Abstract
Purpose
The volume-outcomes relationship has led many to advocate centralization of cancer procedures at high volume hospitals (HVH). We hypothesized that in response cancer surgery has become increasingly centralized and that this centralization has resulted in increased travel burden for patients.
Patients and Methods
Using 1996 to 2006 discharge data from NY, NJ, PA, all patients ≥ 18 years old treated with extirpative surgery for colorectal, esophageal, or pancreatic cancer were examined. Patients and hospitals were geocoded. Annual hospital procedure volume for each tumor site was examined, and multiple quantile and logistic regressions were used to compare changes in centralization and distance traveled.
Results
Five thousand two hundred seventy-three esophageal, 13,472 pancreatic, 202,879 colon, and 51,262 rectal procedures were included. A shift to HVH occurred to varying degrees for all tumor types. The odds of surgery at a low volume hospital decreased for esophagus, pancreas and colon: per year odds ratios (ORs) were 0.87 (95% CI, 0.85 to 0.90), 0.85 (95% CI, 0.84 to 0.87), and 0.97 (95% CI, 0.97 to 0.98). Median travel distance increased for all sites: esophagus 72%, pancreas 40%, colon 17%, and rectum 28% (P < .0001). Travel distance was proportional to procedure volume (P < .0001). The majority of the increase in distance was attributable to centralization.
Conclusion
There has been extensive centralization of complex cancer surgery over the past decade. While this process should result in population-level improvements in cancer outcomes, centralization is increasing patient travel. For some subsets of the population, increasing travel requirements may pose a significant barrier to access to quality cancer care.
INTRODUCTION
Over the past 30 years, a wealth of publications documenting relationships between hospital procedure volume and clinical outcomes has emerged.1,2 Most recently, there is increasing focus on the volume-outcome relationships for cancer care.3–6 The evidence documenting an inverse relationship between mortality and hospital procedure volume for pancreatic and esophageal surgery has caused many to advocate centralization of these procedures at high volume hospitals (HVH).3,7–12 Others worry centralization may create access problems for a substantial portion of patients and worsen existing disparities between those who are treated at HVH versus low volume hospitals (LVH).2,13–23 Despite general acceptance of the volume-outcome relationships for complex cancer operations, little is known about how patterns of care in the United States have changed in response to recently published studies.24–28
In this observational study, we do not attempt to reconfirm the volume-outcome relationships for cancer surgery, but rather we examine changes in the distribution of cases among hospitals with differing procedure volumes and document how this has evolved over time. We address the impact changes have had on travel distance for patients with cancer and investigate whether existing disparities have been exacerbated by centralization of cancer surgery. We hypothesize that centralization has substantially increased the travel distance for patients and has exacerbated existing disparities relating to treatment at high versus low volume centers.
PATIENTS AND METHODS
A secondary data analysis was performed using discharge data from NJ, NY, and PA. Available data included demographic information (including race as documented in the medical record), clinical diagnoses, procedure codes, length of stay, disposition at discharge, and hospital charges.
All patients ≥ 18 years of age undergoing procedures in the service area (NJ, NY, PA) from 1996 to 2006 were included. From 1996 to 1999, only NY and PA data were available. Starting in 2000, procedures from all three states were included. The most common primary gastrointestinal malignancies were chosen for study. International Classification of Diseases 9th revision procedure codes captured all pancreatectomies, esophagectomies, colectomies, and proctectomies, as well as local surgical excision of masses at these sites (ie, transanal excision of rectal mass). Endoscopic resections were excluded. The study group was further limited to cases with an International Classification of Diseases 9th revision diagnosis code for neoplasm.
Cases were geocoded to zip code centroid and linked to publicly available area-based sociodemographic data. Hospitals were geocoded to actual street address. Straight-line distance was calculated between cases and the hospitals at which the procedures were performed as well as between cases and the nearest HVH or very high volume hospital (VHVH).29
Hospital procedure volume of extirpative procedures for each organ site was calculated for each year individually, allowing for the possibility that volume at a given hospital could change over time. Five roughly equal-sized groups of patients were created for each organ site in 1996 based on procedure volume of the treating hospital. These quintiles were designated very low, low, medium, high, and very high volume. The 1996 cut points were then applied to each subsequent year in order to determine changes in the distribution of patients among volume categories. Similarly, 1996 tercile cut points were created for each organ site. Multiple logistic regressions provided estimates of the odds of receiving care at a hospital in the lowest tercile over time, examining year as a continuous variable and controlling for the overall number of cases performed in the study area annually. Using the same data set, annual market-wide in-hospital mortality was calculated for each organ site.
We used multiple quantile regressions to investigate median time trends in distance traveled. We added an indicator variable of treatment in the highest volume tercile to investigate if coefficients of the year main effects and interaction terms changed after controlling for high volume tercile. We used similar multiple quantile regressions to investigate if time trends in distance varied by rurality status and to investigate the relationship between median distance and the number of available HVH.
Using multiple logistic regressions, we investigated the association between sociodemographic factors and the odds of having a surgery at a LVH in 1996 to 1997 and 2005 to 2006. Interaction models were used to investigate if the strength of associations changed between the two time points.
All hypothesis tests were two sided, and the criterion for statistical significance was a P < .05. Data were deidentified, and the study was approved by the institutional review board of Fox Chase Cancer Center.
RESULTS
Two hundred seventy-two thousand eight hundred eighty-six procedures met criteria for inclusion (5,273 esophagus, 13,472 pancreas, 202,879 colon, 51,262 rectum). Inward border crossers (patients residing outside the study area who had surgery within the study area) comprised 1.8% of the study population: esophagus 7.2% (382 of 5,273), pancreas 4.9% (666 of 13,472), colon 1.4% (2,857 of 202,879), rectum 2.0% (1,014 of 51,262).
Centralization
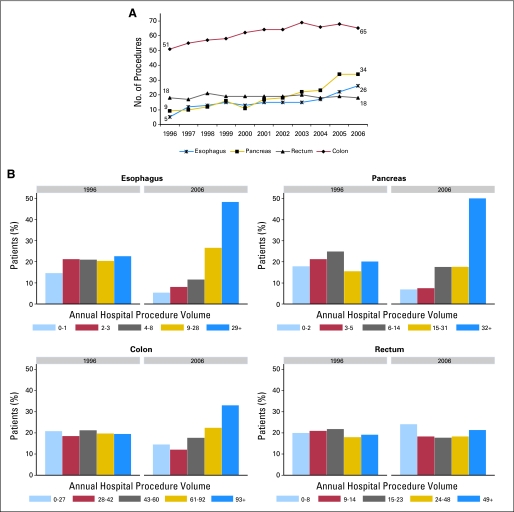
A shift from LVH to HVH occurred to varying degrees for esophageal, pancreatic, and colon cancer procedures (Figs 1A, 1B). For esophageal and pancreatic procedures, this process is ongoing without evidence of abating. In contrast to the other organ sites, the distribution of rectal cancer procedures between volume groups did not change substantially over time.
Fig 1.
Procedure volume over time. (A) Median procedure volume. Values should be interpreted as volume for the 50th percentile of all cases. For example, in 1996, half of all resections for esophageal cancer were performed at a hospital that did ≤ 5 such procedures that year. In contrast, in 2006, half of all esophageal cancer resections were performed at a hospital that did ≥ 26 resections. (B) Distribution of procedures among volume categories based on 1996 quintile cut points.
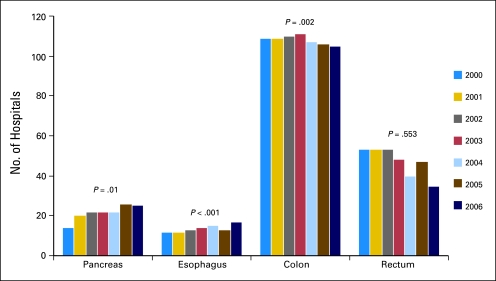
To accommodate the centralization, there was an increase in the number of HVH and VHVH (Fig 2) as well as an increase in the number of procedures performed at existing HVH and VHVH. Average procedure volume for the top five HVH for pancreatic cancer surgery increased from 62.8 to 125 cases/year from 2000 to 2006. Similarly, for esophageal cancer, average procedure volume for the top five HVH increased from 36.6 to 60 cases/year from 2000 to 2006.
Fig 2.
Number of available high- or very high–volume hospitals. Total number of hospitals meeting 1996 quintile cut points for high- or very high–volume hospitals.
Adjusting for population-wide changes in organ site-specific procedure volume over time, the odds of surgery at a LVH decreased annually for esophageal (≤ 3/year) and pancreatic (≤ 4/year) procedures: per year odds ratio (OR) of 0.87 (95% CI, 0.85 to 0.90) and 0.85, respectively (95% CI, 0.84 to 0.87). There were small changes in the odds of having surgery at a LVH for colon (≤ 37/year) and rectal (≤ 11/year) cancer procedures, as well: per year OR 0.97 (95% CI 0.97 to 0.98) and 1.02 (95% CI, 1.01 to 1.03), respectively. Over the entire study period, this translated to substantial decreases in the likelihood of surgery at a LVH for esophageal (OR, 0.25) and pancreatic (OR, 0.20) procedures. In absolute terms, the number of esophagectomies performed at LVH (≤ 3/year) decreased from 36% (130 of 362) to 14% (92 of 681), and the number of pancreatectomies performed at LVH (≤ 4/year) decreased from 36% (280 of 777) to 12% (209 of 1,718).
Mortality
For the study area, in-hospital mortality for esophageal resections declined from 8.15% to 3.12% from 1996 to 2006 (P = .038). Similarly, in-hospital mortality for pancreatic resections declined from 7.31% to 3.84% (P = .001). In-hospital mortality declined from 3.33% to 2.64% (P = .002) for colon cancer surgery. Mortality for rectal resections was very low and did not change significantly over time (1.84% in 1996, 1.57% in 2006; P = .703).
Travel Distance
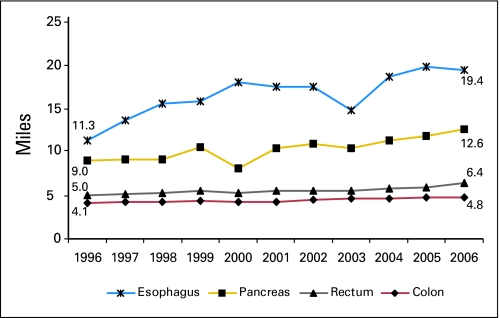
Throughout the study period, travel distance was proportional to hospital procedure volume (Table 1). For each site, an increase in median travel distance, roughly proportional to the degree of centralization, was observed over time: esophagus 72% (P < .001), pancreas 40% (P < .001), colon 17% (P < .001), and rectum 28% (P < .001; Fig 3).
Table 1.
Distance Traveled (miles) by Hospital Procedure Volume (1996 to 2006)
| Organ | Volume |
P | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Very Low |
Low |
Medium |
High |
Very High |
|||||||
| Median | IQ Range | Median | IQ Range | Median | IQ Range | Median | IQ Range | Median | IQ Range | ||
| Esophagus | 3.6 | 1.7-9.9 | 6.2 | 2.5-14.6 | 11.4 | 4.4-30.7 | 22.7 | 9.0-46.0 | 29.8 | 13.4-57.5 | < .001 |
| Pancreas | 3.5 | 1.6-8.3 | 4.7 | 2.1-9.9 | 7.0 | 3.0-18.0 | 13.4 | 6.3-30.4 | 20.4 | 9.6-43.4 | < .001 |
| Colon | 3.2 | 1.3-8.3 | 3.3 | 1.5-8.1 | 4.3 | 1.9-9.3 | 4.6 | 2.1-9.1 | 5.9 | 2.9-11.9 | < .001 |
| Rectum | 3.6 | 1.4-8.9 | 4.3 | 1.9-9.2 | 4.8 | 2.2-9.9 | 6.4 | 3.0-14.3 | 10.3 | 4.8-24.5 | < .001 |
Abbreviation: IQ, interquartile.
Fig 3.
Travel distance over time. Median straight-line distance from patient zip code to treating facility in miles.
The 10.2% of patients (27,848 of 271,986) who lived in rural counties traveled further than other patients for all disease sites. In 1996 to 1997, rural patients traveled a median 35.9, 35.9, 5.4, and 6.2 miles further than nonrural patients for esophageal, pancreatic, colon, and rectal resections, respectively (P < .001 in all cases). In 2005 to 2006, the differences between rural and nonrural travel were 38.9, 35.9, 6.5, and 9.4 miles, respectively (P < .001 in all cases). The increasing effect of rurality on travel distance over time was significant for all sites, except pancreas (esophagus P = .028; pancreas P = .977; colon P < .001; rectum P < .001).
We performed additional analyses to examine whether the increase in travel distance could be attributed directly to centralization. The median distance traveled increased by 0.73 miles from 1996 to 1997 to 2005 to 2006 for rectal cancer (P < .001), 5.02 miles for esophageal cancer (P < .001), 3.14 miles for pancreatic cancer (P < .001), and 0.47 miles for colon cancer (P < .001). After controlling for treatment in the highest volume tercile, distance traveled still increased by 0.83 miles for rectal cancer (P < .001), but only 1.16 miles for esophageal cancer (P = .001), and 0.25 miles for colon cancer. After controlling for treatment at a HVH, the median distance traveled for pancreatic cancer actually decreased 0.48 miles between 1996 to 1997 and 2005 to 2006 (P = .025). The attenuation of the year effect after controlling for volume was statistically significant in all cases (P < .001), providing strong evidence for a causal relationship between centralization and the increasing travel distance.
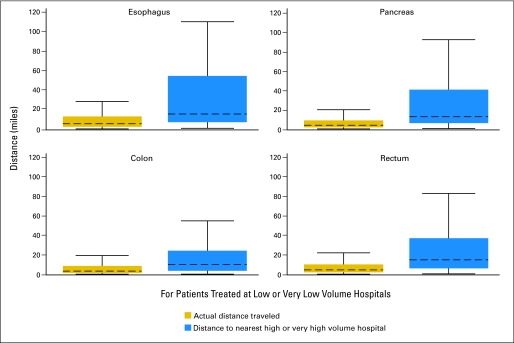
For each organ site, a substantial portion of patients (esophagus 14.8%, pancreas 13.3%, colon 22.6%, rectum 17.5%) treated at LVH or VLVH in 2006 traveled equal or further distance to reach that hospital than they would have had to travel to reach the nearest existing HVH or VHVH in the study area. The majority of patients (esophagus 54%, pancreas 59%, colon 62%, rectum 55%) who had surgery at a LVH or VLVH in 2006 could have reached an existing HVH or VHVH by traveling ≤ 10 additional miles. For patients treated at LVH and VLVH, the median travel distance to the nearest HVH or VHVH in the study area was less than 15.5 miles (esophagus 15.3, pancreas 12.5, colon 10.1, rectum 14.6 miles; Fig 4). Still, in 2006, a modest percentage of all patients from the study area lived ≥ 60 miles from the nearest HVH or VHVH. The less common the procedure, the greater the percentage of patients who lived ≥ 60 miles from the nearest HVH or VHVH: esophagus 11.9% (75 of 628), pancreas 9.0% (146 of 1,631), colon 1.4% (258 of 18,928), rectum 5.5% (217 of 3,925).
Fig 4.
Difference between actual distances traveled and distances to nearest high volume hospital. Comparison of actual distances traveled to reach treating facility and distances to nearest high or very high volume hospital in 2006 for patients from NJ, NY, and PA who underwent surgery at a low or very low volume hospital in the study area. Dashed line represents median. Box represents interquartile (IQ) range. Outliers (values > 75th percentile + 1.5 × IQ range) were omitted from the graphic for ease of interpretation.
Disparities
For each organ site, there were differences in race, payer, socioeconomic status, and rurality between patients treated at LVH and those treated at higher volume hospitals (Table 2). In general, patients at LVH were more likely to be black, have Medicaid, Medicare, or no insurance, be from nonmetropolitan areas, and be from areas with higher poverty than patients treated at higher volume hospitals. No consistent pattern of improvement or deterioration over time could be identified.
Table 2.
Sociodemographic Differences in Odds Ratios of Having Surgery at Low Volume Hospital
| Variable | Rectum |
Colon |
Esophagus |
Pancreas |
||||
|---|---|---|---|---|---|---|---|---|
| 1996 to 1997 | 2005 to 2006 | 1996 to 1997 | 2005 to 2006 | 1996 to 1997 | 2005 to 2006 | 1996 to 1997 | 2005 to 2006 | |
| Metropolitan | ||||||||
| Yes | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-metropolitan | 4.42*† | 2.86*† | 4.54* | 4.95* | 0.70 | 1.07 | 1.62‡ | 0.86 |
| Race | ||||||||
| White | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Black | 1.47§ | 1.48* | 1.36* | 1.36* | 0.93∥ | 3.22*∥ | 1.61∥ | 2.16*∥ |
| Asian | 0.89 | 0.72 | 0.96 | 1.29‡ | 0.50 | 1.25 | 1.60 | 0.89 |
| Unknown | 1.29*¶ | 0.97¶ | 0.83*† | 1.26*† | 0.93 | 1.17 | 1.42§ | 1.18 |
| Insurance | ||||||||
| Private insurance | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Medicaid | 2.20* | 2.01* | 1.96* | 1.97* | 1.39 | 1.82 | 3.61* | 2.78* |
| Medicare | 1.26* | 1.44* | 1.26* | 1.31* | 1.39 | 1.58‡ | 1.27‡ | 1.35‡ |
| Uninsured | 2.34* | 1.70‡ | 2.57*¶ | 3.85*¶ | 2.42 | 2.71 | 3.22§ | 4.51* |
| Unknown insurance | 1.08∥ | 1.94§∥ | 1.97* | 1.83* | 1.28 | 2.59 | 0.55 | 3.04§ |
| Sex | ||||||||
| Female | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Male | 1.09 | 1.04 | 0.99 | 1.02 | 0.95 | 0.74 | 0.94 | 1.09 |
| Poverty, %# | ||||||||
| 0-5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6-20 | 1.46* | 1.50* | 1.70* | 1.69* | 1.23 | 1.61‡ | 1.26 | 1.38‡ |
| > 20% | 1.68* | 1.72* | 1.71*† | 2.23*† | 3.20* | 2.39§ | 0.86 | 1.13 |
NOTE. Multivariable analysis examining odds of having surgery at a low volume hospital (1996 tercile cutpoints) in 1996 to 1997 compared with 2005 to 2006 for various sociodemographic factors.
Odds ratio is significant (P ≤ .001) compared to referent category for given site and year.
Change in odds ratio for category is significant (P ≤ .001) over time for given site.
Odds ratio is significant (P ≤ .05) compared to referent category for given site and year.
Odds ratio is significant (P ≤ .01) compared to referent category for given site and year.
Change in odds ratio for category is significant (P ≤ .05) over time for given site.
Change in odds ratio for category is significant (P ≤ .01) over time for given site.
Percentage of residents in census tract living at or below 100% poverty line.
DISCUSSION
Centralization of cancer care has been documented to varying degrees throughout Canada and Europe,31–34 but little information has previously been available regarding the degree to which cancer surgery has been centralized in the United States.24–28 We demonstrated that over the past decade in the United States, the majority of procedures for esophageal and pancreatic cancer have been centralized to HVH. Meanwhile for colorectal cancer, there has been little change.
The volume-outcome relationships for esophagectomy and pancreatectomy have received more consistent national and international attention than the volume-outcome relationships for colorectal surgery, possibly one explanation for the differing degrees to which these procedures have been centralized.8,9,33,34 The focus of studies addressing the more complex procedures has been differences in perioperative mortality,2,4,5,35,36 while most of the compelling data for colorectal surgery have addressed intermediate outcomes such as nodal yield,17,37–39 complication rates,40–42 length of stay,41,42 ostomy rates,17,37,42–44 and local recurrence rates.43,45 Stakeholders have placed more emphasis on minimizing perioperative mortality than on improving other clinical outcomes.8,9
The degree to which cancer surgery has become centralized is likely dependent on many factors. For example, the scope of practice for surgeons, in general, is narrowing.46 Less frequent, more complex operations are increasingly deferred to a small group of subspecialists, while more common operations, such as colectomy, remain the purview of a larger group of surgeons, including general surgeons, surgical oncologists, minimally invasive surgeons, and colorectal surgeons. As a result, surgeons, themselves, may be an influential force behind the degree to which cancer care is centralized.
Other difficult to quantify factors also contribute to the process. Health care systems encourage referral to in-system providers as a means of maintaining market share; physicians establish referral patterns, based on factors such as ease of referral and likelihood of retaining patients after surgery; and, payers designate preferred in-network providers. Each of these factors may alternately promote or impede referral to HVH depending on the situation. Ultimately, individual patient choice is only one of many factors influencing where a procedure is performed, and quality improvement initiatives need to be targeted accordingly.
An unresolved issue is the question of where to set the bar.47,48 Studies have been unable to determine threshold volumes above which outcomes are generally regarded as acceptable. While few would contend that one to two esophagectomies or pancreatectomies per year is adequate volume, it is possible that eight proctectomies or 25 colectomies may be sufficient, even though these numbers would have placed hospitals in the very low volume category for this study. In addition, which clinical outcomes should be used to determine benchmark volume standards needs to be determined. While perioperative mortality is easy to study and suitable for some procedures, other outcomes (ie, local control or morbidity) may be more appropriate for procedures with low expected mortality. The Leapfrog group set volume standards for pancreatectomy (11/year) and esophagectomy (13/year).9 If national quality initiatives set similar volume standards for colorectal surgery, increased centralization of these more common procedures might ensue. In contrast, if studies demonstrate that the bar does not need to be set high to achieve acceptable clinical outcomes, then adequate volume may be achieved by many hospitals without the need for major changes in cancer care delivery. Until validated minimum volume standards are established, the need for and appropriateness of further centralization cannot be fully assessed.
Previous studies speculate about changes in travel distance using existing hospital volume and location measurements. These methods do not account for changes in hospital volume over time and may underestimate or misjudge the quantity and distribution of HVH.21,49,50 In this observational study, we documented that the number of hospitals meeting HVH criteria changed considerably over time. However, even with significant increases in HVH, overall travel distance for pancreas and esophageal cancer patients increased substantially with centralization. While most patients remaining at LVH could reach an existing HVH with little to no increase in travel, there are a modest number of patients who travel or would have to travel very long distances (≥ 60 miles) to reach the nearest HVH. Thus far, travel distance has not prevented centralization of esophagectomy or pancreatectomy, but the implications of increasing distance should not be ignored. Even small increases in distance may impose a substantial barrier for subsets of the population. As the cost of travel increases, the extent to which travel distance serves as a barrier to cancer care is likely to increase.
Centralization has the potential to introduce a capacity problem. To accommodate more patients at HVH without increasing wait times, existing HVH need to increase capacity or new HVH need to be developed. Figure 2 demonstrates that for esophageal and pancreatic cancer, the number of HVH is increasing. Meanwhile the caseload of the existing HVH also increased over time. Although wait times need to be monitored, previous work suggests that extensive centralization is possible without increases in wait time.32
As reported in previous studies, the majority of patients remaining at LVH could reach an existing HVH with minimal increase in travel distance.49 Other barriers aside from travel distance have been consistently demonstrated.2,13–19 We found marked differences in race, socioeconomic status, and payer among patients treated at HVH versus LVH. We hypothesized that centralization would exacerbate existing disparities as empowered patients shifted to HVH leaving only disadvantaged patients at LVH, where volume would decrease further over time. While the data do not support this a priori hypothesis, the significance of existing disparities should not be understated.
Our study included all hospital discharges for a large geographically diverse area. Consequently, we were able to study shifts in patterns of care over time. Since the examined area was a large, contiguous geographic region with little border crossing, the influence of adjacent, noncaptured centers was negligible. While these are strengths of the data, there are limitations. For example, only 10% of our study population lived in counties designated as rural. As a result, this study may underestimate the impact of centralization on travel distance for patients from more rural areas of the country.
Since discharge data do not provide staging information, we were unable to examine the likelihood of receiving standard of care therapy. It is possible that patients facing long travel distances may have been more likely to forgo or delay surgery. Since only patients who underwent surgical resection were examined, this study cannot completely assess the degree to which travel burden served as a barrier to care.
Straight-line distance is an adequate proxy for travel time,52,53 but these measurements are known to underestimate road distance by at least 20% to 30%.29,54 Examining a random sample of all patients from our study, we estimate that straight-line distance underestimated true travel distance by 35% to 40%. Consequently, for this study, travel distance should be viewed in terms of relative mileage differences and percent change over time, rather than absolute mile measurements.
This study was not designed to identify a causal relationship between centralization of cancer surgery and clinical outcomes. As a result, one cannot know whether population-wide improvements in perioperative mortality are due directly to centralization of care. However, there have been significant improvements in in-hospital mortality for complex cancer procedures coincident with increasing centralization of cancer surgery, and it is quite plausible that these gains are attributable to centralization.
Although there is no uniform consensus regarding what surgical volumes are sufficient for adequate clinical outcomes, the wealth of published literature supports a relevant relationship between procedure volume and outcomes for cancer surgery. Accordingly, there has been extensive centralization of complex cancer surgery over the past decade. Centralization continues without evidence of remitting. While this process can be expected to result in population level improvements in cancer outcomes, centralization is substantially increasing the travel distance for patients with cancer. Thus far, travel burden has not prevented centralization of complex cancer surgery; however, the impact of increasing travel distances needs to be monitored. The persistence of other sociodemographic barriers to HVH also needs to be addressed. The challenge remains to find ways to improve access to high quality cancer care for all patients.
Appendix
Detailed methods.
A secondary data analysis was performed using inpatient discharge data from NJ, NY, and PA. Each state mandates reporting of claims in a deidentified format from all nonfederal hospitals and surgery centers. (In NJ, freestanding surgery centers are not required to participate.) Databay Resources (Warrendale, PA) acquires the claims data from the individual state agencies (State of NJ Department of Health and Senior Services, State of NY Statewide Planning and Research Cooperative System, and PA Healthcare Cost Containment Council) and produces a database product suitable for secondary analysis. Available data include demographic information (including race as documented in the medical record), clinical diagnoses, procedure codes, length of stay, disposition at discharge, and hospital charges.
All patients ≥ 18 years of age undergoing procedures at nonfederal hospitals in the service area (NJ, NY, PA) from 1996 to 2006 were included. Patients residing in the service area who underwent surgery outside of the service area (outward border crossers) were not captured; however, patients residing outside the service area who had surgery in the service area (inward border crossers) were included. From 1996 to 1999, only NY and PA data were available. Starting in 2000, procedures from all three states were included. To account for the limited years of data for NJ, regression analyses were adjusted for state.
The most common primary gastrointestinal malignancies were chosen for study. International Classification of Diseases 9th revision procedure codes (423, 423.2, 424-424.2, 457-457.9, 458, 483, 483.5, 484, 484.1, 484.9, 485, 486-486.9, 522, 522.2, 525-525.9, 526, 527) captured all pancreatectomies, esophagectomies, colectomies, and proctectomies, as well as local surgical excision of masses at these sites (ie, transanal excision of rectal mass). Endoscopic resections were excluded. The study group was further limited to cases with an International Classification of Diseases 9th revision diagnosis code for neoplasm (140-239.9).
Cases were geocoded to zip code centroid. Zip code of residence was available for every case. Using a publically available geocoding tool (Excel geocoding tool version 2, Juice Analytics, Herndon, VA; referencing Yahoo! Maps Web Services-Geocoding API, Yahoo, Sunnyvale, CA), zip codes were assigned a latitude and longitude. Once geocoded, patients were matched to publicly available area-based sociodemographic data, specifically year 2000 census tract poverty rate (% of census tract at or below 100% poverty line) and county-level United States Department of Agriculture rural-urban continuum codes. Hospitals were geocoded to actual street address. Using the great-circle distance formula [d = acos(sin(lat1) · sin(lat2) + cos(lat1) · cos(lat2) · cos(long2−long1)) · R], straight-line distance was calculated between cases and the hospitals at which the procedures were performed. (Fortney J, Rost K, Warren J: Health Serv Outcomes Res Methodol 1:173-184, 2000) The distance from patient zip code centroid to the nearest high or very high volume hospital was also calculated.
Hospital procedure volume of extirpative procedures for each organ site was calculated for each year individually, allowing for the possibility that volume at a given hospital could change over time. Although all cases were included for determination of hospital procedure volume, inward border crossers (cases for which the patient's address was outside of the service area [NJ, NY, PA]) were excluded from the distance and disparities analyses in order to minimize the impact of outliers on inferences. The mean travel distance for this group, which included overseas travelers, was more than 500 miles, and inclusion of this small, distinct group would have disproportionately influenced the results.
Previous studies (Billingsley KG, Morris AM, Dominitz JA, et al: Arch Surg 142:23-31, 2007; discussion 32, 2007; Finlayson EV, Goodney PP, Birkmeyer JD: Arch Surg 138:721-725, 2003; discussion 726, 2003; Hannan EL, Radzyner M, Rubin D, et al: Surgery 131:6-15, 2002; Kuo EY, Chang Y, Wright CD: Ann Thorac Surg 72:1118-1124, 2001; Lerut T, Nafteux P, Moons J, et al: Eur J Surg Oncol 31:587-594, 2005; Meyerhardt JA, Catalano PJ, Schrag D, et al: Ann Intern Med 139:649-657, 2003; Rabeneck L, Davila JA, Thompson M, et al: Am J Gastroenterol 99:668-675, 2004; Steyerberg EW, Neville BA, Koppert LB, et al: J Clin Oncol 24:4277-4284, 2006; Swisher SG, Deford L, Merriman KW, et al: J Thorac Cardiovasc Surg 119:1126-1132, 2000) examining the relationship between hospital procedure volume and outcomes for esophageal, pancreatic, and colorectal cancer surgery have used a wide variety of methods to define low and high volume hospitals. Since standard cut points and methodology do not exist, we chose to define volume categories based on actual 1996 hospital procedure volume in the study area. Five roughly equal-sized groups of patients were created for each organ site in 1996 based on procedure volume of the treating hospital. Integer cut points were chosen that allowed for the most even distribution of cases between the five groups. These quintiles were designated very low, low, medium, high, and very high volume. The 1996 cut points were then applied to each subsequent year in order to determine changes in the distribution of patients among volume categories. Similarly, 1996 tercile integer cut points were created for each organ site. While the quintile measures were generally more descriptive, the tercile cut points were used in the regression analyses to minimize the impact of outlier hospitals.
Multiple logistic regressions provided estimates of the odds of receiving care at a hospital in the lowest volume tercile over time, examining year as a continuous variable. Since changes in overall market procedure volume could impact individual hospital volume, we also controlled for the overall number of cases performed in the study area annually. For example, the odds of undergoing pancreatic resection for cancer at a low volume hospital were adjusted for the total number of extirpative pancreatic cancer procedures performed in NJ, NY, and PA during the given year.
Using the same data set, annual market-wide in-hospital mortality was calculated for each organ site. Since inpatient discharge databases provide information only on discrete inpatient encounters and cannot be linked to other admissions or external clinical data sources, mortality numbers include only deaths that occurred in the course of the admission during which the operative procedure was performed. Deaths that occurred after the initial postoperative discharge are not captured.
We used multiple quantile regressions to investigate median time trends in distance traveled. A multiple quantile regression is similar to multiple linear regression, but models the median rather than mean outcome. Since the distance variable was highly skewed, the results are most accurately reported as medians. To establish time trends, the first models included cancer site, year (1996 to 1997 v 2005 to 2006), and interactions between site and year. We examined trends using year entered as a dichotomous term (1996 to 1997 v 2005 to 2006, with only individuals from those years included in the analysis). We added an indicator variable of treatment in the highest volume tercile to investigate if coefficients of the year main effects and interaction terms changed after controlling for high volume tercile. Wald tests with bootstrap SEs (1,000 bootstrap samples) were used to test statistical significance (Efron B, Tibshirani RJ: New York, Chapman & Hall/CRC, 1993).
We used similar multiple quantile regressions to investigate if time trends in distance varied by rurality status and to investigate the relationship between median distance and the number of available high volume hospitals. Using multiple logistic regressions, we investigated the association between sociodemographic factors (census tract poverty rate, county rurality, sex, age, race, and primary payer) and the odds of having a surgery at a low volume hospital in 1996 to 1997 and 2005 to 2006 by cancer site. Interaction models were used to investigate if the strength of associations changed between the two time points.
All hypothesis tests were two sided, and the criterion for statistical significance was a P value ≤ .05. Data were deidentified, and the study was approved by the institutional review board of Fox Chase Cancer Center.
Footnotes
Supported by the John A. Ridge, MD, PhD, Surgical Oncology Fellowship at Fox Chase Cancer Center (K.B.S.); and by Grant No. P30 CA 06927 from the National Institutes of Health (B.L.E.). These funding sources had no role in the design and conduct of the study nor the collection, management, analysis, and interpretation of the data, nor the preparation, review, or approval of the manuscript.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Karyn B. Stitzenberg, Neal J. Meropol
Administrative support: Karyn B. Stitzenberg, Russell B. Starkey
Collection and assembly of data: Karyn B. Stitzenberg, Russell B. Starkey
Data analysis and interpretation: Karyn B. Stitzenberg, Elin R. Sigurdson, Brian L. Egleston, Russell B. Starkey, Neal J. Meropol
Manuscript writing: Karyn B. Stitzenberg, Elin R. Sigurdson, Brian L. Egleston, Russell B. Starkey, Neal J. Meropol
Final approval of manuscript: Karyn B. Stitzenberg, Elin R. Sigurdson, Brian L. Egleston, Russell B. Starkey, Neal J. Meropol
REFERENCES
- 1.Luft HS, Bunker JP, Enthoven AC. Should operations be regionalized? The empirical relation between surgical volume and mortality. N Engl J Med. 1979;301:1364–1369. doi: 10.1056/NEJM197912203012503. [DOI] [PubMed] [Google Scholar]
- 2.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Sun Y, Wong SL, et al. Hospital volume and late survival after cancer surgery. Ann Surg. 2007;245:777–783. doi: 10.1097/01.sla.0000252402.33814.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman MD, Kilburn H, Lindsey M, et al. Relation of perioperative deaths to hospital volume among patients undergoing pancreatic resection for malignancy. Ann Surg. 1995;222:638–645. doi: 10.1097/00000658-199511000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flood AB, Scott WR, Ewy W. Does practice make perfect? Part I: The relation between hospital volume and outcomes for selected diagnostic categories. Med Care. 1984;22:98–114. [PubMed] [Google Scholar]
- 7.Balzano G, Zerbi A, Capretti G, et al. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95:357–362. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt M, Petitti D. Interpreting the Volume-Outcome Relationship in the Context of Cancer Care. Washington, DC: National Academies Press; 2001. p. 32. [PubMed] [Google Scholar]
- 9.The Leapfrog Group. Evidenced Based Hospital Referral. Washington, DC: The Leapfrog Group c/o Academy Health; 2008. pp. 1–3. [Google Scholar]
- 10.van Lanschot JJ, Hulscher JB, Buskens CJ, et al. Hospital volume and hospital mortality for esophagectomy. Cancer. 2001;91:1574–1578. doi: 10.1002/1097-0142(20010415)91:8<1574::aid-cncr1168>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Topal B, Van de Sande S, Fieuws S, et al. Effect of centralization of pancreaticoduodenectomy on nationwide hospital mortality and length of stay. Br J Surg. 2007;94:1377–1381. doi: 10.1002/bjs.5861. [DOI] [PubMed] [Google Scholar]
- 12.Epstein AM. Volume and outcome: It is time to move ahead. N Engl J Med. 2002;346:1161–1164. doi: 10.1056/NEJM200204113461512. [DOI] [PubMed] [Google Scholar]
- 13.Schrag D, Cramer LD, Bach PB, et al. Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA. 2000;284:3028–3035. doi: 10.1001/jama.284.23.3028. [DOI] [PubMed] [Google Scholar]
- 14.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–592. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosa JA, Bowman HM, Gordon TA, et al. Importance of hospital volume in the overall management of pancreatic cancer. Ann Surg. 1998;228:429–438. doi: 10.1097/00000658-199809000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harmon JW, Tang DG, Gordon TA, et al. Hospital volume can serve as a surrogate for surgeon volume for achieving excellent outcomes in colorectal resection. Ann Surg. 1999;230:404–411. doi: 10.1097/00000658-199909000-00013. discussion 411-413, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodgson DC, Zhang W, Zaslavsky AM, et al. Relation of hospital volume to colostomy rates and survival for patients with rectal cancer. J Natl Cancer Inst. 2003;95:708–716. doi: 10.1093/jnci/95.10.708. [DOI] [PubMed] [Google Scholar]
- 18.Morris AM, Wei Y, Birkmeyer NJ, et al. Racial disparities in late survival after rectal cancer surgery. J Am Coll Surg. 2006;203:787–794. doi: 10.1016/j.jamcollsurg.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Liu JH, Zingmond DS, McGory ML, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296:1973–1980. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 20.Ward MM, Jaana M, Wakefield DS, et al. What would be the effect of referral to high-volume hospitals in a largely rural state? J Rural Health. 2004;20:344–354. doi: 10.1111/j.1748-0361.2004.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 21.Dimick JB, Finlayson SR, Birkmeyer JD. Regional availability of high-volume hospitals for major surgery. Health Aff (Millwood) Web Exclusives. 2004;(suppl):VAR45–VAR53. doi: 10.1377/hlthaff.var.45. [DOI] [PubMed] [Google Scholar]
- 22.Finlayson EV, Birkmeyer JD. Effects of hospital volume on life expectancy after selected cancer operations in older adults: A decision analysis. J Am Coll Surg. 2003;196:410–417. doi: 10.1016/S1072-7515(02)01753-2. [DOI] [PubMed] [Google Scholar]
- 23.Effective health care. Hospital volume and health care outcomes, costs and patient access. NHS Centre for Reviews and Dissemination. 1996;2:1–14. [Google Scholar]
- 24.Riall TS, Eschbach KA, Townsend CM, Jr, et al. Trends and disparities in regionalization of pancreatic resection. J Gastrointest Surg. 2007;11:1242–1251. doi: 10.1007/s11605-007-0245-5. discussion 1251-1252, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Ho V, Heslin MJ, Yun H, et al. Trends in hospital and surgeon volume and operative mortality for cancer surgery. Ann Surg Oncol. 2006;13:851–858. doi: 10.1245/ASO.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Gordon TA, Bowman HM, Tielsch JM, et al. Statewide regionalization of pancreaticoduodenectomy and its effect on in-hospital mortality. Ann Surg. 1998;228:71–78. doi: 10.1097/00000658-199807000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodney PP, Siewers AE, Stukel TA, et al. Is surgery getting safer? National trends in operative mortality. J Am Coll Surg. 2002;195:219–227. doi: 10.1016/s1072-7515(02)01228-0. [DOI] [PubMed] [Google Scholar]
- 28.McPhee JT, Hill JS, Whalen GF, et al. Perioperative mortality for pancreatectomy: A national perspective. Ann Surg. 2007;246:246–253. doi: 10.1097/01.sla.0000259993.17350.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fortney J, Rost K, Warren J. Comparing alternative methods of measuring geographic access to health services. Health Serv Outcomes Res Methodol. 2000;1:173–184. [Google Scholar]
- 30. Reference deleted.
- 31.Al-Sarira AA, David G, Willmott S, et al. Oesophagectomy practice and outcomes in England. Br J Surg. 2007;94:585–591. doi: 10.1002/bjs.5805. [DOI] [PubMed] [Google Scholar]
- 32.Forshaw MJ, Gossage JA, Stephens J, et al. Centralisation of oesophagogastric cancer services: Can specialist units deliver? Ann R Coll Surg Engl. 2006;88:566–570. doi: 10.1308/003588406X130624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer B. Role of volume outcome data in assuring quality in HPB surgery. HPB (Oxford) 2007;9:330–334. doi: 10.1080/13651820701611234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Heek NT, Kuhlmann KF, Scholten RJ, et al. Hospital volume and mortality after pancreatic resection: A systematic review and an evaluation of intervention in the Netherlands. Ann Surg. 2005;242:781–788. doi: 10.1097/01.sla.0000188462.00249.36. discussion 788-790, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patti MG, Corvera CU, Glasgow RE, et al. A hospital's annual rate of esophagectomy influences the operative mortality rate. J Gastrointest Surg. 1998;2:186–192. doi: 10.1016/s1091-255x(98)80011-5. [DOI] [PubMed] [Google Scholar]
- 36.Glasgow RE, Mulvihill SJ. Hospital volume influences outcome in patients undergoing pancreatic resection for cancer. West J Med. 1996;165:294–300. [PMC free article] [PubMed] [Google Scholar]
- 37.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of hospital procedure volume on surgical operation and long-term outcomes in high-risk curatively resected rectal cancer: Findings from the Intergroup 0114 study. J Clin Oncol. 2004;22:166–174. doi: 10.1200/JCO.2004.04.172. [DOI] [PubMed] [Google Scholar]
- 38.Miller EA, Woosley J, Martin CF, et al. Hospital-to-hospital variation in lymph node detection after colorectal resection. Cancer. 2004;101:1065–1071. doi: 10.1002/cncr.20478. [DOI] [PubMed] [Google Scholar]
- 39.Rogers SO, Jr, Wolf RE, Zaslavsky AM, et al. Relation of surgeon and hospital volume to processes and outcomes of colorectal cancer surgery. Ann Surg. 2006;244:1003–1011. doi: 10.1097/01.sla.0000231759.10432.a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Farber BF, Kaiser DL, Wenzel RP. Relation between surgical volume and incidence of postoperative wound infection. N Engl J Med. 1981;305:200–204. doi: 10.1056/NEJM198107233050405. [DOI] [PubMed] [Google Scholar]
- 41.Kuhry E, Bonjer HJ, Haglind E, et al. Impact of hospital case volume on short-term outcome after laparoscopic operation for colonic cancer. Surg Endosc. 2005;19:687–692. doi: 10.1007/s00464-004-8920-z. [DOI] [PubMed] [Google Scholar]
- 42.Marusch F, Koch A, Schmidt U, et al. Hospital caseload and the results achieved in patients with rectal cancer. Br J Surg. 2001;88:1397–1402. doi: 10.1046/j.0007-1323.2001.01873.x. [DOI] [PubMed] [Google Scholar]
- 43.Ptok H, Marusch F, Kuhn R, et al. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol. 2007;33:854–861. doi: 10.1016/j.ejso.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 44.Simons AJ, Ker R, Groshen S, et al. Variations in treatment of rectal cancer: The influence of hospital type and caseload. Dis Colon Rectum. 1997;40:641–646. doi: 10.1007/BF02140891. [DOI] [PubMed] [Google Scholar]
- 45.Wibe A, Eriksen MT, Syse A, et al. Effect of hospital caseload on long-term outcome after standardization of rectal cancer surgery at a national level. Br J Surg. 2005;92:217–224. doi: 10.1002/bjs.4821. [DOI] [PubMed] [Google Scholar]
- 46.Stitzenberg KB, Sheldon GF. Progressive specialization within general surgery: Adding to the complexity of workforce planning. J Am Coll Surg. 2005;201:925–932. doi: 10.1016/j.jamcollsurg.2005.06.253. [DOI] [PubMed] [Google Scholar]
- 47.Metzger R, Bollschweiler E, Vallbohmer D, et al. High volume centers for esophagectomy: What is the number needed to achieve low postoperative mortality? Dis Esophagus. 2004;17:310–314. doi: 10.1111/j.1442-2050.2004.00431.x. [DOI] [PubMed] [Google Scholar]
- 48.Christian CK, Gustafson ML, Betensky RA, et al. The Leapfrog volume criteria may fall short in identifying high-quality surgical centers. Ann Surg. 2003;238:447–455. doi: 10.1097/01.sla.0000089850.27592.eb. discussion 455-457, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003;290:2703–2708. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]
- 50.Dudley RA, Johansen KL, Brand R, et al. Selective referral to high-volume hospitals: Estimating potentially avoidable deaths. JAMA. 2000;283:1159–1166. doi: 10.1001/jama.283.9.1159. [DOI] [PubMed] [Google Scholar]
- 51. Reference deleted.
- 52.Phibbs CS, Luft HS. Correlation of travel time on roads versus straight line distance. Med Care Res Rev. 1995;52:532–542. doi: 10.1177/107755879505200406. [DOI] [PubMed] [Google Scholar]
- 53.McGuirk MA, Porell FW. Spatial patterns of hospital utilization: The impact of distance and time. Inquiry. 1984;21:84–95. [PubMed] [Google Scholar]
- 54.Williams AP, Schwartz WB, Newhouse JP, et al. How many miles to the doctor? N Engl J Med. 1983;309:958–963. doi: 10.1056/NEJM198310203091606. [DOI] [PubMed] [Google Scholar]