we propose the alternative term DCML deficiency. DC depletion is almost universal in blood and skin, with the notable exception of epidermal LCs. We have shown that this syndrome is related to complete absence of MLP and depletion of GMP in the BM. In association with these cellular defects we found massively elevated Flt3L, supporting its role in vivo as a key “DC-poietin.” M-CSF was also increased. Finally, we provided correlative in vivo data of DC and T reg cell coregulation in humans, which is consistent with a homeostatic model recently proposed in mice.
MATERIALS AND METHODS
Cell isolation and culture.
Human peripheral blood, BM, and skin were obtained with informed consent and favorable ethical opinion from Newcastle and North Tyneside and North London Research Ethics Committees. Normal skin was obtained from mammoplasty surgery and 4-mm punch or shave skin biopsies were taken from subjects. Epidermal and dermal sheets were prepared by digestion with 1 mg/ml dispase (Invitrogen) and dermal sheets were treated with 0.8 mg/ml collagenase (Type IV; Worthington) as previously described (Haniffa et al., 2009). PBMCs and BM mononuclear cells were prepared by density-gradient centrifugation according to standard protocols.
IFN-γ and IL-12 production in vitro.
Whole blood was diluted 1:5 with Rosewell Park Memorial Institute medium (RPMI) into 96-well F plates (Corning) and stimulated with IL-12 (20 ng/ml; R&D Systems; Abingdon), phytohemagglutinin (PHA; 10 µg/ml; Sigma-Aldrich), bacillus Calmette-Guerin (106/well; Statens Serum Institut, Copenhagen, Denmark), and LPS (1 µg/ml; List Biochemicals). Supernatants were taken at 12 h (for TNF and IL-12 production), 24 h (for IL-6 production), and 48 h (for IFN-γ production). Cytokines were measured using standard ELISA according to the manufacturer’s recommendations (IFN-γ, TNF, IL-6, and IL-12; R&D Systems).
Flow cytometry.
PBMCs, BM, and normal skin preparations, were stained in aliquots of 106 cells in 50 µl of buffer according to standard protocols. For subject skin biopsies, all available cells were prepared. Flow cytometry was performed on an LSRII cytometer (BD), and data was analyzed with FlowJo (Tree Star, Inc.). Viability was usually >95% by DAPI (Partec) or LIVE/DEAD dead cell stain (Invitrogen) exclusion. The following antibodies were obtained from BD unless otherwise indicated: antigen fluorochrome (clone); CD1a FITC (NA1/34; Dako); CD1a APC (HI 149); CD3 FITC PE APC (UCHT1) PerCPCy5.5 (SK7); CD4 FITC (SK3); CD8 APCCy7 (SK1); CD10 APC (HI10a); CD11c APC V450 (B-ly6); CD14 PE PECy7 (M5E2) and QDOT605 (TuK4; Invitrogen); CD16 FITC PE PECy7 (3G8); CD19 FITC PE PECy7 (SJ25C1); CD25 PE PECy7 (2A3); CD34 APC (8G12) and APCCy7 (581; BioLegend); CD38 PE-Cy7 (HB7); CD45 APCCy7 V450 (2D1); CD45RA FITC (L48) PE (HI100); CD56 FITC PE (NCAM16.2); CD90 Qdot605 (5E10; eBioscience); CD123 PE (9F5); CD135 PE (4G8); HLA-DR PerCP Cy5.5 (L243); and Langerin PE (DCGM4; Beckman Coulter). Intracellular staining with FoxP3 APC (PCH101; eBioscience) and Ki-67 PE FITC (B56) was performed with 2 × 106 cells per tube according to manufacturers’ protocols. Absolute counting was performed using 50 µl whole blood in BD Trucount Tubes according to manufacturer’s protocol.
Microscopy.
Fluorescence microscopy was performed using a TCS SP2 (Leica) confocal microscope with HCX Plan Apo ×40 NA 0.85 lens and PMTs running Leica LCS v2.61 software, or using an Axioplan 2 microscope (Carl Zeiss, Inc.) EC Plan-Neofluar ×40 NA 0.75 lens and Axiocam with running Axiovision v2.8 software (Carl Zeiss, Inc.). Separated epidermal sheets were fixed in acetone for 20 min and rehydrated in PBS for 15 min before staining. The following primary or directly labeled antibodies were used: anti-CD1a FITC (NA1/34; Dako); anti–HLA-DR FITC (L243 or G46-6; BD); and anti–Ki-67 (rabbit monoclonal SP6; Vector Laboratories) followed by Alexafluor 555–conjugated goat anti–rabbit IgG (H+L; Invitrogen). Specimens were mounted in VectaShield containing DAPI (Vector Laboratories).
ELISA.
Serum Flt3L and SCF were measured by ELISA kits according to manufacturer’s instructions (Quantikine Human Flt3/Flk-2 Ligand Immunoassay and Quantikine Human SCF Immunoassay; R&D Systems).
Statistics.
Graphs were plotted with Prism version 4 or 5 (GraphPad Software Inc). Mean and SD calculation, paired Student’s t tests, and linear regression analysis were performed within the graphing software.
Online supplemental material.
Fig. S1 shows the proliferative capacity of LCs and macrophages. Fig. S2 shows the Assessment of tissue macrophage populations in subject 3. Fig. S3 shows BM histology. Fig. S4 shows DC analysis after hematopoietic stem cell transplantation at 3 mo in subject 1 and at 40 d in subject 3. Fig. S5 shows Q-PCR analysis of peripheral blood CD34+ cells from subjects 3 and 4 compared with healthy controls. Table S1 shows a summary of clinical details. Table S2 shows automated peripheral blood count. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101459/DC1.
Acknowledgments
We thank Ken Brigham and the Newcastle Stem Cell Bank; Trevor Booth and Bioimaging at Newcastle University; Rebecca Stewart for assistance with flow cytometry; and Steven Holland for comments on the manuscript.
This study was funded by a Medical Research Council (MRC) Clinical Training Fellowship (V. Bigley), Wellcome Intermediate Fellowship (M. Haniffa), MRC Clinician Scientist Award (S. Hambleton), and LLR Bennett Senior Fellowship in Experimental Haematology (M. Collin).
The authors declare no competing financial interests.
Footnotes
Abbreviations used:
- DCML
- dendritic cell, monocyte, B and NK lymphoid deficiency
- Flt3
- fms-like tyrosine kinase
- Flt3L
- Flt3 ligand
- LC
- Langerhans cell
- MLP
- multilymphoid progenitor
- MSMD
- Mendelian susceptibility to mycobacterial disease
- Q-PCR
- quantitative
- SCF
- stem cell factor
References
- Angel C.E., George E., Brooks A.E., Ostrovsky L.L., Brown T.L., Dunbar P.R. 2006. Cutting edge: CD1a+ antigen-presenting cells in human dermis respond rapidly to CCR7 ligands. J. Immunol. 176:5730–5734 [DOI] [PubMed] [Google Scholar]
- Birnberg T., Bar-On L., Sapoznikov A., Caton M.L., Cervantes-Barragán L., Makia D., Krauthgamer R., Brenner O., Ludewig B., Brockschnieder D., et al. 2008. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 29:986–997 10.1016/j.immuni.2008.10.012 [DOI] [PubMed] [Google Scholar]
- Carotta S., Dakic A., D’Amico A., Pang S.H., Greig K.T., Nutt S.L., Wu L. 2010. The transcription factor PU.1 controls dendritic cell development and Flt3 cytokine receptor expression in a dose-dependent manner. Immunity. 32:628–641 10.1016/j.immuni.2010.05.005 [DOI] [PubMed] [Google Scholar]
- Casanova J.L., Abel L. 2007. Primary immunodeficiencies: a field in its infancy. Science. 317:617–619 10.1126/science.1142963 [DOI] [PubMed] [Google Scholar]
- Chicha L., Jarrossay D., Manz M.G. 2004. Clonal type I interferon–producing and dendritic cell precursors are contained in both human lymphoid and myeloid progenitor populations. J. Exp. Med. 200:1519–1524 10.1084/jem.20040809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin M.P., Hart D.N., Jackson G.H., Cook G., Cavet J., Mackinnon S., Middleton P.G., Dickinson A.M. 2006. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J. Exp. Med. 203:27–33 10.1084/jem.20051787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrasse-Jèze G., Deroubaix S., Mouquet H., Victora G.D., Eisenreich T., Yao K.H., Masilamani R.F., Dustin M.L., Rudensky A., Liu K., Nussenzweig M.C. 2009. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J. Exp. Med. 206:1853–1862 10.1084/jem.20090746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doulatov S., Notta F., Eppert K., Nguyen L.T., Ohashi P.S., Dick J.E. 2010. Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development. Nat. Immunol. 11:585–593 10.1038/ni.1889 [DOI] [PubMed] [Google Scholar]
- Galy A., Travis M., Cen D., Chen B. 1995. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 3:459–473 10.1016/1074-7613(95)90175-2 [DOI] [PubMed] [Google Scholar]
- Geissmann F., Manz M.G., Jung S., Sieweke M.H., Merad M., Ley K. 2010. Development of monocytes, macrophages, and dendritic cells. Science. 327:656–661 10.1126/science.1178331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Tacke F., Angeli V., Bogunovic M., Loubeau M., Dai X.M., Stanley E.R., Randolph G.J., Merad M. 2006. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7:265–273 10.1038/ni1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Liu K., Helft J., Bogunovic M., Greter M., Hashimoto D., Price J., Yin N., Bromberg J., Lira S.A., et al. 2009. The origin and development of nonlymphoid tissue CD103+ DCs. J. Exp. Med. 206:3115–3130 10.1084/jem.20091756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimond M., Freud A.G., Mao H.C., Yu J., Blaser B.W., Leong J.W., Vandeusen J.B., Dorrance A., Zhang J., Mackall C.L., Caligiuri M.A. 2010. In vivo role of Flt3 ligand and dendritic cells in NK cell homeostasis. J. Immunol. 184:2769–2775 10.4049/jimmunol.0900685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haniffa M., Ginhoux F., Wang X.N., Bigley V., Abel M., Dimmick I., Bullock S., Grisotto M., Booth T., Taub P., et al. 2009. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J. Exp. Med. 206:371–385 10.1084/jem.20081633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochweller K., Miloud T., Striegler J., Naik S., Hämmerling G.J., Garbi N. 2009. Homeostasis of dendritic cells in lymphoid organs is controlled by regulation of their precursors via a feedback loop. Blood. 114:4411–4421 10.1182/blood-2008-11-188045 [DOI] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Laouar Y., Welte T., Fu X.Y., Flavell R.A. 2003. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 19:903–912 10.1016/S1074-7613(03)00332-7 [DOI] [PubMed] [Google Scholar]
- Larregina A.T., Morelli A.E., Spencer L.A., Logar A.J., Watkins S.C., Thomson A.W., Falo L.D.J., Jr 2001. Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat. Immunol. 2:1151–1158 10.1038/ni731 [DOI] [PubMed] [Google Scholar]
- Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. 2009. In vivo analysis of dendritic cell development and homeostasis. Science. 324:392–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg S., Schaerli P., Knezevic-Maramica I., Köllnberger M., Tubo N., Moseman E.A., Huff I.V., Junt T., Wagers A.J., Mazo I.B., von Andrian U.H. 2007. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 131:994–1008 10.1016/j.cell.2007.09.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna H.J., Stocking K.L., Miller R.E., Brasel K., De Smedt T., Maraskovsky E., Maliszewski C.R., Lynch D.H., Smith J., Pulendran B., et al. 2000. Mice lacking flt3 ligand have deficient hematopoiesis affecting hematopoietic progenitor cells, dendritic cells, and natural killer cells. Blood. 95:3489–3497 [PubMed] [Google Scholar]
- Merad M., Manz M.G. 2009. Dendritic cell homeostasis. Blood. 113:3418–3427 10.1182/blood-2008-12-180646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J., Summer R., Wilson A.A., Kotton D.N., Fine A. 2008. The prolonged life-span of alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 38:380–385 10.1165/rcmb.2007-0224RC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnmacht C., Pullner A., King S.B., Drexler I., Meier S., Brocker T., Voehringer D. 2009. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 206:549–559 10.1084/jem.20082394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G., Mattei F., Sestili P., Borghi P., Venditti M., Morse H.C., III, Belardelli F., Gabriele L. 2002. ICSBP is essential for the development of mouse type I interferon–producing cells and for the generation and activation of CD8α+ dendritic cells. J. Exp. Med. 196:1415–1425 10.1084/jem.20021263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R.M., Banchereau J. 2007. Taking dendritic cells into medicine. Nature. 449:419–426 10.1038/nature06175 [DOI] [PubMed] [Google Scholar]
- Swee L.K., Bosco N., Malissen B., Ceredig R., Rolink A. 2009. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 113:6277–6287 10.1182/blood-2008-06-161026 [DOI] [PubMed] [Google Scholar]
- Tussiwand R., Onai N., Mazzucchelli L., Manz M.G. 2005. Inhibition of natural type I IFN-producing and dendritic cell development by a small molecule receptor tyrosine kinase inhibitor with Flt3 affinity. J. Immunol. 175:3674–3680 [DOI] [PubMed] [Google Scholar]
- Vinh D.C., Patel S.Y., Uzel G., Anderson V.L., Freeman A.F., Olivier K.N., Spalding C., Hughes S., Pittaluga S., Raffeld M., et al. 2010. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 115:1519–1529 10.1182/blood-2009-03-208629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskow C., Liu K., Darrasse-Jèze G., Guermonprez P., Ginhoux F., Merad M., Shengelia T., Yao K., Nussenzweig M. 2008. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat. Immunol. 9:676–683 10.1038/ni.1615 [DOI] [PMC free article] [PubMed] [Google Scholar]