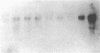
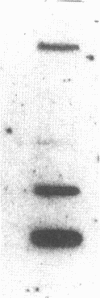
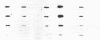Abstract
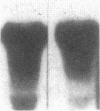
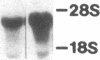
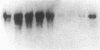
Multiple isoenzymes of the Na+,K+-ATPase (alpha, alpha+, and alpha 3) have been identified by molecular cloning (Shull, G. E., J. Greeb, and J. B. Lingrel. 1986. Biochemistry. 25:8125-8132; and Schneider, J. W., R. W. Mercer, and E. J. Benz, Jr. 1987. Clin. Res. 35:585A. [Abstr.]). At least one of these, the alpha 3 chain, represents a novel form for which protein products and enzymatic activities are just beginning to be defined in rodents. We have recently demonstrated that expression of alpha 3 is largely confined to neuromuscular tissues of fetal and adult rats (Schneider, J. W., R. W. Mercer, M. Gilmore-Hebert, M. F. Utset, C. Lai, A. Greene, and E. J. Benz, Jr. 1988. Proc. Natl. Acad. Sci. USA. 85:284-288). We now report that certain human leukemia cell lines including HL60, HEL, and Molt 4 express mRNA for both alpha and alpha 3 isoforms of Na+,K+-ATPase; mRNA was not detected in several other cell lines, including K562 and U937; no cell lines expressed alpha+ mRNA. In uninduced HL60 cells, alpha 3 mRNA comprised 20-30% of total Na+,K+-ATPase mRNA. Furthermore, in HL60 and HEL cells, both alpha and alpha 3 mRNA declined after induction of maturation by DMSO, retinoic acid, or hemin. However, the reduction in alpha 3 mRNA was far more dramatic. alpha 3 mRNA virtually disappeared, but alpha mRNA declined by only approximately 50%. In contrast, when maturation of HL60 cells along the monocyte/macrophage lineage was induced by exposure to phorbol esters, alpha 3 mRNA remained abundant. Moreover, mRNA for the beta subunit of the Na+,K+-ATPase increased dramatically. Our results demonstrate that the alpha 3 isoform, formerly thought to be confined to neuromuscular tissues, is expressed in restricted lineages of hematopoietic origin. These leukemia cell lines should provide a useful model for analyzing regulation of the alpha 3 isoform gene and characterization of alpha 3 isoform activities.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Martinotti S., Franchini G., Papas T. S., Gallo R. C., Wong-Staal F. Cloning and characterization of different human sequences related to the onc gene (v-myc) of avian myelocytomatosis virus (MC29). Proc Natl Acad Sci U S A. 1982 Nov;79(21):6497–6501. doi: 10.1073/pnas.79.21.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargus J. J., Adelberg E. A., Slayman C. W. Rapid changes in bidirectional K+ fluxes preceding DMSO-induced granulocytic differentiation of HL-60 human leukemic cells. J Cell Physiol. 1984 Jul;120(1):83–90. doi: 10.1002/jcp.1041200112. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y., Nikamoto A., Kojima T., Matsumoto A., Nakao M. Expression of sodium pump activities in BALB/c 3T3 cells transfected with cDNA encoding alpha 3-subunits of rat brain Na+,K+-ATPase. FEBS Lett. 1988 Sep 26;238(1):27–30. doi: 10.1016/0014-5793(88)80218-7. [DOI] [PubMed] [Google Scholar]
- High K. A., Stolle C. A., Schneider J. W., Hu W., Benz E. J., Jr c-myc gene inactivation during induced maturation of HL-60 cells. Transcriptional repression and loss of a specific DNAse I hypersensitive site. J Clin Invest. 1987 Jan;79(1):93–99. doi: 10.1172/JCI112814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladoux A., Abita J. P., Geny B. Retinoic-acid-induced differentiation of HL-60 cells is associated with biphasic activation of the Na+-K+ pump. Differentiation. 1986;33(2):142–147. doi: 10.1111/j.1432-0436.1986.tb00419.x. [DOI] [PubMed] [Google Scholar]
- Ladoux A., Geny B., Marrec N., Abita J. P. [3H]Ouabain binding and 86 rubidium uptake during the dimethyl sulfoxide-induced differentiation of human promyelocytic leukemia HL-60 cells. FEBS Lett. 1984 Oct 29;176(2):467–472. doi: 10.1016/0014-5793(84)81220-x. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Mummery C. L., van der Saag P. T., de Laat S. W. Rapid ionic events and the initiation of growth in serum-stimulated neuroblastoma cells. Cell. 1981 Mar;23(3):789–798. doi: 10.1016/0092-8674(81)90443-8. [DOI] [PubMed] [Google Scholar]
- Schneider J. W., Mercer R. W., Gilmore-Hebert M., Utset M. F., Lai C., Greene A., Benz E. J., Jr Tissue specificity, localization in brain, and cell-free translation of mRNA encoding the A3 isoform of Na+,K+-ATPase. Proc Natl Acad Sci U S A. 1988 Jan;85(1):284–288. doi: 10.1073/pnas.85.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shull G. E., Greeb J., Lingrel J. B. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–8132. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- Shull M. M., Lingrel J. B. Multiple genes encode the human Na+,K+-ATPase catalytic subunit. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4039–4043. doi: 10.1073/pnas.84.12.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. M., Lingrel J. B. Tissue distribution of mRNAs encoding the alpha isoforms and beta subunit of rat Na+,K+-ATPase. Biochem Biophys Res Commun. 1987 May 29;145(1):52–58. doi: 10.1016/0006-291x(87)91286-1. [DOI] [PubMed] [Google Scholar]