Abstract
The extracellular Ca2+-sensing receptor (CaR) is increasingly implicated in the regulation of multiple cellular functions in the gastrointestinal tract, including secretion, proliferation and differentiation of intestinal epithelial cells. However, the signaling mechanisms involved remain poorly defined. Here we examined signaling pathways activated by the CaR, including Ca2+ oscillations, in individual human colon epithelial cells. Single cell imaging of colon-derived cells expressing the CaR, including SW-480, HT-29 and NCM-460 cells, shows that stimulation of this receptor by addition of aromatic amino acids or by an elevation of the extracellular Ca2+ concentration promoted striking intracellular Ca2+ oscillations. The intracellular calcium oscillations in response to extracellular Ca2+ were of sinusoidal pattern and mediated by the phospholipase C/diacylglycerol/inositol 1,4,5-trisphosphate pathway as revealed by a biosensor that detects the accumulation of diacylglycerol in the plasma membrane. The intracellular calcium oscillations in response to aromatic amino acids were of transient type, i. e. Ca2+ spikes that returned to baseline levels, and required an intact actin cytoskeleton, a functional Rho, Filamin A and the ion channel TRPC1. Further analysis showed that re-expression and stimulation of the CaR in human epithelial cells derived from normal colon and from colorectal adenocarcinoma inhibits their proliferation. This inhibition was associated with the activation of the signaling pathway that mediates the generation of sinusoidal, but not transient, intracellular Ca2+ oscillations. Thus, these results indicate that the CaR can function in two signaling modes in human colonic epithelial cells offering a potential link between gastrointestinal responses and food/nutrients uptake and metabolism.
Keywords: extracellular calcium sensing receptor, human colon epithelial cells, colorectal cancer, GPCR, intracellular calcium oscillations
INTRODUCTION
The progression of colorectal cancer (CRC) from normal colonic epithelium to the malignant phenotype is associated with diverse genetic and epigenetic alterations, including mutations of the adenomatous polyposis coli (APC) or the β-catenin genes (Kinzler and Vogelstein, 1996). Extensive experimental and epidemiological evidence also suggest that individual differences in dietary intake contribute to more variation in cancer incidence than any other factor (Doll and Peto, 1981). For example, several animal experimental studies support the notion that dietary Ca2+ prevents the development of intestinal cancer (Lipkin and Newmark, 1995b; Newmark et al., 2009; Newmark et al., 2001; Yang et al., 2008). In line with animal experiments, many observational studies in humans suggested that Ca2+-rich diets are associated with reduced risk of CRC and reduced risk of colorectal adenomas (Baron et al., 1999a; Baron et al., 1999b; Duris et al., 1996; Garland et al., 1985; Huncharek et al., 2009; Lipkin and Newmark, 1995a; McCullough et al., 2003; Park et al., 2009; Pence, 1993; Pietinen et al., 1999; Slattery et al., 1988; Terry et al., 2002; Wu et al., 2002; Zheng et al., 1998). Although the precise mechanisms mediating the chemopreventive properties of Ca2+ remain incompletely understood, its mode of action in colon epithelial cells is likely associated with the activation of signal transduction pathways that regulate their proliferation and differentiation (Ahnen and Byers, 1998; Holt, 1999; Holt et al., 1998; Lamprecht and Lipkin, 2003).
The extracellular Ca2+-sensing receptor (CaR), a member of the C family of heptahelical G protein-coupled receptors (GPCR), was originally cloned from parathyroid chief cells (Brown and MacLeod, 2001). Inactivating and activating mutations of the CaR in humans (Hendy et al., 2000) and genetic disruption of the CaR gene in mice (Ho et al., 1995) established that the CaR functions in the control of Ca2+ homeostasis. In addition to its role as a sensor of extracellular Ca2+ ([Ca2+]o), the activity of the CaR expressed in HEK-293 cells is also regulated by amino acids that, like [Ca2+]o, stimulate increases in intracellular Ca2+ ([Ca2+]i) (Conigrave et al., 2000; Young and Rozengurt, 2002). These findings indicated that the CaR is an allosteric protein that recognizes and responds to two different agonists, namely Ca2+ and aromatic amino acids. Although a major physiological role of the CaR is to correct small changes in [Ca2+]o concentration by regulating parathyroid hormone secretion, subsequent studies demonstrated that the CaR is also expressed in other tissues and organs not directly involved in Ca2+ homeostasis (Quarles, 2003) including the entire gastrointestinal (GI) tract (Conigrave and Brown, 2006; Hebert et al., 2004). However, little is known about the regulation of this receptor and the signaling pathways it activates in colon epithelial cells. Interestingly, CaR expression in differentiated colonic carcinomas is greatly reduced or completely lost (Kallay et al., 2000; Sheinin et al., 2000), suggesting that the suppression of its expression may be associated with abnormal differentiation and malignant progression.
Oscillatory changes in [Ca2+]i in response to receptor stimulation is a fundamental mechanism of cell signaling in both non-excitable and excitable cells that can protect cells from the cytotoxic effects of prolonged increases in [Ca2+]i. There are two predominant patterns in [Ca2+]i oscillations termed transient and sinusoidal. Transient oscillations are represented by repetitive, low frequency, [Ca2+]i spikes that return to the base-line level whereas sinusoidal ones correspond to high frequency [Ca2+]i oscillations upon a raised plateau level. We previously demonstrated that the stimulation of the CaR by aromatic amino acids and [Ca2+]o in human fibroblasts promoted, respectively, transient and sinusoidal [Ca2+]i oscillations and that each type of oscillation is associated with the activation of a different signaling pathway (Rey et al., 2006b; Rey et al., 2005; Young and Rozengurt, 2002; Young et al., 2002). As the frequency of [Ca2+]i oscillations plays a key role in signal transduction, an allosteric interaction of [Ca2+]o and amino acids on the production of [Ca2+]i oscillations by the CaR could provide a novel mechanism that could distinguish and integrate the effect of these luminal and basolateral agonists in the GI tract. However, it is not known whether [Ca2+]o and amino acids induce different patterns of [Ca2+]i oscillations via the CaR in human colon-derived epithelial cells.
The results presented here demonstrate, for the first time, that the CaR can initiate transient and sinusoidal [Ca2+]i oscillations in human epithelial cells derived from normal colon and from colorectal adenocarcinoma and that these oscillations are mediated by distinct signal transduction pathways. Furthermore, our results indicate that re-expression and [Ca2+]o-induced stimulation of the CaR inhibits cell proliferation, a response associated with sinusoidal, but not transient, [Ca2+]i oscillations.
MATERIALS AND METHODS
Cell culture, transfection and Western blot
The human colorectal adenocarcinoma cell lines SW-480 and HT-29 were obtained from ATCC. NCM-460 cells, an immortalized cell line derived from normal human colon mucosal epithelium (Moyer et al., 1996), were obtained from Incell Corp. All the cell lines were maintained as recommended by the providers. SW-480, HT-29 and NCM-460 cells were transiently transfected as previously described (Rey et al., 2005) with the human CaR expression vector pCR3.1-CaR (0.5 μg/21 cm2 dish) (Ray et al., 1997). A SW-480 cell line constitutively expressing the CaR was established by transfection with pCR3.1-CaR and selection with 800 μg/ml of Geneticin™ (Invitrogen Corp.). The vectors encoding a fusion protein between protein kinase D (PKD) and red fluorescent protein (pPKD-RFP), Clostridium botulinum C3 exoenzyme and the 14 and 15 domains of human filamin-A were previously described (Rey et al., 2001; Rey et al., 2005; Yuan et al., 2001). Western blot analysis was performed as described (Rey et al., 2006a) and images captured using a luminescent image analyzer LAS-4000 mini (Fujifilm Life Sciences).
mRNA amplification
To detect the expression of sequences encoding CaR (accession U20759) and actin (accession V01217J00691) we employed reverse transcriptase-PCR (RT-PCR) with specific oligonucleotides primers designed using MacVector v11.02 (MacVector, Inc.). The cDNA sequences used to design the primers were for CaR forward primer (843-864) 5′-GAGCCCCTCACAAGGAGATTG-3′, CaR reverse primer (1448-1470) 5′-CCAGGTCACCACACTCATCAAAG-3′; Actin forward primer (134-153) 5′-TGGGTATGGGTCAGAAGGAC-3′, Actin reverse primer (618-636) 5′-AATGTCACGCACGATTTCC-3′. RT-PCR was performed on 5 μg of total RNA extracted from semi-confluent SW-480, HT-29 and NCM-460 cells using TRIzol reagent (Invitrogen Corp.). First-strand cDNA was synthesized at 45°C by using the designed CaR and actin antisense oligonucleotides described above and ThermoScript Reverse Transcriptase II (Invitrogen Corp.) under the conditions suggested by the manufacturer. A fraction of the obtained cDNAs were amplified by PCR using Platinum Taq DNA Polymerase High Fidelity (Invitrogen Corp.), as suggested by the manufacturer, and the sense and antisense primers specific for CaR and actin indicated above. As control, in a set of reactions the sense primers for CaR and actin were not added to the obtained cDNAs during the PCR amplification step. The products of PCR were resolved in 1.4% Agarose (Invitrogen Corp.)-1XTBE buffer (100 mM Tris, 90 mM boric acid, 1 mM EDTA, pH 8.4). The gel was stained for 60 min with ethidium bromide (0.5 μg/ml) in 1XTBE, followed by two 15 min washes with distilled water. The gel was viewed and images captured using a luminescent image analyzer LAS-4000 mini (Fujifilm Life Sciences). The predicted sizes of the RT-PCR products for CaR and actin are 627 and 502 bp, respectively.
Cell imaging
[Ca2+]i was measured in single cells loaded with the calcium indicator fura-2 as previously described (Young and Rozengurt, 2002). Briefly, cells were incubated in saline solution containing 138 mM NaCl, 4 mM NaHCO3, 0.3 mM Na2HPO4, 5 mM KCl, 0.3 mM KH2PO4, 1.5 mM CaCl2, 0.5 mM MgCl2, 0.4 mM MgSO4, 5.6 mM D-glucose, 20 mM HEPES, pH: 7.4 which was supplemented with 5 μM fura-2 AM for 45-60 min at 37°C before [Ca2+]i imaging. The cells were then washed and placed in an experimental chamber that was perfused with saline solution at 1.5 ml/min at 37°C. The chamber in turn was placed on the stage of an inverted microscope connected to a digital imaging system. Ratios of images (340 nm excitation/ 380 nm excitation, emission filter 520 nm) were obtained at 1.5 sec intervals. A region of interest covering 15 μm X 15 μm was defined over each cell, and the average ratio intensity over the region was converted to [Ca2+]i using an standard curve constructed with a series of calibrated buffered calcium solutions (Calcium Calibration Buffer Kit #2, Invitrogen Corp.). Identification of cells transiently transfected with pCR3.1-CaR (0.5 μg/21 cm2 dish), the plasmid encoding Clostridium botulinum C3 exoenzyme (0.5 μg/21 cm2 dish) or pFilA14/15 (1.0 μg/21 cm2 dish) was achieved by co-transfection with pDsRed-Express (BD Biosciences) (0.1 μg/21 cm2 dish), a vector that encodes a red fluorescent protein. Single live-cell imaging of the fluorescent biosensor for diacylglycerol (RFP-PKD) was performed as previously described (Rey et al., 2005) analyzing 50 cells/experiment, with each experiment done at least in duplicate. The selected cells displayed in the figures were representative of 90% of the population of RFP-PKD positive cells.
Production of retroviruses and establishment of a CaR-inducible expression NCM-460 cell line
The complete CaR cDNA coding sequence, spanning nucleotides −14 to +3277, was excised from pCR3.1-CaR (Ray et al., 1997) using HindIII/AflIII and the ends filled with Klenow polymerase. This cDNA fragment was subcloned into the Klenow-filled SpeI/XbaI sites of the entry vector pEN_TmiRc3 (Shin et al., 2006) immediately downstream of the tetracycline (Tet) responsive element effectively eliminating the microRNA related sequences in the new entry pEN-CaR vector. The pEN-CaR vector was used in a recombination cloning reaction with pSLIK-Venus (Shin et al., 2006) to achieve Tet-ON regulated expression of CaR in a lentiviral vector. The resulting lentiviral expression vector pSLIK-CaR was transfected along with third-generation lentivirus packaging vectors into HEK-293T cells using Lipofectamine 2000 (Invitrogen Corp.). Plasmids were cotransfected by using 10 μg of pSLIK-CaR plasmid, 7.5 μg of each of the two packaging plasmids pMDLg/pRRE and pRSVREV, and 5 μg of the vesicular stomatitis virus (VSV) G envelope plasmid. The transfection medium was replaced after 12 h with fresh DMEM medium with 10% FBS and the viral supernatant collected 48 h after transfection and concentrated by using a Centricon Plus-70 filter unit (Millipore). Logarithmically growing NCM-460 cells were infected at a low MOI to ensure <30% infection frequency such that the majority of transduced cells contained single viral integrants. Cells were collected 48–72 h later, and Venus (YFP)-positive cells were FACS-sorted using a Becton Dickinson FACStar PLUS machine. Venus-positive cells were propagated, and multiple aliquots frozen.
Materials
Antibodies were obtained from: GE Healthcare, horseradish peroxidase-conjugated donkey anti-rabbit or anti-mouse IgG; Cell Signaling Technology, anti-phospho Ser-916 that recognizes the phosphorylation of Ser-916 in PKD and Ser-876 in PKD2; Alomone Labs, Israel, anti-TRPC1; BD Biosciences, anti-RFP; Affinity Bioreagents, anti-CaR; Abcam, anti-αtubulin. Fura-2 AM was purchased from Invitrogen Corp. All the other reagents were the highest grade commercially available.
RESULTS
Generation of [Ca2+]i oscillations by the CaR in colon-derived epithelial cells
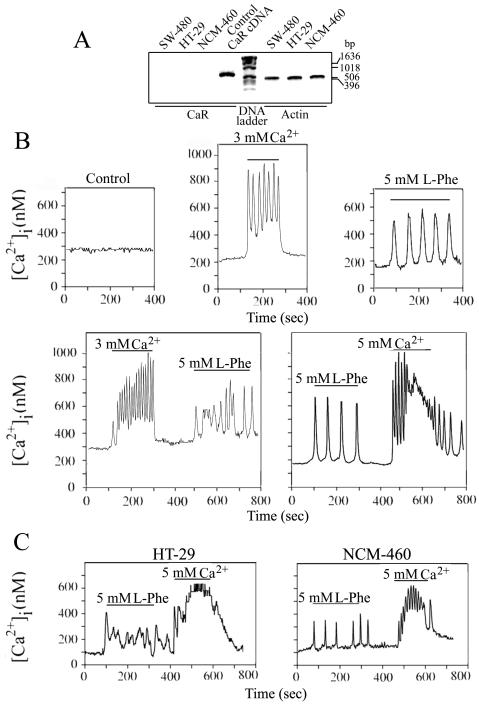
In order to determine whether [Ca2+]i oscillations were elicited in response to CaR stimulation in human colon-derived epithelial cells, SW-480 cells, a cell line derived from human colorectal adenocarcinoma in which the expression of endogenous CaR is non-detectable by RT-PCR (Fig. 1A), were transiently co-transfected with a plasmid encoding the human CaR (pCR3.1-CaR) and a plasmid encoding a red fluorescent protein (pDsRed-Express) to facilitate the identification of the transfected cells. After 16 h, the cultures were loaded with the Ca2+ indicator fura-2 before the addition of either Ca2+ or L-Phe, potent activators of the CaR (Conigrave and Brown, 2006; Conigrave et al., 2000; Young and Rozengurt, 2002; Young et al., 2002). Single cell imaging of fura-2 loaded SW-480 cells expressing the CaR revealed that a rise in [Ca2+]o from 1.5 mM to 3.0 stimulate, in the transfected cells, an increase in [Ca2+]i in 84% of the examined cells (n=90). Further analysis of SW-480 cells expressing the CaR indicate that a rise in [Ca2+]o from 1.5 mM to 3.0 promoted sinusoidal [Ca2+]i oscillations (Fig. 1B) with a mean frequency of 3.5 ± 0.8 min-1 (n=36). Sinusoidal [Ca2+]i oscillations were also elicited by 5 mM [Ca2+]o and halted when the [Ca2+]o was returned to 1.5 mM (Fig. 1B). Addition of 3 mM or 5 mM [Ca2+]o to non-transfected SW-480 cells did not induce any detectable change in [Ca2+]i in the examined cells (n=50) (data not shown).
Fig. 1. Expression of CaR in SW-480, HT-29 and NCM-460 cells.
(A) RT-PCR was performed using specific primers for mRNAs encoding the human CaR and actin (see Materials and Methods) on 5 μg of total RNA isolated from semi-confluent cultures of each cell line. PCR products were resolved on 1.4% agarose and the gel was then stained with ethidium bromide. Products of the predicted size for the CaR (627 bp) and actin (502 bp) were detected only when both antisense and sense primers were included during the PCR reaction. Human CaR cDNA (50 ng) was used as control for the CaR RT-PCR; bp: base pairs. The results are representative of two independent experiments. CaR-mediated [Ca2+]i oscillation in human colon-derived epithelial cells. (B, C) SW-480, HT-29 and NCM-460 cells transiently co-transfected with a plasmid encoding the human CaR (pCR3.1-CaR) and a plasmid encoding a red fluorescent protein (pDsRed-Express) to facilitate the identification of the transfected cells were loaded after 16 h with the Ca2+ indicator fura-2 and perfused with a solution containing the indicated Ca2+ or L-Phe concentrations during the times denoted by the horizontal bars. Control: SW-480 cells transiently co-transfected with pCR3.1-CaR and pDsRed-Express loaded after 16 h with the Ca2+ indicator fura-2 and perfused with a solution containing 1.5 mM Ca2+.
We next examined whether L-Phe-mediated stimulation of the CaR in SW-480 cells elicited a different type of [Ca2+]i oscillations than the ones initiated by [Ca2+]o. Single-cell intracellular Ca2+ imaging revealed that 5 mM L-Phe promoted an increase in [Ca2+]i in 28% of the cells (n=98). In agreement with previous reports (Conigrave et al., 2000), L-Trp, but not L-Leu, also promoted an [Ca2+]i increment in 26% of the cells (n=40). Although the precise mechanism mediating the difference in responsiveness to [Ca2+]o and L-Phe or L-Trp needs further investigation, it is tempting to speculate that the response to these aromatic amino acids may involve the formation on the cell surface of less abundant CaR oligomers (Bai et al., 1998), e. g. tetramers, whereas [Ca2+]o-mediated responses could be elicited by both dimers and tetramers. Further analysis of SW-480 cells expressing the CaR indicate that L-Phe promoted transient [Ca2+]i oscillations with a mean frequency of 1.5 ± 0.2 min-1 (n=36) (Fig. 1B). The transient [Ca2+]i oscillations elicited by L-Phe were dependent on the continued presence of L-Phe and a threshold [Ca2+] above 1.0 mM (data not shown). Addition of 5 mM L-Phe or 5 mM L-Trp to non-transfected SW-480 cells did not induce any detectable change in [Ca2+]i in the analyzed cells (n=50) (data not shown). Our results also indicate that the same cell could be sequentially stimulated to generate transient and sinusoidal [Ca2+]i oscillations (Fig. 1B), indicating that neither type of [Ca2+]i oscillations interfere with the subsequent cellular response to the alternative CaR agonist (Fig. 1B)
To extend these observations, we examined whether stimulation of the CaR in HT-29 and NCM-460 cells, two human colon-derived epithelial cell lines with non-detectable endogenous CaR (Fig. 1A), also generate transient and sinusoidal [Ca2+]i oscillations. HT-29 and NCM-460 cells were transiently co-transfected with pCR3.1-CaR and pDsRed-Express and loaded with fura-2 before stimulation. As shown in Fig. 1C, intracellular Ca2+ imaging revealed that stimulation of CaR-expressing HT-29 and NCM-460 cells with 5 mM L-Phe or 5 mM [Ca2+]o also promoted transient and sinusoidal [Ca2+]i oscillations, respectively. In agreement with the results obtained with SW-480 cells, [Ca2+]o-mediated stimulation of transiently expressed CaR elicited sinusoidal oscillations in 75% of HT-29 (n=20) and in 90% of NCM-460 cells (n=30) whereas L-Phe promoted an increment in [Ca2+]i in 25% (n=20) and 35% (n=30) of HT-29 and NCM-460 cells, respectively. Addition of 5 mM L-Phe or 5 mM [Ca2+]o to non-transfected HT-29 or NCM-460 cells did not induce any detectable change in [Ca2+]i in the examined cells (data not shown). These studies provide, for the first time, a strong indication that the CaR can function in two signaling modes in human colon-derived epithelial cells. These results also indicate that the signaling pathways that regulate the generation of transient and sinusoidal [Ca2+]i oscillations in response to the stimulation of the CaR are functional in human cells derived from normal colon mucosal epithelium and colorectal adenocarcinomas.
PKC inhibition interferes with CaR-mediated [Ca2+]i oscillations
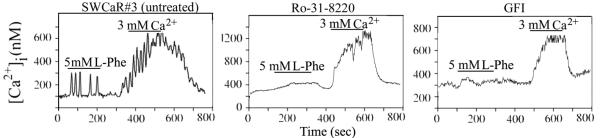
In order to avoid the limitations associated with transient transfection, i. e. variable number of transfected cells and fluctuating amounts of expressed protein, we established a cell line constitutively expressing the CaR. SW-480 cells were transfected with a plasmid encoding the human CaR under the control of a cytomegalovirus promoter and the cells were selected in the presence of the antibiotic Geneticin™ (Invitrogen Corp.). Clones were isolated and further propagated in order to examine the activity of the CaR. CaR stimulation by L-Phe or [Ca2+]o generated transient and sinusoidal [Ca2+]i oscillations in several clones. We used in the subsequent studies presented here the clone SWCaR#3 (Fig. 2) because it expresses a relatively low amount of CaR (data not shown) compared to a cell model of HEK-293 cells stably transfected with the same CaR encoding plasmid (Rey et al., 2006b; Rey et al., 2005; Young et al., 2002).
Fig. 2. Effect of PKC inhibitors in CaR-mediated [Ca2+]i oscillations.
SW-480 cells constitutively expressing the CaR (SWCaR#3) were incubated in the presence of the selective PKC inhibitors Ro 31-8220 (1.25 μM) or GFI (3.5 μM) simultaneously with the Ca2+ indicator fura-2 for 1 h before being perfused with a solution containing 5 mM L-Phe or 3 mM Ca2+ during the times denoted by the horizontal bars.
We next examined some fundamental properties of CaR-mediated [Ca2+]i oscillations in colonic epithelial cells. Previous studies demonstrated that inhibition of PKC activity blocked the transient and sinusoidal [Ca2+]i oscillations in HEK-293 cells expressing the CaR (Young and Rozengurt, 2002; Young et al., 2002). Accordingly, we examined whether exposure to selective PKC inhibitors eliminates L-Phe and [Ca2+]o-elicited oscillatory changes in [Ca2+]i. Pretreatment of SWCaR#3 cells with the PKC inhibitor Ro-31-8220 inhibited the [Ca2+]i oscillations in response to 5 mM L-Phe (n=30) (Fig. 2). Pretreatment of SWCaR#3 cells with Ro-31-8220 also blocked the generation of sinusoidal [Ca2+]i oscillations in response to 3 mM [Ca2+]o (n=30). However, and in contrast to the results obtained with L-Phe, up to 50% of the cells exhibited a non-oscillatory increase in [Ca2+]i from an average baseline of 297 nM in Ro-31-8220 treated-cells compared to a baseline of 119 nM in untreated cells (n=30). The amplitude of the oscillations at the peak of the wave elicited by 3 mM [Ca2+]o was dramatically reduced from 95.2 ± 14.0 nM in control SWCaR#3 cells (n=30) to 3.5 ± 2.0 nM in Ro-31-8220-treated cells (n=30). Similar results were obtained when 5 mM [Ca2+]o was used to stimulate the Ro-31-8220-treated cells (data not shown). Pretreatment of SWCaR#3 cells with GFI, another selective PKC inhibitor, also suppressed the generation of transient and sinusoidal [Ca2+]i oscillations but it did not block the nonoscillatory increase in [Ca2+]i in response to [Ca2+]o stimulation. A small number of GFI-treated cells (3.8%, n=50) still responded to L-Phe with a non-oscillatory increase in [Ca2+]i (Fig. 2) very likely due to the different IC50 of Ro-31-8220 and GFI for the PKC isoforms (Martiny-Baron et al., 1993; Toullec et al., 1991; Wilkinson et al., 1993). Thus, these results suggest that PKC negatively modulates the coupling of the CaR to intracellular signaling systems in colon-derived epithelial cells.
CaR stimulation by [Ca2+]o, but not by L-Phe, triggers DAG production
We previously employed a set of sensors developed in our laboratory to monitor in real-time the synthesis of different second messengers in response to GPCRs stimulation (Kisfalvi et al., 2007; Rey et al., 2005). For example, to detect diacylglycerol (DAG) synthesis we monitored the intracellular distribution of a fusion protein consisting of protein kinase D (PKD) and a red fluorescent protein (PKD-RFP) (Kisfalvi et al., 2007; Rey et al., 2005). PKD is a Ca2+-insensitive serine/threonine kinase that rapidly translocates from the cytosol to the plasma membrane in response to phospholipase C β (PLCβ)-mediated DAG production following GPCR stimulation (Rey et al., 2004; Rey et al., 2001). Accordingly, we decided to employ the DAG sensor to further dissect the signaling pathways activated by the CaR.
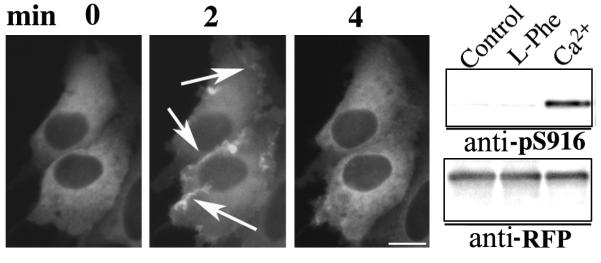
SWCaR#3 cells transfected with the DAG sensor encoding PKD-RFP were stimulated by increasing the [Ca2+]o from 1.5 to 5 mM and the intracellular distribution of the sensor monitored in live cells as previously described (Rey et al., 2001). In non-stimulated cells, the DAG sensor was distributed throughout the cytoplasm but excluded from the nucleus (Fig. 3). [Ca2+]o-mediated CaR stimulation induced a rapid translocation of the DAG sensor from the cytosol to the plasma membrane that peaked after 2 min (Fig. 3, arrows). The plasma membrane localization of the sensor was transient, returning within 4-6 min to the cellular compartment, i. e. cytoplasm, it occupied before stimulation (Fig. 3). In contrast to the results obtained after [Ca2+]o-mediated CaR stimulation, 5 or 10 mM L-Phe did not induce any detectable redistribution of the DAG sensor in SWCaR#3 cells (data not shown).
Fig. 3. CaR stimulation promotes DAG synthesis in human colon-derived epithelial cells.
SWCaR#3 cells transiently transfected with a plasmid encoding a sensor for DAG (PKD-RFP) were perfused with a saline solution at 37°C containing 5 mM Ca2+ during the indicated times and the intracellular distribution of the sensor monitored using a fluorescence microscope. The selected cells displayed in the figure were representative of 90% of the population of cells expressing PKD-RFP. Alternatively, SWCaR#3 cells transiently transfected with pPKD-RFP were incubated with a saline solution containing 5 mM Ca2+ or 5 mM L-Phe for 10 min, lysed and analyzed by Western blot using an antibody that detects the phosphorylation of Ser-916 in PKD (anti-pS916) and antibody against RFP (anti-RFP) for expression and loading control. Bar: 10 μm.
The plasma membrane translocation of PKD, in response to GPCR-induced DAG synthesis, is associated with its activation as revealed by the phosphorylation of Ser-916, a major autophosphorylation site on PKD (Matthews et al., 1999; Rey et al., 2004). In view of the results presented above, we hypothesized that [Ca2+]o, but not L-Phe, stimulation of the CaR should promote the phosphorylation of Ser-916 in the PKD moiety of the DAG biosensor. Accordingly, SWCaR#3 cells transiently transfected with pPKD-RFP were incubated in a saline solution containing 5 mM Ca2+ or 5 mM L-Phe for 10 min before being analyzed by Western blot with an antibody that detects the phosphorylation of Ser-916 in PKD. In agreement with the real-time imaging results, we found that [Ca2+]o, but not L-Phe, induced a marked increase in Ser-916 phosphorylation denoting PKD catalytic activation (Fig. 3). Thus, these results indicate that although both CaR agonists initiate [Ca2+]i oscillations, only [Ca2+]o stimulates DAG synthesis in colon epithelial cells suggesting that the transient [Ca2+]i oscillations elicited by L-Phe are mediated by a PLCβ-independent signaling pathway.
Disruption the actin cytoskeleton interferes with CaR-mediated [Ca2+]i oscillations
Our results suggest that in colon-derived epithelial cells, CaR-mediated [Ca2+]i oscillations in response to aromatic amino acids could be elicited by a different signaling pathway than the one generating sinusoidal [Ca2+]i oscillations. We hypothesized that if aromatic amino acids and extracellular calcium were activating two different signaling pathways, distinct signaling transducers should also be associated with each pathway. A number of studies have shown that the organization of the actin cytoskeleton plays a role in the generation of [Ca2+]i oscillations in at least some cell types (Patterson et al., 1999; Rey et al., 2005; Ribeiro et al., 1997). Accordingly, we treated SWCaR#3 cells with cytochalasin D (CytD), which induces F-actin depolymerization through the disruption of actively turning over actin stress fibers, to determine whether disruption of the actin cytoskeleton affected the [Ca2+]i oscillations mediated by the CaR.
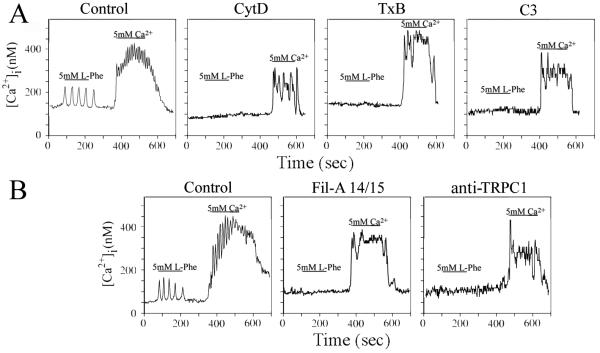
Pretreatment of SWCaR#3 cells with CytD completely abolished the production of L-Phe mediated transient [Ca2+]i oscillations (n=40) (Fig. 4A). However, the same treatment did not affect the percentage of SWCaR#3 cells (n=50), that exhibited [Ca2+]i oscillations after [Ca2+]o-mediated CaR stimulation (Fig. 4A) when compared to untreated SWCaR3# cells, Disruption of microtubules with nocodazole, an agent that prevents the formation of microtubules by binding to tubulin monomers, did not interfere with the production of either transient or sinusoidal [Ca2+]i oscillations (data not shown). These results indicate that, in colon-derived epithelial cells, the organization of the actin cytoskeleton is necessary for CaR-mediated generation of transient, but not sinusoidal, [Ca2+]i oscillations.
Fig. 4. CaR-mediated [Ca2+]i oscillations in human colon-derived epithelial cells requires the actin cytoskeleton, Rho GTPases, Filamin A and TRPC1.
(A, B) SWCaR#3 cells pretreated for 1 h with 2 μM cytochalasin-D (CytD) or with toxin B from Clostridium difficile (TxB) (40 ng/ml) were loaded with the Ca2+ indicator fura-2 and subsequently perfused with a saline solution containing the 5 mM L-Phe or 5 mM Ca2+ during the times indicated by the horizontal bars. The [Ca2+]i oscillations were measured in individual cells. Parallel cultures of SWCaR#3 cells were transiently co-transfected with plasmids encoding Clostridium botulinum C3 exoenzyme (C3) and pDsRed-Express or with plasmids encoding the 14 and 15 domains of human filamin-A (FilA14/15) and pDsRed-Express. psDsRed-express was used to facilitate the identification of the transfected cells. After 18 h, the transfected cells were loaded with fura-2 before determining the [Ca2+]i oscillations. Alternatively, SWCaR#3 cells loaded with fura-2 were incubated for 10 min with 15 μg of anti-TRPC1 (anti-TRPC1) before the [Ca2+]i oscillations were measured in individual cells. Control: untreated SWCaR#3 cells expressing DsRed Express.
Rho inhibition interferes with CaR-mediated [Ca2+]i oscillations
The small GTP-binding proteins of the Rho family, including Rho, Rac and Cdc-42, play a critical role in regulating the organization of the actin cytoskeleton (Burridge and Wennerberg, 2004). Because disruption of the actin cytoskeleton prevented the production of transient [Ca2+]i oscillations by the CaR, we examined whether Rho GTPases are required for the generation of [Ca2+]i oscillations in colon-derived epithelial cells. Treatment of SWCaR#3 cells with Clostridium difficile toxin B (TxB), which inactivates Rac, Cdc-42 and Rho (Aktories, 1997; Just et al., 1994), or with Clostridium botulinum C3 exoenzyme (C3), which specifically inactivates Rho (Sekine et al., 1989), did not affect the [Ca2+]i response mediated by [Ca2+]o stimulation of the CaR (n=50) (Fig. 4A). However, the same toxins abrogated up to 90% the transient [Ca2+]i oscillations evoked by L-Phe (n=50), indicating that Rho is a downstream effector in a signaling cascade triggered by L-Phe stimulation of the CaR. Thus, these results show that in colon-derived epithelial cells transient, but not sinusoidal, [Ca2+]i oscillations are Rho-dependent further reinforcing the concept that the CaR can function in two signaling modes.
CaR-mediated transient [Ca2+]i oscillations generation involves Filamin-A and TRPC1
Filamin-A, a large scaffold protein that interacts with the C-terminal region of the CaR (Awata et al., 2001; Hjalm et al., 2001; Pi et al., 2002) has been implicated in CaR-mediated Rho signaling (Pi et al., 2002). In view of our results, we examined whether this interaction played a role in the production of [Ca2+]i oscillations mediated by CaR. We transiently expressed the 14 and 15 domains of filamin-A (Fil-A14/15) to disrupt the interaction between endogenous filamin-A and the CaR (Awata et al., 2001; Hjalm et al., 2001; Pi et al., 2002) and determined the [Ca2+]i oscillations in response to L-Phe or [Ca2+]o stimulation. As shown in Fig. 4B, the expression of this truncated form of filamin-A selectively prevented up to 85% of transfected SWCaR#3 cells (n=50) from responding to L-Phe. However, the expression of Fil-A14/15 in SWCaR#3 cells (n=50) did not interfere with the [Ca2+]i response after [Ca2+]o stimulation of the CaR (Fig. 4B). These results suggest that in colon-derived epithelial cells the interaction of the C-terminus of the CaR with filamin-A play is involved in the generation of transient [Ca2+]i oscillations.
Transient receptor potential channel 1 (TRPC1), which is a highly conserved and widely expressed membrane protein that allows the flux of small cations including sodium and calcium (Beech et al., 2003), is involved in CaR-mediated generation of transient [Ca2+]i oscillations in HEK-293 cells (Rey et al., 2006b). To test whether TRPC1, which is expressed in SW-480 cells (data not shown), also plays a role in CaR-mediated [Ca2+]i oscillations in colon-derived epithelial cells, we interfered in vivo with the activity of this ion channel with an antibody that binds a peptide sequence located in the pore region of human TRPC1 (Rey et al., 2006b; Rosado et al., 2002). In agreement with previous reports from our laboratory using HEK-293 cells (Rey et al., 2006b), we found that exposure of SWCaR#3 cells to the TRPC1 antibody blocked L-Phe-elicited transient [Ca2+]i oscillations in up to 90% of the cells (n=45) (Fig. 4B) without preventing the [Ca2+]o-mediated [Ca2+]i responses (Fig. 4B). SWCaR#3 cells incubated with a control rabbit anti-c-myc (15 μg) responded to L-Phe and [Ca2+]o stimulation with the production of transient and sinusoidal [Ca2+]i oscillations (data not shown). Thus, these results show that in colon-derived epithelial cells TRPC1 is a component of a functional signaling complex formed in the presence of the CaR that mediates transient [Ca2+]i oscillations.
CaR-mediated signaling impairs colon-derived epithelial cells proliferation
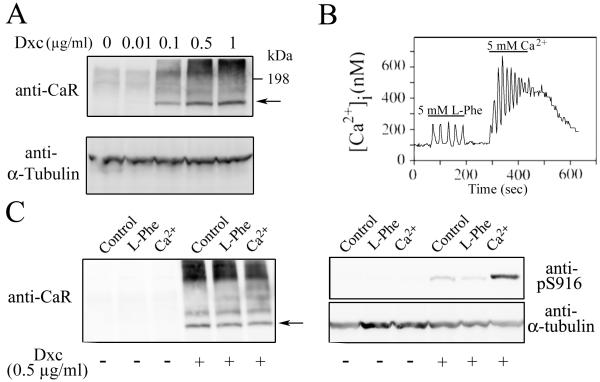
In the normal GI tract exist a gradient of CaR expression from non-detectable, in the proliferating cells at the bottom of the crypt, to highly expressed in the differentiated cells at the top of the crypt (Chakrabarty et al., 2005). In order to mimic this CaR expression gradient, NCM-460 cells were transduced with a retrovirus encoding the human CaR under the control of an inducible Tet promoter (Tet-ON). Western blot analysis of the obtained NCMiCaR cell line indicates that the CaR is not detectable in the absence, or a very low concentrations (0.01 μg/ml), of the inducer doxycycline, a member of the tetracycline antibiotics group (Fig. 5A). Addition of higher concentrations of doxycycline increased, in a dose-dependent manner, the synthesis of a protein with the predicted MW for the nonglycosylated monomeric CaR (121 kDa, arrow) and additional heavier bands corresponding to the glycosylated receptor (Fig. 5A) (Bai et al., 1996; Brown et al., 1993; Ward et al., 1998). As shown in Fig. 5B, NCMiCaR cells responded to L-Phe and [Ca2+]o with the generation of transient and sinusoidal [Ca2+]i oscillations. In agreement with the results obtained with SWCaR#3 cells, only [Ca2+]o stimulation of the CaR promoted the activation and autophosphorylation of Ser-876 in PKD2, the predominant PKD isoform expressed in NCM-460 cells (Chiu et al., 2007; Papazyan et al., 2008) (Fig. 5C).
Fig. 5. Characterization of NCMiCaR cells.
(A) NCMiCaR cells were incubated with the indicated concentrations of doxycycline (Dxc), as inducer of the CaR expression, for 16 h before being lysed and analyzed by Western blot using a rabbit polyclonal antibody against the CaR and a mouse monoclonal antibody against α-tubulin for expression and loading control. (B) NCMiCaR incubated for 16 h with 0.5 μg/ml of doxycycline were loaded with the Ca2+ indicator fura-2 and subsequently perfused with a saline solution containing the 5 mM L-Phe or 5 mM Ca2+ during the times indicated by the horizontal bars. (C) NCMiCaR incubated for 16 h with 0.5 μg/ml of doxycycline were challenged with 5 mM Ca2+ or 5 mM L-Phe for 10 min, lysed and analyzed by Western blot using a rabbit polyclonal antibody (anti-pS916) that detects the phosphorylation of the equivalent serine residues in PKD (Ser-916) and PKD2 (Ser-876) and a mouse monoclonal antibody against α-tubulin for expression and loading control.
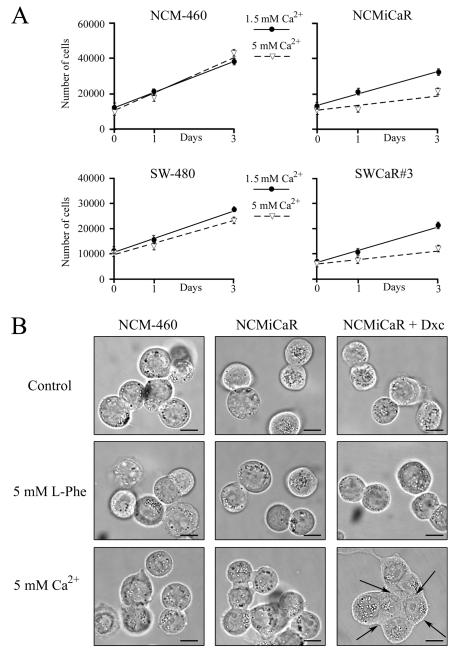
Several lines of evidence suggest that Ca2+ can exert, through the CaR, an anti-proliferative effect in normal colonic epithelium and in colorectal carcinomas-derived cells (Saidak et al., 2009). In order to examine whether CaR-mediated sinusoidal and/or transient [Ca2+]i oscillations are associated with proliferative responses in cells derived from intestinal epithelium, NCM-460, NCMiCaR, SW-480 and SWCaR#3 cells were incubated in the presence of DMEM containing 10% fetal bovine serum supplemented with 1.5 mM or 5 mM Ca2+. In the case of the NCMiCaR cells, the DMEM media was supplemented with 0.5 μg/ml of doxycycline to promote the synthesis of the CaR. The different cell lines were collected at the indicated times and their numbers determined with a Z1 Coulter particle counter. As shown in Fig. 6A, no significant difference was detected in the proliferation of NCM-460 or SW-480 cells incubated in the presence of 1.5 mM or 5 mM Ca2+. However, the proliferation response of the cell lines expressing the CaR indicates that 5 mM Ca2+ promotes a significant inhibition in their proliferation. No differences were detected in the proliferation of NCM-460, NCMiCaR, SW-480 or SWCaR#3 cells incubated in the presence of DMEM containing 10% fetal bovine serum supplemented with 1.5 mM Ca2+ and 5 mM L-Phe (data not shown).
Fig. 6. Effect of CaR stimulation on colon-derived epithelial cells proliferation and morphology.
(A) NCM-460 cells, NCMiCaR, SW-480 and SWCaR#3 cells incubated in DMEM containing 10% FBS supplemented 1.5 mM (closed circles) or 5 mM Ca2+ (open triangles) and 0.5 μg/ml of doxycycline for NCMiCaR cells were collected at the indicated times and counted with a Z1 Coulter particle counter. Mean values ± standard error of the mean, n=6. (B) NCM-460 cells and NCMiCaR incubated for 16 h without or with 0.5 μg/ml of doxycycline (+ Dxc) were challenged with 5 mM L-Phe or 5 mM Ca2+ for 10 min and examined by bright field microscopy. Images were acquired using a Spot Pursuit digital camera (Diagnostic Instruments Inc.). Bar: 10 μm.
In addition to proliferation inhibition, NCMiCaR cells adopted a flattened morphology and extended cell-to-cell contacts (arrows) in response to 5 mM Ca2+ (Fig. 6B), a phenotype also observed in SWCaR#3 cell incubated under the same conditions (data not shown). In contrast, L-Phe stimulation of NCMiCaR (Fig. 6B) or SWCaR#3 cells (data not shown) did not result in any morphological changes compared to cells incubated in DMEM containing 10% fetal bovine serum supplemented with 1.5 mM Ca2+. Thus, these results indicate that in colon-derived epithelial cells sinusoidal, but not transient, [Ca2+]i oscillations promote morphological changes and proliferation inhibition and suggest that colorectal adenocarcinomas have the complex machinery necessary for downstream events triggered by the stimulation of the CaR including the regulation of proliferation responses.
DISCUSSION
In mammals, the CaR is expressed along the entire GI tract. CaR transcripts and/or protein have been found in parietal, mucous and gastrin secreting cells of the stomach, villus cells of the small intestine and in different types of epithelial cells present in the colon (Conigrave and Brown, 2006; Hebert et al., 2004). In contrast to normal GI cells, the expression of the CaR is greatly reduced or completely lost in differentiated colorectal carcinomas (Kallay et al., 2000; Sheinin et al., 2000), suggesting that loss of this GPCR is associated with abnormal differentiation and malignant progression (Chakrabarty et al., 2003). Because it has been proposed that the CaR plays an important role in the regulation of intestinal epithelial cell proliferation and differentiation (Lamprecht and Lipkin, 2003; Saidak et al., 2009), a critical question is whether re-expression of the CaR in intestinal epithelial cells result in a receptor capable of mediating early (e. g. Ca2+ signaling) and late (e. g. proliferation) responses.
Previous studies in human fibroblast lead us to propose a model to explain the mechanism by which the CaR can function in two different modes, triggering sinusoidal oscillations in response to an increase in [Ca2+]o or transient oscillations in response to aromatic amino acids (Rey et al., 2006b; Rey et al., 2005; Young and Rozengurt, 2002; Young et al., 2002). In this model, [Ca2+]o-induced CaR activation stimulates PLCβ which in turn promotes the synthesis of inositol 1,4,5-trisphosphate (Ins (1,4,5)P3) and DAG. Ins (1,4,5)P3 mobilizes Ca2+ from the internal stores and DAG activates PKC. Activated PKC then phosphorylates the CaR providing the negative feedback needed to cause sinusoidal [Ca2+]i oscillations. Accordingly, inhibition of PKC abolished the [Ca2+]i oscillations mediated by [Ca2+]o without affecting the increase in [Ca2+]i. In contrast to [Ca2+]o, the binding of aromatic amino acids to a topographically distinct site of the Ca2+ binding sites in the extracellular domain of the CaR does not stimulates PLCβ but engages, through its cytoplasmic tail, a multiprotein complex that includes Rho, filamin-A and TRPC1. This interaction leads to TRPC1 channel opening and extracellular Ca2+ entry, which is further stimulated by PKC, leading to the generation of transient [Ca2+]i oscillations.
In the present study, we present several lines of experimental evidence indicating that the proposed model of CaR as a mediator of transient and sinusoidal [Ca2+]i oscillations operates in colon-derived epithelial cells in which the CaR was re-expressed. The notion that the CaR can act in colon-derived epithelial cells through PLCβ-dependent and -independent pathways was substantiated by the difference in the ability of the CaR agonists to induce translocation of the DAG biosensor PKD-RFP from the cytosol to the plasma membrane. Specifically, [Ca2+]o-elicited CaR stimulation induced a rapid translocation of PKD-RFP from the cytoplasm to the plasma membrane indicative of DAG production. [Ca2+]o-elicited CaR activation also induced a remarkable increase in the phosphorylation of PKD Ser-916 and PKD2 Ser-876 in SW-480 and NCM-460 cells, respectively, another indication of DAG production (Matthews et al., 1999; Rey et al., 2004; Rey et al., 2005). In striking contrast, parallel experiments demonstrated that addition of L-Phe, at concentrations that induced the production of robust and lasting transient [Ca2+]i oscillations, did not stimulate any detectable DAG synthesis in these cell lines as revealed by the lack of plasma membrane translocation of the DAG biosensor and of PKD Ser-916 or PKD2 Ser-876 phosphorylation. We also showed that PKC inhibition abolishes sinusoidal and transient CaR-mediated [Ca2+]i oscillations without blocking the nonoscillatory increase in [Ca2+]i in response to [Ca2+]o stimulation, indicating that PKC negatively modulates the coupling of the CaR to intracellular signaling systems. The results presented in this study also demonstrate that L-Phe-elicited transient [Ca2+]i oscillations were selectively abolished in colon-derived epithelial cells by multiple approaches targeting key signal transducer proposed in our model. These include pharmacological disruption of the actin cytoskeleton with cytochalasin D, inactivation of Rho GTPases with Clostridium difficile toxin B and with Clostridium botulinum C3 exoenzyme, interference with the binding of filamin-A to the CaR and obstruction of the activity of TRPC1 via an antibody that binds the pore region of this ion channel. In agreement with the proposed model of CaR regulation, we demonstrated in each case suppression of [Ca2+]i signaling in response to amino acid stimulation but retention of [Ca2+]i signaling induced by an elevation of [Ca2+]o. The capacity of the CaR to couple to PLCβ or to TRPC1 in response to Ca2+ and aromatic amino acids could explain the remarkable ability of this receptor to mediate sinusoidal and transient patterns of [Ca2+]i oscillations.
The Wnt/β-catenin signalling pathway is activated in 90% of human colon cancers by nuclear accumulation of β-catenin protein due to its own mutation or to that of APC. In the nucleus, β-catenin regulates gene expression promoting cell proliferation, migration and invasiveness. SW-480 cells, which were established from a primary human colon adenocarcinoma, harbor most of the genetic abnormalities that characterize advanced colon cancers including a non-functional APC (Leibovitz et al., 1976; Schwarte-Waldhoff et al., 1999; Tomita et al., 1992). Nevertheless, re-expression and [Ca2+]o-mediated stimulation the CaR was sufficient to inhibit SW-480 proliferation to the same extent as observed in NCM-460 cells derived from normal human colon mucosal epithelium expressing functional APC and β-catenin proteins (Hope et al., 2008). This remarkable finding suggest that sinusoidal, but not transient, [Ca2+]i oscillations are associated with growth inhibition. Although further experiments are needed to characterize the precise mechanism by which the CaR promotes proliferation inhibition, alternative pathways that regulate the activity of β-catenin, such as downregulation of its expression via Wnt5 secretion (MacLeod et al., 2007) or regulation of its transcriptional activity by dephosphorylation (Fang et al., 2007; Taurin et al., 2006), could be responsible for the observed effects.
Collectively, our results show that in human colon-derived epithelial cells stimulation of the CaR by aromatic amino acids or by extracellular Ca2+ promotes transient and sinusoidal [Ca2+]i oscillations by activating distinct signal transduction pathways. The extensive presence of the CaR in the GI tract and its unique capability to recognize and differentially respond to aromatic amino acids and extracellular Ca2+ provides a potential link between dietary metabolism and GI responses including growth, differentiation and colon cancer progression.
AKNOWDLEGMENTS
This work was supported by NIH awards K22CA128883 and K22CA128883-03S1 to O. R., a recipient of a Margaret E. Early Medical Research Trust award. E. R., the Ronald S. Hirshberg Professor of Translational Pancreatic Cancer Research, is a recipient of NIH awards R21CA137292, RO1DK55003, RO1DK56930 and P30DK41301. Support from the Morphology/Imaging Core of the CURE Center Grant P30DK41301 is gratefully acknowledged.
Contract grant sponsor: National Institute of Health; Grant numbers: K22CA128883, K22CA128883-03S1 & P30DK41301
LITERATURE CITED
- Ahnen DJ, Byers T. Proliferation happens. Jama. 1998;280(12):1095–1096. doi: 10.1001/jama.280.12.1095. [DOI] [PubMed] [Google Scholar]
- Aktories K. Bacterial toxins that target Rho proteins. Journal of Clinical Investigation. 1997;99(5):827–829. doi: 10.1172/JCI119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awata H, Huang C, Handlogten ME, Miller RT. Interaction of the calcium-sensing receptor and filamin, a potential scaffolding protein. J Biol Chem. 2001;276(37):34871–34879. doi: 10.1074/jbc.M100775200. [DOI] [PubMed] [Google Scholar]
- Bai M, Quinn S, Trivedi S, Kifor O, Pearce SH, Pollak MR, Krapcho K, Hebert SC, Brown EM. Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem. 1996;271(32):19537–19545. doi: 10.1074/jbc.271.32.19537. [DOI] [PubMed] [Google Scholar]
- Bai M, Trivedi S, Brown EM. Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J Biol Chem. 1998;273(36):23605–23610. doi: 10.1074/jbc.273.36.23605. [DOI] [PubMed] [Google Scholar]
- Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Bond JH, Greenberg ER. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999a;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, Rothstein R, Summers RW, Snover DC, Beck GJ, Frankl H, Pearson L, Bond JH, Greenberg ER. Calcium supplements and colorectal adenomas. Polyp Prevention Study Group. Ann N Y Acad Sci. 1999b;889:138–145. doi: 10.1111/j.1749-6632.1999.tb08731.x. [DOI] [PubMed] [Google Scholar]
- Beech DJ, Xu SZ, McHugh D, Flemming R. TRPC1 store-operated cationic channel subunit. Cell Calcium. 2003;33(5-6):433–440. doi: 10.1016/s0143-4160(03)00054-x. [DOI] [PubMed] [Google Scholar]
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, Sun A, Hediger MA, Lytton J, Hebert SC. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81(1):239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- Chakrabarty S, Radjendirane V, Appelman H, Varani J. Extracellular calcium and calcium sensing receptor function in human colon carcinomas: promotion of E-cadherin expression and suppression of beta-catenin/TCF activation. Cancer Res. 2003;63(1):67–71. [PubMed] [Google Scholar]
- Chakrabarty S, Wang H, Canaff L, Hendy GN, Appelman H, Varani J. Calcium sensing receptor in human colon carcinoma: interaction with Ca(2+) and 1,25-dihydroxyvitamin D(3) Cancer Res. 2005;65(2):493–498. [PubMed] [Google Scholar]
- Chiu TT, Leung WY, Moyer MP, Strieter RM, Rozengurt E. Protein kinase D2 mediates lysophosphatidic acid-induced interleukin 8 production in nontransformed human colonic epithelial cells through NF-kappaB. Am J Physiol Cell Physiol. 2007;292(2):C767–777. doi: 10.1152/ajpcell.00308.2006. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Brown EM. Taste receptors in the gastrointestinal tract. II. L-amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physiol Gastrointest Liver Physiol. 2006;291(5):G753–761. doi: 10.1152/ajpgi.00189.2006. [DOI] [PubMed] [Google Scholar]
- Conigrave AD, Quinn SJ, Brown EM. L-Amino acid sensing by the extracellular Ca2+ -sensing receptor. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4814–4819. doi: 10.1073/pnas.97.9.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66(6):1191–1308. [PubMed] [Google Scholar]
- Duris I, Hruby D, Pekarkova B, Huorka M, Cernakova E, Bezayova T, Ondrejka P. Calcium chemoprevention in colorectal cancer. Hepatogastroenterology. 1996;43(7):152–154. [PubMed] [Google Scholar]
- Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, Mills GB, Kobayashi R, Hunter T, Lu Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1(8424):307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- Hebert SC, Cheng S, Geibel J. Functions and roles of the extracellular Ca2+-sensing receptor in the gastrointestinal tract. Cell Calcium. 2004;35(3):239–247. doi: 10.1016/j.ceca.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Hendy GN, D’Souza-Li L, Yang B, Canaff L, Cole DE. Mutations of the calcium-sensing receptor (CASR) in familial hypocalciuric hypercalcemia, neonatal severe hyperparathyroidism, and autosomal dominant hypocalcemia. Hum Mutat. 2000;16(4):281–296. doi: 10.1002/1098-1004(200010)16:4<281::AID-HUMU1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Hjalm G, MacLeod RJ, Kifor O, Chattopadhyay N, Brown EM. Filamin-A binds to the carboxyl-terminal tail of the calcium-sensing receptor, an interaction that participates in CaR-mediated activation of mitogen-activated protein kinase. J Biol Chem. 2001;276(37):34880–34887. doi: 10.1074/jbc.M100784200. [DOI] [PubMed] [Google Scholar]
- Ho C, Conner DA, Pollak MR, Ladd DJ, Kifor O, Warren HB, Brown EM, Seidman JG, Seidman CE. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11(4):389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- Holt PR. Studies of calcium in food supplements in humans. Ann N Y Acad Sci. 1999;889:128–137. doi: 10.1111/j.1749-6632.1999.tb08730.x. [DOI] [PubMed] [Google Scholar]
- Holt PR, Atillasoy EO, Gilman J, Guss J, Moss SF, Newmark H, Fan K, Yang K, Lipkin M. Modulation of abnormal colonic epithelial cell proliferation and differentiation by low-fat dairy foods: a randomized controlled trial. Jama. 1998;280(12):1074–1079. doi: 10.1001/jama.280.12.1074. [DOI] [PubMed] [Google Scholar]
- Hope C, Planutis K, Planutiene M, Moyer MP, Johal KS, Woo J, Santoso C, Hanson JA, Holcombe RF. Low concentrations of resveratrol inhibit Wnt signal throughput in colon-derived cells: implications for colon cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S52–61. doi: 10.1002/mnfr.200700448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: a meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61(1):47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- Just I, Fritz G, Aktories K, Giry M, Popoff MR, Boquet P, Hegenbarth S, von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. Journal of Biological Chemistry. 1994;269(14):10706–10712. [PubMed] [Google Scholar]
- Kallay E, Bajna E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Dietary calcium and growth modulation of human colon cancer cells: role of the extracellular calcium-sensing receptor. Cancer Detect Prev. 2000;24(2):127–136. [PubMed] [Google Scholar]
- Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87(2):159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- Kisfalvi K, Rey O, Young SH, Sinnett-Smith J, Rozengurt E. Insulin potentiates Ca2+ signaling and phosphatidylinositol 4,5-bisphosphate hydrolysis induced by Gq protein-coupled receptor agonists through an mTOR-dependent pathway. Endocrinology. 2007;148(7):3246–3257. doi: 10.1210/en.2006-1711. [DOI] [PubMed] [Google Scholar]
- Lamprecht SA, Lipkin M. Chemoprevention of colon cancer by calcium, vitamin D and folate: molecular mechanisms. Nat Rev Cancer. 2003;3(8):601–614. doi: 10.1038/nrc1144. [DOI] [PubMed] [Google Scholar]
- Leibovitz A, Stinson JC, McCombs WB, 3rd, McCoy CE, Mazur KC, Mabry ND. Classification of human colorectal adenocarcinoma cell lines. Cancer Res. 1976;36(12):4562–4569. [PubMed] [Google Scholar]
- Lipkin M, Newmark H. Calcium and the prevention of colon cancer. J Cell Biochem Suppl. 1995a;22:65–73. doi: 10.1002/jcb.240590810. [DOI] [PubMed] [Google Scholar]
- Lipkin M, Newmark H. Development of clinical chemoprevention trials. J Natl Cancer Inst. 1995b;87(17):1275–1277. doi: 10.1093/jnci/87.17.1275. [DOI] [PubMed] [Google Scholar]
- MacLeod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by the extracellular calcium-sensing receptor inhibits defective Wnt signaling in colon cancer cells. Am J Physiol Gastrointest Liver Physiol. 2007;293(1):G403–411. doi: 10.1152/ajpgi.00119.2007. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268(13):9194–9197. [PubMed] [Google Scholar]
- Matthews SA, Rozengurt E, Cantrell D. Characterization of serine 916 as an in vivo autophosphorylation site for protein kinase D/Protein kinase Cmu. Journal of Biological Chemistry. 1999;274(37):26543–26549. doi: 10.1074/jbc.274.37.26543. [DOI] [PubMed] [Google Scholar]
- McCullough ML, Robertson AS, Rodriguez C, Jacobs EJ, Chao A, Carolyn J, Calle EE, Willett WC, Thun MJ. Calcium, vitamin D, dairy products, and risk of colorectal cancer in the Cancer Prevention Study II Nutrition Cohort (United States) Cancer Causes Control. 2003;14(1):1–12. doi: 10.1023/a:1022591007673. [DOI] [PubMed] [Google Scholar]
- Moyer MP, Manzano LA, Merriman RL, Stauffer JS, Tanzer LR. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell Dev Biol Anim. 1996;32(6):315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- Newmark HL, Yang K, Kurihara N, Fan K, Augenlicht LH, Lipkin M. Western-style diet-induced colonic tumors and their modulation by calcium and vitamin D in C57Bl/6 mice: a preclinical model for human sporadic colon cancer. Carcinogenesis. 2009;30(1):88–92. doi: 10.1093/carcin/bgn229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki H. A Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22(11):1871–1875. doi: 10.1093/carcin/22.11.1871. [DOI] [PubMed] [Google Scholar]
- Papazyan R, Doche M, Waldron RT, Rozengurt E, Moyer MP, Rey O. Protein kinase D isozymes activation and localization during mitosis. Exp Cell Res. 2008;314(16):3057–3068. doi: 10.1016/j.yexcr.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169(4):391–401. doi: 10.1001/archinternmed.2008.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98(4):487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Pence BC. Role of calcium in colon cancer prevention: experimental and clinical studies. Mutat Res. 1993;290(1):87–95. doi: 10.1016/0027-5107(93)90036-f. [DOI] [PubMed] [Google Scholar]
- Pi M, Spurney RF, Tu Q, Hinson T, Quarles LD. Calcium-sensing receptor activation of rho involves filamin and rho-Guanine nucleotide exchange factor. Endocrinology. 2002;143(10):3830–3838. doi: 10.1210/en.2002-220240. [DOI] [PubMed] [Google Scholar]
- Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, Virtamo J. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control. 1999;10(5):387–396. doi: 10.1023/a:1008962219408. [DOI] [PubMed] [Google Scholar]
- Quarles LD. Extracellular calcium-sensing receptors in the parathyroid gland, kidney, and other tissues. Curr Opin Nephrol Hypertens. 2003;12(4):349–355. doi: 10.1097/00041552-200307000-00002. [DOI] [PubMed] [Google Scholar]
- Ray K, Fan GF, Goldsmith PK, Spiegel AM. The carboxyl terminus of the human calcium receptor. Requirements for cell-surface expression and signal transduction. J Biol Chem. 1997;272(50):31355–31361. doi: 10.1074/jbc.272.50.31355. [DOI] [PubMed] [Google Scholar]
- Rey O, Papazyan R, Waldron RT, Young SH, Lippincott-Schwartz J, Jacamo R, Rozengurt E. The nuclear import of protein kinase D3 requires its catalytic activity. J Biol Chem. 2006a;281(8):5149–5157. doi: 10.1074/jbc.M508014200. [DOI] [PubMed] [Google Scholar]
- Rey O, Reeve JR, Jr., Zhukova E, Sinnett-Smith J, Rozengurt E. G Protein-coupled Receptor-mediated Phosphorylation of the Activation Loop of Protein Kinase D: Dependence on Plasma Membrane Translocation and Protein Kinesa C{epsilon} J Biol Chem. 2004;279(33):34361–34372. doi: 10.1074/jbc.M403265200. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Cantrell D, Rozengurt E. Rapid protein kinase D translocation in response to G protein-coupled receptor activation: dependence on protein kinase Ce. J Biol Chem. 2001;276(35):32616–32626. doi: 10.1074/jbc.M101649200. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Papazyan R, Shapiro MS, Rozengurt E. Requirement of the TRPC1 Cation Channel in the Generation of Transient Ca2+ Oscillations by the Calcium-sensing Receptor. J Biol Chem. 2006b;281(50):38730–38737. doi: 10.1074/jbc.M605956200. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/Ins(1,4,5)P3-independent pathway that requires G12, Rho, filamin-A and the actin cytoskeleton. J Biol Chem. 2005;280(24):22875–22882. doi: 10.1074/jbc.M503455200. [DOI] [PubMed] [Google Scholar]
- Ribeiro CM, Reece J, Putney JW., Jr. Role of the cytoskeleton in calcium signaling in NIH 3T3 cells. An intact cytoskeleton is required for agonist-induced [Ca2+]i signaling, but not for capacitative calcium entry. J Biol Chem. 1997;272(42):26555–26561. doi: 10.1074/jbc.272.42.26555. [DOI] [PubMed] [Google Scholar]
- Rosado JA, Brownlow SL, Sage SO. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277(44):42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- Saidak Z, Mentaverri R, Brown EM. The role of the calcium-sensing receptor in the development and progression of cancer. Endocr Rev. 2009;30(2):178–195. doi: 10.1210/er.2008-0041. [DOI] [PubMed] [Google Scholar]
- Schwarte-Waldhoff I, Klein S, Blass-Kampmann S, Hintelmann A, Eilert C, Dreschers S, Kalthoff H, Hahn SA, Schmiegel W. DPC4/SMAD4 mediated tumor suppression of colon carcinoma cells is associated with reduced urokinase expression. Oncogene. 1999;18(20):3152–3158. doi: 10.1038/sj.onc.1202641. [DOI] [PubMed] [Google Scholar]
- Sekine A, Fujiwara M, Narumiya S. Asparagine residue in the rho gene product is the modification site for botulinum ADP-ribosyltransferase. J Biol Chem. 1989;264(15):8602–8605. [PubMed] [Google Scholar]
- Sheinin Y, Kallay E, Wrba F, Kriwanek S, Peterlik M, Cross HS. Immunocytochemical localization of the extracellular calcium-sensing receptor in normal and malignant human large intestinal mucosa. J Histochem Cytochem. 2000;48(5):595–602. doi: 10.1177/002215540004800503. [DOI] [PubMed] [Google Scholar]
- Shin KJ, Wall EA, Zavzavadjian JR, Santat LA, Liu J, Hwang JI, Rebres R, Roach T, Seaman W, Simon MI, Fraser ID. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci U S A. 2006;103(37):13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery ML, Sorenson AW, Ford MH. Dietary calcium intake as a mitigating factor in colon cancer. Am J Epidemiol. 1988;128(3):504–514. doi: 10.1093/oxfordjournals.aje.a114998. [DOI] [PubMed] [Google Scholar]
- Taurin S, Sandbo N, Qin Y, Browning D, Dulin NO. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J Biol Chem. 2006;281(15):9971–9976. doi: 10.1074/jbc.M508778200. [DOI] [PubMed] [Google Scholar]
- Terry P, Baron JA, Bergkvist L, Holmberg L, Wolk A. Dietary calcium and vitamin D intake and risk of colorectal cancer: a prospective cohort study in women. Nutr Cancer. 2002;43(1):39–46. doi: 10.1207/S15327914NC431_4. [DOI] [PubMed] [Google Scholar]
- Tomita N, Jiang W, Hibshoosh H, Warburton D, Kahn SM, Weinstein IB. Isolation and characterization of a highly malignant variant of the SW480 human colon cancer cell line. Cancer Res. 1992;52(24):6840–6847. [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. Journal of Biological Chemistry. 1991;266(24):15771–15781. [PubMed] [Google Scholar]
- Ward DT, Brown EM, Harris HW. Disulfide bonds in the extracellular calcium-polyvalent cation-sensing receptor correlate with dimer formation and its response to divalent cations in vitro. J Biol Chem. 1998;273(23):14476–14483. doi: 10.1074/jbc.273.23.14476. [DOI] [PubMed] [Google Scholar]
- Wilkinson SE, Parker PJ, Nixon JS. Isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase C. Biochem J. 1993;294(Pt 2):335–337. doi: 10.1042/bj2940335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K, Willett WC, Fuchs CS, Colditz GA, Giovannucci EL. Calcium intake and risk of colon cancer in women and men. J Natl Cancer Inst. 2002;94(6):437–446. doi: 10.1093/jnci/94.6.437. [DOI] [PubMed] [Google Scholar]
- Yang K, Kurihara N, Fan K, Newmark H, Rigas B, Bancroft L, Corner G, Livote E, Lesser M, Edelmann W, Velcich A, Lipkin M, Augenlicht L. Dietary induction of colonic tumors in a mouse model of sporadic colon cancer. Cancer Res. 2008;68(19):7803–7810. doi: 10.1158/0008-5472.CAN-08-1209. [DOI] [PubMed] [Google Scholar]
- Young SH, Rozengurt E. Amino acids and Ca2+ stimulate different patterns of Ca2+ oscillations through the Ca2+-sensing receptor. Am J Physiol Cell Physiol. 2002;282(6):C1414–1422. doi: 10.1152/ajpcell.00432.2001. [DOI] [PubMed] [Google Scholar]
- Young SH, Wu SV, Rozengurt E. Ca2+-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor require negative feedback by protein kinase C. J Biol Chem. 2002;277(49):46871–46876. doi: 10.1074/jbc.M207083200. [DOI] [PubMed] [Google Scholar]
- Yuan J, Slice LW, Rozengurt E. Activation of protein kinase D by signaling through Rho and the alpha subunit of the heterotrimeric G protein G13. J Biol Chem. 2001;276(42):38619–38627. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- Zheng W, Anderson KE, Kushi LH, Sellers TA, Greenstein J, Hong CP, Cerhan JR, Bostick RM, Folsom AR. A prospective cohort study of intake of calcium, vitamin D, and other micronutrients in relation to incidence of rectal cancer among postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1998;7(3):221–225. [PubMed] [Google Scholar]