Abstract
CD44 is a cell surface receptor for the extracellular matrix macromolecule hyaluronan. In addition, CD44 mediates the endocytosis of hyaluronan leading to its subsequent degradation within lysosomes. Using model systems of COS-7 and Flp-293 cells, we demonstrate that the association of CD44 with lipid rafts is essential for the endocytosis of hyaluronan but not the extracellular binding. Further, we demonstrate that palmitoylation of CD44 on two highly conserved cysteine residues is essential for the association with lipid rafts as determined by density gradient ultracentrifugation. Mutations of either cysteine residues or pretreatment of cells with the palmitic acid analog 2-bromopalmitate, reduced the [3H]palmitic acid incorporation into CD44 and prevented CD44-lipid rafts association. Preventing CD44 palmitoylation had no effect on the binding of hyaluronan but inhibited hyaluronan internalization. The turnover of the CD44 receptor itself was also affected by blocking its association with lipid rafts. Using cycloheximide to prevent de novo protein synthesis, palmitoylation-deficient cysteine mutants underwent slower turnover from cell surface compared with the palmitoylation-intact wild type, as determined by immunofluorescence and Western blotting. These results indicate that palmitoylation of CD44 is a critical driving determinant to CD44 association with lipid rafts and, concomitantly, the rates of hyaluronan endocytosis and CD44 turnover from cell surface.
CD44 is a single pass transmembrane glycoprotein expressed by a wide variety of cells. On many of these cells, CD44 serves as the principal receptor for the extracellular matrix macromolecule hyaluronan. For example, in articular chondrocytes CD44-hyaluronan interactions are critical to the cellular retention of the proteoglycan-rich extracellular matrix (1). CD44 also participates in receptor-mediated internalization of hyaluronan, resulting in hyaluronan degradation within low pH intracellular compartments (2–4). CD44-mediated internalization of hyaluronan occurs independent of clathrin-coated vesicles or the caveolin or pinocytosis pathways (3). In addition, the cytoplasmic domain of CD44 does not contain an AP-2 adaptor binding site (YQRL or LL) indicative of interactions with clathrin or caveolin-binding domains such as ΦXΦXXXXΦ, ΦXXXΦXXΦ, and ΦXΦXXXXΦXXΦ, where X is any amino acid and Φ is an aromatic residue (5). As such, it remains unclear as to the mechanism cells use to regulate the extent of CD44-mediated hyaluronan internalization. Although we have found various regions within the cytoplasmic domain of CD44 that are required for the mechanics of CD44-mediated endocytosis of hyaluronan (6–9), none of these interaction sites appear to regulate the process. In this study, the differential association of CD44 with lipid rafts was determined to be the major mechanism by which cells segregate pools of CD44 destined for internalization.
Lipid rafts are specialized plasma membrane microdomains rich in cholesterol and sphingolipids such as sphingomyelin and gangliosides. Various lipids as well as proteins associate with lipid rafts. Lipid rafts can have widespread function in cells, including signal transduction, receptor endocytosis, apoptosis, and cholesterol esterification (10). The association of CD44 with lipid rafts has previously been reported in other systems. For example, in EpH4 cells (immortalized mouse mammary epithelial cell line) CD44 was shown to associate with lipid rafts along with caveolin/VIP21 and an actin-binding protein annexin II, and this interaction was dependent on the presence of cholesterol and an intact cytoskeleton (11). Similarly in T cells, antibody-induced cross-linking of CD44 was capable of rearranging the actin-based cytoskeletal network followed by targeting of CD44 and certain CD44-associated kinases like Fyn and Lck into lipid rafts (12).
Certain fatty acid modifications can lend proteins the necessary hydrophobicity for such an association with lipid rafts to take place (13). One such modification is fatty acid acylation, especially palmitoylation, a dynamic, reversible, post-translational modification involving the formation of thioesters between one or more palmitate to cysteine residues on proteins. The cysteine residues optimal for palmitoylation are believed to reside on the transmembrane domain or the cytoplasmic domain or the boundary between the transmembrane/cytoplasmic domains (13). Interestingly, CD44 has two cysteine residues located in regions of the protein accessible to acylation. One of these cysteines is within the highly conserved transmembrane domain, Cys286, and another, within the highly conserved proximal cytoplasmic domain, Cys295. CD44 palmitoylation has previously been reported in human peripheral blood lymphocytes (14). In these cells, CD44 acylation was stimulated by the addition of particular cross-bridging anti-CD44 monoclonal antibodies. Such a fatty acid modification on CD44 was found to be important to the CD44-hyaluronan-mediated signal transduction. Palmitoylation can be involved in dictating several functions in cells. For example, hRas undergoes dual palmitoylation where both the palmitate residues were shown to have distinct roles with respect to membrane affinity, lateral segregation, and protein trafficking from the Golgi to the plasma membrane (15). Similarly, CCR5, a chemokine co-receptor for HIV-1,2 undergoes palmitoylation, which in turn affects the degradation of the molecule (16). In this case, the loss of palmitoylation led to faster endocytosis and degradation of the mutant protein compared with the palmitoylation-intact wild-type receptor (16). These examples present the evidence for a role of palmitoylation in protein membrane affinity and receptor turnover.
In this report, we demonstrate that a pool of CD44 is associated with a cholesterol-rich microdomain of the plasma membrane and, that this association is dependent on the dual acylation of Cys286 and Cys295. Prevention of CD44 localization within lipid rafts does not interfere with the ability of the receptor to bind to hyaluronan but blocks hyaluronan internalization as well as the turnover/cycling of the receptor itself.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was obtained from Cellgro (Herndon, VA). Fetal bovine serum was purchased from HyClone (South Logan, UT). Specific primers for CD44 detection and mutagenesis were custom made by Integrated DNA Technologies (Coralville, IA). The expression plasmid construct, human pCD44H, was the same as described previously (7). pCDM8 and pTracer-SV40 vectors, the Flp-In stable transfectants system, l-glutamine, hygromycin, Lipofectamine 2000, and the anti-V5 monoclonal antibody were all obtained from Invitrogen. The pDsRed2-C1 vector was purchased from Clontech (Palo Alto, CA). QuikChange site-directed mutagenesis kit was purchased from Stratagene (La Jolla, CA). Anti-human CD44 antibody BU-52 was purchased from ID Labs (London, Ontario, Canada). Luminol reagent was obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and Clear Blue x-ray film from Pierce (Rockford, IL). The [3H]palmitate (45 Ci/mmol) was from PerkinElmer Life Sciences. The nuclear stain, 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) and NeutrAvidin-Texas Red were obtained from Molecular Probes, Inc. (Eugene, OR). Cyanine-conjugated goat anti-mouse IgG (Cy3-AffiPure H + L IgG) was purchased from Jackson ImmunoResearch (West Grove, PA). The anti-flotillin-1 antibodies were obtained from BD Pharmingen (San Jose, CA). Biotinylated hyaluronan-binding protein was purchased from Seikagaku (Falmouth, MA). All other enzymes and chemicals, either for molecular biology or as reagent grade materials, were purchased from Sigma.
Cell Culture and Transfections
COS-7 cells (simian virus 40-transformed African green monkey epithelial cells, ATCC) and Flp-HEK 293 (human embryonic kidney epithelial kidney cells (Invitrogen) were cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics. The COS-7 cells were transiently transfected as previously described (7) using Lipofectamine 2000 with cDNA encoding the hematopoietic isoform of human CD44 (CD44H) as well as various point mutation isoforms of CD44H (CD44-C286A, CD44-C286S, or CD44-C286,295A). In this study, CD44H will be denoted as wild-type CD44 or CD44wt. The CD44 cDNAs were all subcloned into pCDM8 plasmid vectors. All experiments in COS-7 cells were initiated 24 h post-transfection.
Generation of CD44 Stable Transfectants
Stable transfectants expressing recombinant CD44H or various CD44H cysteine mutants were established using the Flp-In system. Briefly, sub-confluent HEK-293 cells, containing a single pre-engineered flp recombinase target site (FRT) within the genome, were co-transfected with an insert shuttle vector pCDNA5/FRT (carrying the CD44 cDNA insert) together with a plasmid expressing the Flp-recombinase enzyme (pOG44). The plasmid ratio of pCDNA5/FRT to pOG44 was 1:8, and the co-transfections were facilitated using Lipofectamine 2000. Five hours post-transfection the media was changed with fresh DMEM containing 20% fetal bovine serum. After 48 h of transfection, the cells were trypsinized and plated into four 100-mm dishes with fresh DMEM containing 5 mm l-glutamine and 10% fetal bovine serum. The cells were allowed to recover for 4 h before addition of the selection medium containing DMEM, 5 mm l-glutamine, and 50 µg/ml hygromycin. Stable, hygromycin-resistant cells were selected for at least 2 weeks with media changes every third day. After 2 weeks of selection, small clusters of cell clones were isolated, allowed to expand, and assayed for expression of CD44 protein by immunoblotting. The use of the pCDNA5/FRT shuttle vector plasmid introduces a V5 epitope tag to the amino-terminal of the CD44 as a fusion protein.
Generation of Point Mutations in CD44H
Point mutations were introduced into human CD44H cDNA cloned into a pCDM8 plasmid using the site-directed mutagenesis kit as previously reported (7). Point mutations were used to modify CD44H Cys286 into alanine or serine and Cys295 into alanine. The forward primer for CD44-C286A was 5′-GCTTTGATTCTTGCAGTTGCCATTGCAGTAAGTCG-3′, and the reverse primer was 5′-CGACTGTTGACTGCAATGGCAACTGCAAGAATCAAAGC-3′. The forward primer for CD44-C286S was 5′-GCTTTGATTCTTGCAGTTTCCATTGCAGTCAACAGTCG-3′, and the reverse primer was 5′-CGACTGTTGACTGCAATGGAAACTGCAAGAATCAAAGC-3′. To generate a double mutation, the CD44-C286A was used as a template and modified into a CD44-C286,295A using the forward primer 5′-CAGTCGAAGAAGGGCTGGGCAGAAGAAAAAGC-3′ and the reverse primer 5′-GCTTTTTCTTCTGCCCAGCCCTTCTTCGACTG-3′. All point mutations were confirmed by DNA sequence analysis at the University of Illinois sequencing laboratory core facility.
Incorporation of [3H]Palmitate into CD44
Various Flp-293 stable transfectants were plated at 2 × 106 cells/35-mm dish. Three days after plating the cells were serum-starved for 1 h and then incubated with 250 µCi of [3H]palmitate at 37 °C for 2 h. In some studies, the cells were preincubated with or without 50 µm 2-bromopalmitate (2-BP) at 37 °C overnight prior to incubation with the [3H]palmitate. The labeled cells were washed with DMEM three times and lysed in 1 ml of cold radioimmune precipitation assay buffer (10 mm Tris, 150 mm NaCl, 1% Triton X-100, pH 7.4) containing 1× protease inhibitor mixture for 30 min on ice. Lysates were clarified at 15,000 × g for 10 min at 4 °C. The lysates were immunoprecipitated using anti-V5 antibody and Sepharose-G beads overnight at 4 °C. Immunoprecipitates were collected by centrifugation, suspended in 1× SDS-PAGE sample buffer containing 50 mm dithiothreitol and subjected to SDS-PAGE. The acrylamide gel was then dehydrated in Me2SO, impregnated with 20% (w/v) of 2,5-diphenyloxazole in Me2SO and exposed to x-ray film at −80 °C for periods of 5–30 days (17). Proteins bands were visualized using a Fluor-S Multimager and Quantity One 4.1.1 software (Bio-Rad).
Optiprep Gradient Ultra Centrifugation
Flp-293 HEK stable and COS-7 transient transfectants, cultured in 60-mm dishes, were preincubated overnight with or without 50–100 µm 2-BP or; with or without 20 mm methyl-β-cyclodextrin (Mβ-CD) for 25–50 min. The cells were then washed with cold 1× PBS and lysed with 200 µl of lysis buffer (25 mm Tris-HCl, pH 7.6, 150 mm NaCl, 1 mm dithiothreitol, 10% sucrose, 1% Triton X-100, and 1× protease and phosphatase inhibitor cocktails). The residual cell material was scraped into a microcentrifuge tube and, together with the lysate buffer, incubated on ice for 30 min. The cell lysate was then mixed directly with iodixanol stock solution (60% solution of Optiprep iodixanol) to yield a 40% (v/v) iodixanol concentration and layered at the bottom of the ultracentrifuge tubes. This was overlaid with equal volumes of 35%, 30%, 25%, 20%, and 0% iodixanol solution in lysis buffer without Triton X-100. All these operations were carried out on ice. The samples were centrifuged at 114,000 × g for 4 h at 4 °C in an SW50.1 rotor (Beckman Coulter). Six equal volume fractions were then collected from the top down. Equivalent aliquots of each fraction were subjected to Western blotting using the anti-human CD44 antibody, BU-52 for COS-7 cell lysates, anti-V5 antibody for Flp-293 HEK cell lysates, and anti-flotillin-1 antibodies. Signal detection was performed using the Luminol reagent according to the manufacturer’s protocol. Protein bands were visualized using a Fluor-S Multimager and Quantity One 4.1.1 software.
Immunofluorescence Microscopy
COS-7 cells were co-transfected with various CD44H constructs subcloned into pCDM8 plasmids together with a red fluorescent protein-positive empty vector (pDsRed2-C1). In other experiments, CD44H subcloned into a green fluorescent protein-positive was used. Twenty-four hours post-transfection, the cells were incubated with or without 100 µg/ml cycloheximide for 3 h at 37 °C in serum-free DMEM. At the end of the treatment, cells were released from the monolayer culture with a non-enzymatic cell dissociation solution and fixed with 2% paraformaldehyde for 15 min at room temperature. Cells were then blocked with 2% bovine serum albumin and 10% goat serum in PBS, followed by incubation with BU-52 monoclonal antibody (1:5,000 dilution, 0.0001 µg/m in 2% bovine serum albumin in PBS) for 1 h at room temperature. Bound antibody was detected with cyanine-3-conjugated anti-mouse secondary antibody; 1:20,000 dilution in 2% bovine serum albumin in PBS (Cy3-AffiPure H + L IgG). To study the cellular accumulation of internalized CD44 protein, COS-7 transfectants were preincubated with 100 µg/ml cycloheximide for 2 h followed by co-incubation of 100 µg/ml cycloheximide and 200 µg/ml chloroquine for 3 h at 37 °C. Cells were trypsinized for 30 min, washed, fixed, and permeabilized with 0.2% Triton X-100 in PBS for 10 min at room temperature. At the end of each procedure, the cells were washed with PBS and mounted onto glass slides in media containing DAPI nuclear stain. Cells were visualized by using a Nikon Eclipse E600 microscope equipped with Y-Fl Epi-fluorescence (Melville, NY), a 40× or 60× 1.4 numerical aperture oil-immersion objective and Texas Red (red), fluorescein isothiocyanate (green) and DAPI (blue) filters. Images were captured digitally in real-time using a Spot-RT camera (Diagnostic Instruments, Sterling Heights, MI) and processed using MetaView imaging software (Universal Imaging, West Chester, PA). As a means to quantify the results observed by immunofluorescence, cell lysates (using radioimmune precipitation assay buffer) were prepared from control or cycloheximide-treated COS-7 transfectants as described above. Aliquots of these cell lysates were analyzed by Western blotting using the anti-human CD44 antibody, BU-52.
The binding and internalization of fluorescein-conjugated hyaluronan (Fl-HA) and hyaluronan decorated with biotinylated hyaluronan-binding protein probe (HABP) by COS-7 co-transfectants were as described previously (2). The binding of hyaluronan at the cell surface was visualized after 1 h of incubation of cells with Fl-HA at 4 °C. Internalized hyaluronan was visualized after 3 h of incubation of cells with Fl-HA at 37 °C, followed by trypsinization, fixation, and permeabilization. Biotinylated HABP was detected by the use of 1 µg/ml NeutrAvidin-Texas Red in PBS containing 1% bovine serum albumin for 1 h at 4 °C. At the end of each procedure, the cells were washed with PBS and mounted onto glass slides in media containing the DAPI and visualized by using the Nikon Eclipse E600 microscope.
RESULTS
CD44-mediated Internalization of Hyaluronan Is Dependent on Cholesterol-rich Lipid Rafts
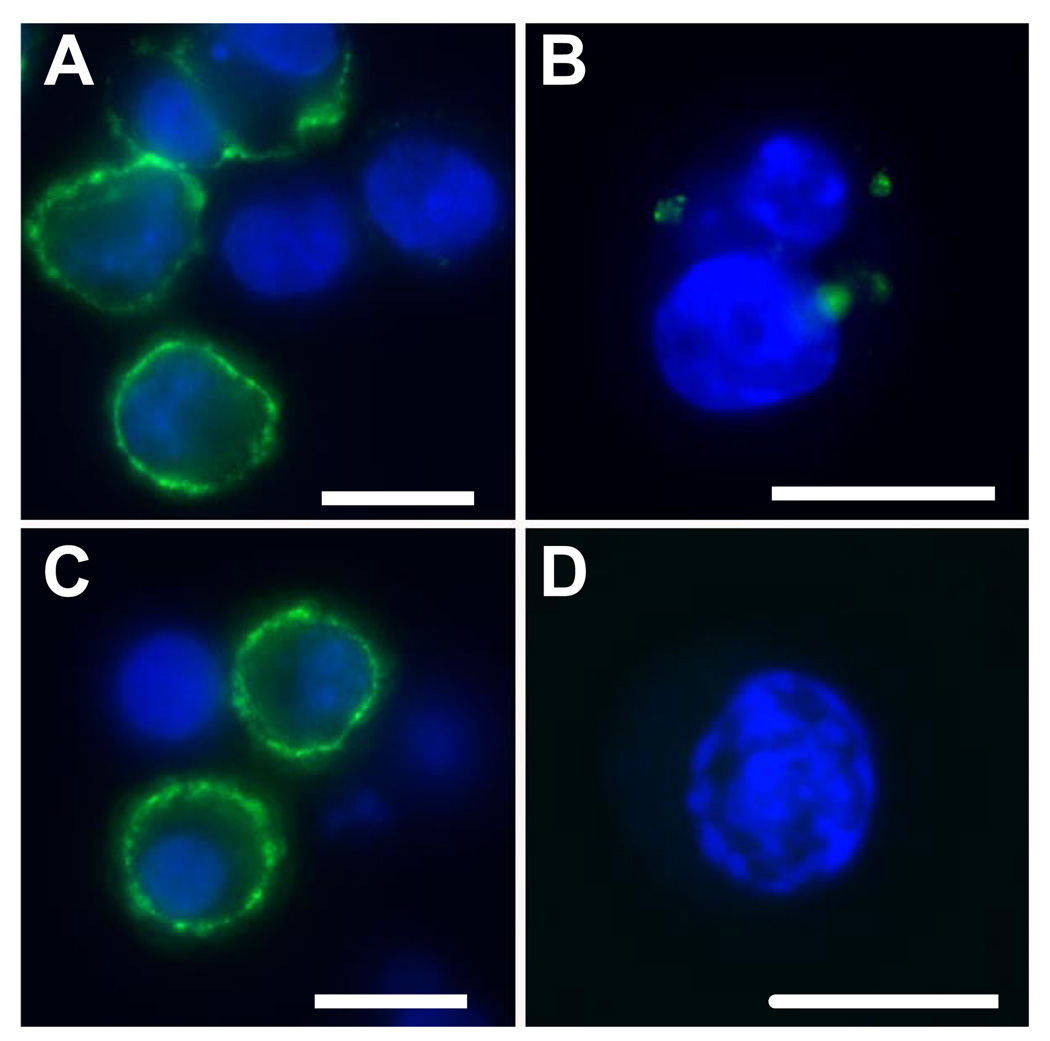
To determine whether CD44 association with lipid rafts was required for hyaluronan endocytosis, the effect of cyclic polysaccharides that bind and chelate cholesterol from the plasma membrane, were tested. COS-7 cells were transiently transfected with the full-length CD44H and denoted as CD44wt. Following transfection the cells were pretreated with or without 10 mm Mβ-CD and monitored for the binding and internalization of Fl-HA. Typical Fl-HA binding was observed in control COS-7 transfectants (Fig. 1A, green fluorescence). Following trypsinization, internalization of Fl-HA could also be visualized (Fig. 1B) as we have shown previously (7). Treatment with Mβ-CD appeared to have no obvious effect on Fl-HA binding (Fig. 1C) but completely blocked the internalization of Fl-HA (Fig. 1D). This suggests that cholesterol or cholesterol-rich microdomains within the plasma membrane are essential for the endocytosis of hyaluronan.
FIGURE 1. Membrane cholesterol is required for CD44-mediated internalization of Fl-HA.
COS-7 cell transfectants of CD44wt pre-treated without (A and B) or with (C and D) 10 mm Mβ-CD, were released from monolayers with EDTA and incubated with complete medium containing 15 µg/ml Fl-HA. To visualize cell surface binding (A and C), the cells were incubated for 1 h at 4 °C with Fl-HA, washed, fixed, mounted onto glass slides with DAPI nuclear counterstain and visualized by fluorescence microscopy. To visualize internalized Fl-HA, a corresponding aliquot of cells from each group was incubated with Fl-HA for 3 h at 37 °C followed by extensive trypsinization to remove all extracellularly bound Fl-HA (B and D). Shown are digital overlay images of green and blue fluorescence channels. The bars shown in each panel represent 20 µm.
A Subpopulation of CD44 Is Associated with Lipid Rafts
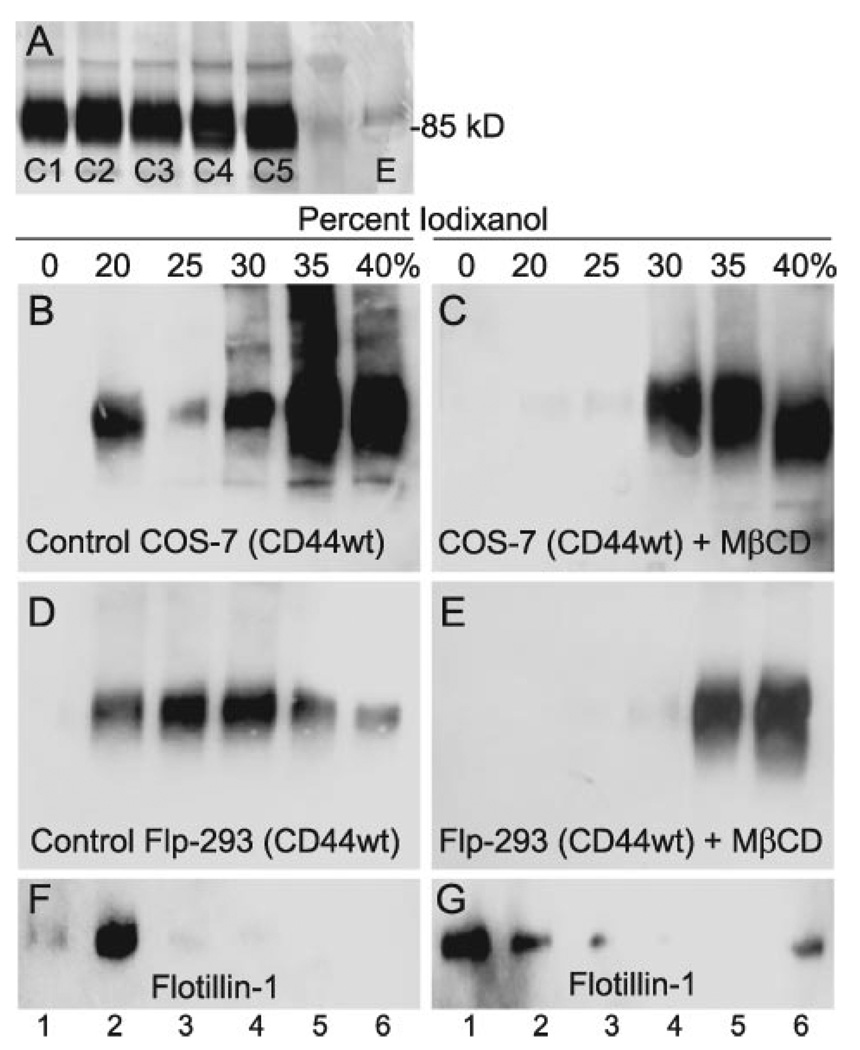
On Western blots, CD44 typically appears as a broad band with an average molecular mass of 85 kDa as seen in direct lysates obtained from five different hygromycin-selected colonies of Flp-293 cells (labeled as C1–C5, Fig. 2A). To determine whether CD44 itself was present in lipid raft microdomains of the plasma membrane, detergent lysates were isolated from transiently transfected COS-7 cells as well as Flp-293 cell-stable transfectants of CD44H. The cold-extracted cell lysates were subjected to ultracentrifugation in step gradients containing iodixanol prepared in lysis buffer without detergent and then analyzed on Western blots. As shown in Fig. 2B, a substantial proportion of the total immunoreactive CD44 isolated from COS-7 cells was present in the 20% iodixanol fraction. CD44 isolated from Flp-293 cells was dispersed throughout the gradient but also was present within the 20% iodixanol fraction (Fig. 2D). Lipid raft-associated proteins are localized within the 0 and 20% iodixanol fractions as illustrated by the standard marker protein, flotillin-1 (Fig. 2F). To further characterize the localization of CD44 in these gradients, the COS-7 and Flp-293 cells were pretreated with Mβ-CD prior to cell lysis. As shown in Fig. 2 (C and E), following the removal of cholesterol, none of the CD44 was present in the low density (0 or 20%) iodixanol fractions. Additionally, under these conditions the distribution of flotillin-1 also shifted to the higher density fractions (Fig. 1G).
FIGURE 2. Association of CD44 with low buoyant density lipid raft membrane fractions.
Panel A represents a typical Western blotting profile for CD44 expressed by five different selected clones of Flp-293 cells stably transfected with CD44wt. The clones are labeled as C1 through C5, whereas E depicts endogenous levels of CD44 expressed by Flp-293 cells. COS-7 transfectants (B) and Flp-293 cells (D) expressing CD44wt were extracted in the presence of 1% Triton X-100 on ice and subjected to ultracentrifugation on step gradients of iodixanol. Equal volume fractions were collected and analyzed by Western blotting for CD44. The 0–20% iodixanol interface (predominately the 20% fraction) represents the low density, lipid raft fraction. In some instances, cells were cholesterol-depleted by pretreatment with 20 mm Mβ-CD at 37 °C for 50 min for COS-7 cells (C) or 25 min for Flp-293 cells (E) prior to ultracentrifugation and Western blotting. The same fractions from the Flp-293 stable transfectants shown in D and E were also assayed for the lipid raft marker flotillin-1 using the anti-flotillin-1 antibody, before (F) and after (G) cholesterol depletion. CD44 was shown to associate with a low buoyant density lipid raft fraction in both COS-7 and Flp-293 cells (verified by the migration of flotillin-1), and this association was dependent on the presence of cholesterol. CD44 was detected using either an anti-CD44 monoclonal antibody, BU-52 (B and C) or the epitope-tag anti-V5 monoclonal antibody (D and E).
Previous studies had suggested that CD44 was palmitoylated through thioesterification at cysteine 286. To explore whether the presence of CD44 in the low density lipid raft-associated fraction was dependent on such acylation of the receptor, both transfected COS-7 and stable Flp-293 cells were pretreated with the palmitic acid analog 2-BP prior to cell lysis. As shown in Fig. 3A and 3B, respectively, the presence of the acylation inhibitor prevented the association of CD44 with the low density fractions. These results suggest that palmitoylation is necessary for CD44 to associate with the cholesterol-rich lipid raft fractions. However, the use of 2-BP is nonspecific and effects the palmitoylation of all membrane proteins. To determine whether CD44 acylation is specifically involved, the potential thioester site present on CD44, Cys286 was mutated to alanine 286 (C286A). In both transfected COS-7 cells and Flp-293 cell-stable transfectants, with CD44 containing the C286A mutation (CD44-C286A), little to no CD44 was present in the lipid raft-associated iodixanol fractions (Fig. 3, C and D, respectively). Nonetheless, a small fraction of CD44 was present in the 0 and 20% iodixanol of the transfected COS-7 cells suggesting that another CD44 amino acid might be palmitoylated. Given its proximity to the inner plasma membrane, CD44 cysteine 295 was considered another potential acylation site. This site was also mutated to an alanine and the double mutated CD44 (denoted as CD44-C286,295A), transfected into COS-7 cells. As shown in Fig. 3E, although the majority of the CD44 from these cells is present in the non-raft fractions, a small but still detectable presence of CD44 can be observed in the 0 and 20% iodixanol fractions. Nonetheless, it is clear that thioesterification of one or more of the cysteines of CD44 contribute to the association of CD44 with the low density lipid raft fractions.
FIGURE 3. Palmitoylation is required for CD44 targeting into lipid rafts.
2-BP pre-treatment prevented CD44 association with low buoyant density iodixanol lipid rafts fractions in both COS-7 transient transfectants (A) and Flp-293 stable transfectants (B) of CD44wt. The palmitoylation mutants of CD44 (pCD44-C286A) expressed in COS-7 transfectants (C) and as Flp-293 stable transfectants (D) also exhibited significantly reduced association with the low density lipid rafts fraction. Similar results were obtained when the double mutation isoform of CD44, CD44-C286,295A, was expressed in COS-7 transfectants (E).
Palmitic Acid Incorporation into CD44
To provide more definitive evidence that CD44 undergoes palmitoylation, 3H-labeled palmitic acid incorporation into CD44 was assessed in Flp-293-stable transfectants of CD44wt. As shown in Fig. 4A, lane 2, fluorography gels of the isolated and immunoprecipitated CD44 depicted a single 3H-labeled band. When the Flp-293 cells were preincubated with 2-BP, no immunoprecipitated 3H-labeled band was detected (Fig. 4A, lane 1). This suggests that the 2-BP does block the acylation of CD44 and that the label is incorporated as palmitate. As a nonspecific control, Flp-293 cells engineered to stably express a different protein, human hyaluronidase-2, were also labeled with [3H]palmitic acid. Only background immunoprecipitated 3H-labeled bands were detected (Fig. 4A, lane 3), none in the size range of CD44.
FIGURE 4. [3H]Palmitate is incorporated into CD44 by acylation of membrane-proximal cysteines.
A, [3H]palmitate incorporation (250 µCi/ml, 2 h at 37 °C) into Flp-293 stable transfectants of CD44wt, pretreated with or without 50 µm 2-BP (lanes 1 and 2, respectively). [3H]Palmitate incorporation into Flp-293 stable transfectants of Hyal-2 followed by immunoprecipitation using anti-V5 monoclonal antibody is shown in lane 3. In B, the extent of [3H]palmitate incorporation in parental Flp-293 cells (lane 1) or Flp-293 cells expressing the wild-type CD44wt (lane 2) were compared with the Flp-293 expressing the cysteine mutants CD44-C286A (lane 3). Lanes 4 and 5 represent a Western blot (detection using an anti-V5 monoclonal antibody) of equivalent aliquots of lysates shown in lanes 2 and 3, respectively. In C, incorporation of [3H]palmitate into wild-type CD44wt (lane 1), CD44-C286,295A (lane 2), and CD44-C286A (lane 3) were compared side-by-side. Lanes 4–6 represent a Western blot (detection using an anti-V5 monoclonal antibody) of equivalent aliquots of lysates shown in lanes 1–3, respectively. Changes in [3H]palmitate incorporation were quantified by digital scanning of x-ray films, and the resultant data are expressed as the average ± S.D. (n = 3) change in pixel density relative to control CD44 wild-type incorporation.
To validate the participation of cysteine residues in the palmitoylation of CD44, Flp-293 cells, stably transfected with CD44wt or CD44-C286A, were labeled with [3H]palmitic acid and processed for palmitate incorporation into CD44. The wild-type CD44 again exhibited a strong single 3H-labeled band (Fig. 4B, lane 2) whereas control Flp-293 cells (without stable CD44 transfection) did not (Fig. 4B, lane 1). Incorporation of [3H]palmitate into CD44-C286A was present but significantly reduced (Fig. 4B, lane 3). Image analysis densitometry determined the incorporation of palmitate into CD44-C286A was 55 ± 9% that of the wild-type CD44. The advantage of the Flp-293 cell system is that each selected line exhibits equivalent copy numbers of the desired transfected gene at the flp recombinase target site. However, to verify the expression equivalence of the two cell lines, the aliquots from the same lysates in Fig. 4, lanes 2 and 3, were probed by Western blotting. As can be seen in Fig. 4B, an equivalent level expression of CD44 is present in both Flp-293 cell-stable transfectants of CD44wt (lane 4) and CD44-C286A (lane 5). Therefore, the reduction in [3H]palmitate incorporation observed in Fig. 4B, lane 3, as compared with lane 2 is not due to differing expression of CD44. Given that CD44 exhibits close to two times the level of [3H]palmitate incorporation as CD44-C286A, there may be two acylated amino acids in the wild-type CD44. To investigate this possibility, [3H]palmitate incorporation into Flp-293 stably expressing the double mutant, CD44-C286,295A was compared with CD44wt and the CD44-C286A single mutant. As shown in Fig. 4C, the incorporation of [3H]palmitate into CD44-C286,295A (lane 2) was reduced by more than 50% (36 ± 7%) of control CD44 (Fig. 4C, lane 1). The incorporation was also substantially lower than that present in the single C286A mutant (Fig. 4C, lane 3) but, interestingly, not completely blocked. It is thus likely that other amino acids in addition to the two membrane-proximal cysteines also participate in CD44 acylation.
Palmitic Acid Incorporation into CD44 Is Necessary for Hyaluronan Internalization
Fig. 1 suggested that the presence of cholesterol was necessary for CD44 internalization. To determine whether CD44 association with lipid rafts was required for hyaluronan internalization, COS-7 cells were transiently transfected with CD44-C286S, another site-directed mutation of CD44 Cys286. COS-7 cells expressing wild-type CD44 bound Fl-HA (Fig. 5A), whereas non-transfected COS-7 cells did not (the red fluorescent protein-positive cells in Fig. 5 insets identify successfully transfected cells). The successfully CD44-transfected COS-7 cells also display trypsin-resistant Fl-HA within what appear as small intracellular vesicles (Fig. 5B). We have previously characterized this hyaluronan as hyaluronan destined for degradation following CD44-mediated endocytosis (2, 8, 18). COS-7 cells expressing CD44-C286S also exhibited the capacity to bind Fl-HA to the cell surface (Fig. 5C), and again, this capacity was limited to the successfully transfected cells (Fig. 5C, inset). However, no internalized Fl-HA was observed in trypsinized CD44-C286S cells (Fig. 5D).
FIGURE 5. Palmitoylation of CD44 is required for HA internalization.
COS-7 cells were co-transfected with red fluorescent protein and the wild-type CD44wt (A and B) or the CD44-C286S mutant isoform (C and D). The transfectants were released from monolayers with EDTA and incubated in complete medium containing 15 µg/ml Fl-HA. To visualize cell surface binding (A and C), the cells were incubated for 1 h at 4 °C with Fl-HA, washed, fixed, mounted onto glass slides with DAPI nuclear counterstain and visualized by fluorescence microscopy (A and C). To visualize internalized Fl-HA, a corresponding aliquot of cells from each group was incubated with Fl-HA for 3 h at 37 °C followed by extensive trypsinization to remove all extracellularly-bound Fl-HA (B and D). Shown are digital overlay images of green and blue fluorescence channels. Insets depict three-channel digital overlays of green, red, and blue fluorescence signals. The bars represent 20 µm.
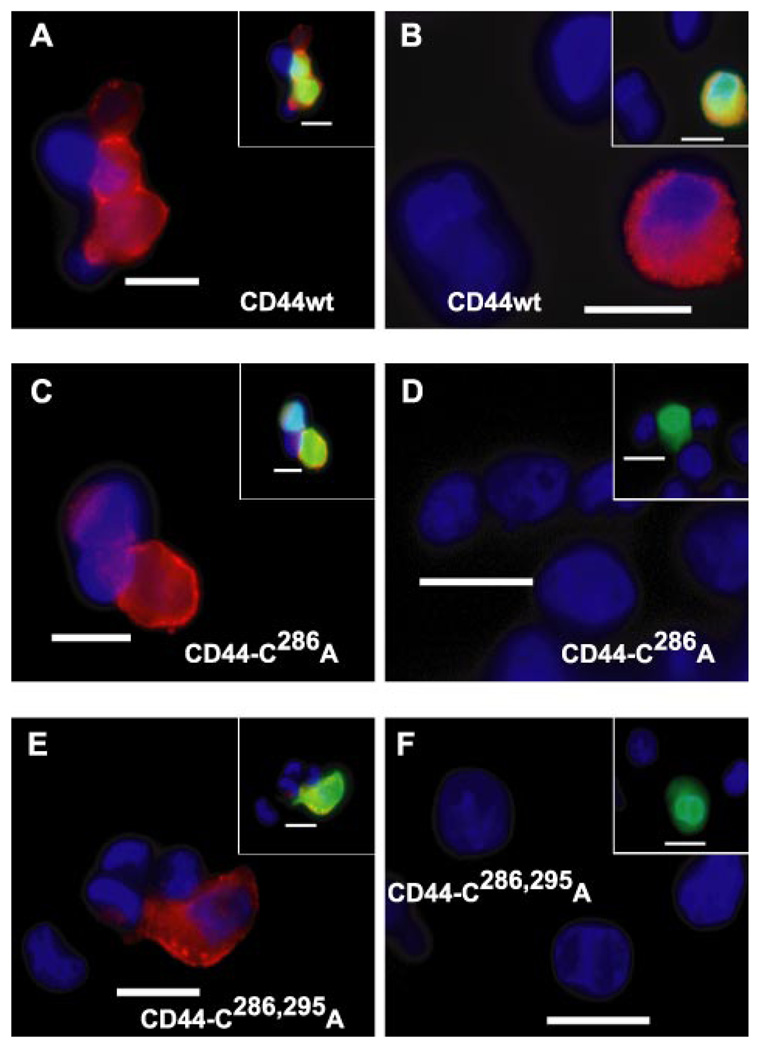
Similar results were obtained when COS-7 cells were assayed for the binding, and internalization of hyaluronan was labeled by an alternative, non-covalent fashion. We have shown previously that CD44-positive cells can bind and internalize high molecular mass hyaluronan decorated with a biotinylated HABP probe (2). COS-7 cells successfully transfected with wild-type CD44 bind (Fig. 6A) and internalize (Fig. 6B) hyaluronan decorated with HABP (visualized using NeutrAvidin-Texas Red). As with the CD44-C286S, COS-7 cells, transfected with either CD44-C286A (Fig. 6C) or the double mutation CD44-C286,295A (Fig. 6E), avidly bound hyaluronan-HABP complexes to the plasma membrane. However, neither of these two transfectants exhibited trypsin-resistant, intracellular fluorescence (Fig. 6, D and F, respectively). Thus, the reduced capacity of the CD44 cysteine mutants to participate in the internalization of the hyaluronan can also be detected using non-covalently labeled hyaluronan.
FIGURE 6. Binding and internalization of HA decorated with biotinylated HABP.
COS-7 cells were co-transfected with green fluorescent protein and the wild-type CD44H (labeled CD44wt, A and B), the CD44-C286A mutant isoform (C and D), or the CD44-C286,295A double mutant isoform (E and F). The transfectants were released from monolayers with EDTA and incubated in complete medium containing 15 µg/ml unlabeled HA pre-decorated with biotinylated HABP. To visualize cell surface binding (A, C, and E), the cells were incubated for 1 h at 4 °C with HA/HABP washed, fixed, mounted onto glass slides with DAPI nuclear counterstain, and visualized by using fluorescence microscopy. To visualize internalized HA/HABP, a corresponding aliquot of cells from each group was incubated with HA/HABP for 3 h at 37 °C followed by extensive trypsinization to remove all extracellularly bound HA/HABP(B, D, and F). Biotinylated HABP was detected using NeutrAvidin-Texas Red. Shown are digital overlay images of red and blue fluorescence channels. Insets depict three-channel digital overlays of green, red, and blue fluorescence signals. The bars represent 20 µm.
Palmitic Acid Incorporation into CD44 Is Necessary for CD44 Turnover/Internalization
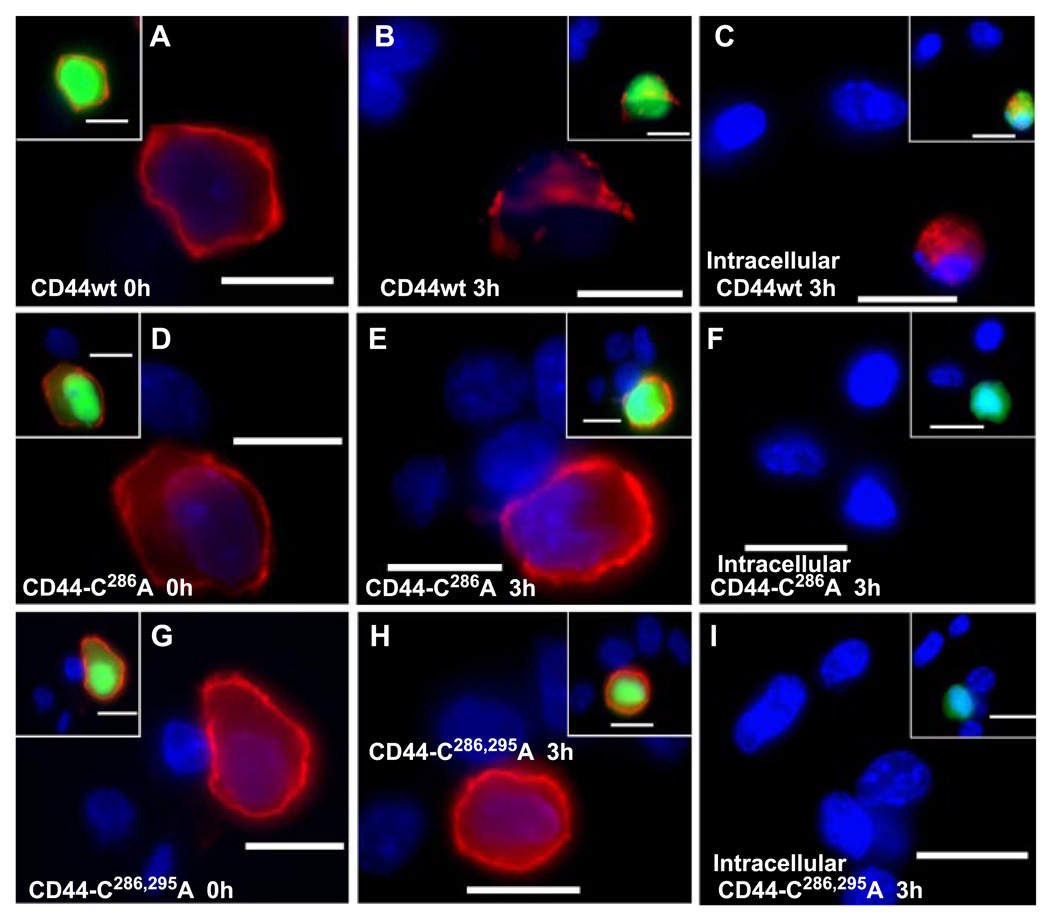
It has previously been shown in chondrocytes that CD44 has a half-life at the cell surface of between 24 and 48 h (19) and that part of this turnover involves the endocytosis of the receptor. Given that CD44-mediated endocytosis of HA was blocked by interference with CD44 mutations that affected CD44 palmitoylation, the same mechanisms may participate in the turnover of CD44. To follow turnover and identify internalized CD44, transfected COS-7 cells were incubated with cycloheximide to block all new synthesis of CD44. At the zero time point, strong staining for CD44 could readily be observed on the surface of COS-7 cells successfully transfected with wild-type CD44wt (Fig. 7A), CD44-C286A (Fig. 7D), and the double mutation CD44-C286,295A (Fig. 7G). After 3 h of incubation in the presence of cycloheximide (prevents replenishment of CD44 to the cell surface), a prominent loss of CD44 receptor was observed in the cells expressing the wild-type CD44wt (Fig. 7B). However, no change in CD44 cell surface expression was observed in COS-7 cells expressing CD44-C286A (Fig. 7E) or CD44-C286,295A (Fig. 7H) in the presence of cycloheximide. Thus, the same conditions that block HA endocytosis also appear to prevent the cycling of the CD44 necessary for receptor turnover.
FIGURE 7. CD44 internalization in COS-7 transfectants is mediated by receptor palmitoylation.
COS-7 cells were co-transfected with green fluorescent protein and the wild-type CD44H (labeled CD44wt, A–C), the CD44-C286A mutant isoform (D–F), or the CD44-C286,295A double mutant isoform (G–I). The transfectants were then preincubated without (A, D, and G) or with 100 µg/ml cycloheximide for 3 h at 37 °C (B, C, E, F, H, and I) as labeled. To detect intracellular staining, the cells were co-incubated with cycloheximide and chloroquine; the latter was used to inhibit lysosomal degradation and enhance visualization of internalized receptor (C, F, and I). Cell surface CD44 was visualized by immunostaining of fixed cells with the anti-CD44 monoclonal antibody, BU-52, and anti-mouse cy-3-conjugated secondary antibody (red fluorescence; A, B, D, E, G, and H). To detect internalized CD44 (C, F, and I), cells were trypsinized, fixed, and permeabilized prior to immunostaining with the BU-52 antibody. Shown are digital overlay images of red and blue fluorescence channels. Insets depict three-channel digital overlays of green, red, and blue fluorescence signals. The bars represent 20 µm.
To determine that the decay in CD44 residency at the cell surface was due to internalization, the COS-7 transfectants, treated with cycloheximide and chloroquine, were trypsinized, permeabilized, and immunostained for intracellularly localized CD44wt. As shown in Fig. 7C, a significant level of trypsin-resistant CD44 was detected in COS-7 cells transfected with the wild-type CD44. However, in the COS-7 cells expressing CD44-C286A (Fig. 7F) or CD44-C286,295A (Fig. 7I) receptors, the level of intracellularly localized CD44 was substantially reduced. The data suggest that turnover of CD44, due to receptor internalization, requires the presence of cysteine residues within the membrane-proximal domains of CD44.
CD44 residency at the cell surface in COS-7 cells transfected with the CD44wt (Fig. 8A) decays after 3 h of cycloheximide treatment (Fig. 8B). This apparent turnover of CD44 then was confirmed by Western blotting. COS-7 cells transfected with wild-type CD44 display a marked 44 ± 1% decrease in the expression of CD44 expression after 6 h of cycloheximide treatment (Fig. 8C, lane 2) as compared with the 0 h, control cells (Fig. 8C, lane 1). In contrast, COS-7 cells expressing CD44-C286A (Fig. 8C, lanes 3 and 4) or CD44-C286,295A (Fig. 8C, lanes 5 and 6) expression displayed no reduction between 0 and 6 h of cycloheximide treatment, respectively, and were even slightly increased (122 ± 19% and 107 ± 14% increase, respectively, as compared with time zero values). In separate experiments, COS-7 cells transfected with wild-type CD44 again displayed a 41.8% decrease due to 3-h treatment with cycloheximide (Fig. 8D, lane 2). However, when the cells were co-incubated with cycloheximide and the lysosomotropic inhibitor, chloroquine (to block lysosomal degradation of internalized CD44), the level of CD44 expression increased (Fig. 8D, lane 3); the level was still 26.5% less than control (lane 1) but more so than when lysosomal degradation was allowed to occur (lane 2). Under the same 3-h cycloheximide alone conditions, no change in the expression of the double cysteine mutant, CD44-C286,295A, was observed (Fig. 8D, lane 5 versus lane 4).
FIGURE 8. CD44 internalization is dependent on receptor palmitoylation.
The two channel fluorescence overlay images of cells (A and B) represent another illustration of the loss of CD44 expression in COS-7 transfectants of CD44wt at time zero (A) as compared with 3 h following pre-treatment with cycloheximide (B). As in Fig. 7, the red fluorescence represents immunostaining of the cells with anti-CD44 monoclonal antibody, BU-52, followed by anti-mouse Cy3-conjugated secondary antibody. C depicts CD44 Western blotting of cell lysates prepared from COS-7 transfectants at time zero (lanes 1, 3, and 5) or after 6 h of treatment with cycloheximide (lanes 2, 4, and 6). Lysates of wild-type CD44 (CD44wt; lanes 1 and 2), CD44-C286A (lanes 3 and 4), or CD44-C286,295A (lanes 5 and 6) were examined. D represents a CD44 Western blot of another experiment that depicts transfectants of CD44H (lanes 1–3) or CD44-C286,295A (lanes 4 and 5) at time zero (lanes 1 and 4), treated with cycloheximide for 3 h (lanes 2 and 5) or treated with cycloheximide and chloroquine for 3 h (lane 3). Changes in band intensity were quantified by digital scanning of x-ray films, and the resultant data are expressed as the average ± S.D. (n = 3) change in pixel density relative to control CD44 wild-type expression.
DISCUSSION
The association of CD44 with lipid rafts has been documented previously as well as the fact that CD44 is a substrate for palmitic acid acylation (11, 20–22). However, in this study, it was demonstrated that an association of CD44 palmitoylation and transit into lipid rafts was required for CD44-mediated hyaluronan endocytosis. The internalization of hyaluronan was inhibited by either sequestration of membrane cholesterol (Fig. 1) or, expression of palmitoylation-defective CD44 isoforms, CD44-C286A, CD44-C286S, and CD44-C286,295A (Figs. 5 and 6). Similarly, the internalization of CD44 itself in the absence of hyaluronan requires palmitoylation and association with lipid rafts (Figs. 7 and 8). The role of acylation with respect to CD44 association with lipid rafts was also documented using iodixanol density gradient ultracentrifugation (Figs. 2 and 3). The sequestration of membrane cholesterol, inclusion of the palmitoylation inhibitor 2-BP, or expression of palmitoylation-defective CD44 isoforms, all prevented CD44 localization in the low density layers, layers that have been shown by several groups to represent the lipid raft fraction of the plasma membrane (23, 24). All together the data suggest that CD44 palmitoylation and association with lipid rafts are events that promote the internalization of the receptor and its principal ligand, hyaluronan.
Several studies have documented a linkage between protein palmitoylation and association with lipid rafts as providing a mechanism for regulation of receptor endocytosis. However, the functional effect of palmitoylation on lipid raft association or endocytosis is inconsistent and varies with each system. For example, palmitoylation of murine leukemia virus envelope protein is essential for its association with lipid rafts (25), whereas palmitoylation of the anthrax toxin receptor acts to exclude the receptor from rafts (26). The chemokine co-receptor for HIV-1, CCR5, undergoes palmitoylation, which allows the co-receptor to transit into cholesterol-rich, lipid raft domains (16). Similarly, palmitoylation of the human luteinizing hormone receptor also favors transit of the receptor into rafts (27). However, in the case of both CCR5 and the human luteinizing hormone receptor, palmitoylation-defective mutants were excluded from lipid rafts and exhibited an enhanced rate of receptor endocytosis. On the contrary, for the anthrax receptor, exclusion from lipid rafts due to palmitoylation decreased receptor endocytosis (26). The seemingly conflicting results concerning rafts and endocytosis are also apparent in results concerning receptor associations with lipid rafts and effects on cell signaling.
Palmitoylation is a post-translational modification that involves the addition of palmitic acid to a free thiol group of cysteine residues present on membrane-spanning proteins, particularly cysteines close to the transmembrane/cytoplasmic domain boundary (13). In many palmitoylated proteins, clusters of hydrophobic and positively charged amino acids may precede or follow one or more of the palmitoylated cysteines creating a domain environment considered as optimal for acylation (13). Interestingly, CD44 includes triplet arginine residues, Arg292–294 and triplet lysines, Lys298–300, near both Cys286 and Cys295. One of our original hypotheses was that the triplet lysines at Lys298–300 were substrates for monoubiquitylation of CD44 and that this post-translational event provided the driving force for CD44 endocytosis as has been found for other receptors (28–31). Our preliminary results documented that CD44 is monoubiquitylated and that mutation of lysine residues affected the rate of CD44-mediated hyaluronan internalization (32). However, the exact lysine residue involved could not be identified as there is considerable redundancy in lysines as acceptor sites for monoubiquitylation (29). Nonetheless, endocytosis of the anthrax receptor was blocked, because the exclusion of the receptor from the lipid rafts prevented its ubiquitylation by E3 ubiquitin ligase Cbl, a ligase that is localized within lipid rafts (26). Thus, it remains possible that monoubiquitylation of CD44 occurs as a downstream driving force for receptor endocytosis once the receptor transits into a lipid raft environment.
The major goal of this study was to determine the regulation of CD44-mediated internalization of hyaluronan. In many cell systems such as chondrocytes, local, cell-mediated endocytosis of hyaluronan is the primary mechanism for turnover of this extracellular matrix macromolecule (8). During osteoarthritis extracellular matrix turnover is substantially elevated, and the question remains as to how these cells affect an increase in hyaluronan internalization. We have documented previously that enhanced endocytosis is not due to changes in CD44 receptor number (33). The results of this study suggest that palmitoylation of CD44 may be the cellular mechanism whereby hyaluronan internalization is regulated. Typically, proteins undergo cycles of palmitoylation and depalmitoylation, although the half-life of palmitate acylation is typically shorter than that of protein (13). Thus, it has been suggested that the steady-state level of protein palmitoylation is regulated predominately by the activity of thioesterases. In addition, the thioesterases that release palmitate from palmitoylated proteins have been far better characterized than putative palmitate transferases. For example, there are two thioesterases termed PPT1 and APT1, known to act on Ras and Gα proteins. Given that PPT1 is localized to the lysosome (34), APT1 is the most likely candidate enzyme for membrane proteins. APT1 is a 230-amino acid cytoplasmic protein that has been shown to depalmitoylate Gα, p21Ras, and endothelial nitric-oxide synthase (35, 36). In preliminary studies, we have found that the expression of APT1 mRNA by chondrocytes was decreased when the cells were treated with the catabolic cytokines that enhance CD44-mediated hyaluronan endocytosis (i.e. interleukin-1α).3 Although future experiments will be necessary to confirm such a mechanism, it allows us to speculate that cells regulate CD44 palmitoylation and the rate of endocytosis by modulating the expression or activity of particular thioesterase.
Acknowledgments
We thank Cheryl B. Knudson for helpful discussions and critical review of the report. We also thank Weihua Wang for her technical assistance.
Footnotes
This work was supported in part by National Institutes of Health Grants RO1-AR43384 and P50-AR39239. This work was submitted as partial fulfillment of a Ph.D. degree (for S. P. T.), from the Dept. of Biochemistry, Graduate College of Rush University, Chicago, IL.
The abbreviations used are: HIV-1, human immunodeficiency virus, type 1; DMEM, Dulbecco’s modified Eagle’s medium; DAPI, 4′,6-diamidino-2-phenylindole; CD44H, human CD44; FRT, flp recombinase target site; PBS, phosphate-buffered saline; Fl-HA, fluorescein-conjugated hyaluronan; HABP, hyaluronan binding protein; CD44wt, CD44 wild-type; 2-BP, 2-bromopalmitate; Mβ-CD, methyl-β-cyclodextrin.
S. P. Thankamony and W. Knudson, unpublished data.
REFERENCES
- 1.Knudson CB. J. Cell Biol. 1993;120:825–834. doi: 10.1083/jcb.120.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Embry J, Knudson W. Arthritis Rheum. 2003;48:3431–3441. doi: 10.1002/art.11323. [DOI] [PubMed] [Google Scholar]
- 3.Tammi R, Rilla K, Pienimaki J-P, MacCallum DK, Hogg M, Luukkonen M, Hascall VC, Tammi M. J. Biol. Chem. 2001;276:35111–35122. doi: 10.1074/jbc.M103481200. [DOI] [PubMed] [Google Scholar]
- 4.Underhill CB, Nguyen HA, Shizari M, Culty M. Dev. Biol. 1993;155:324–336. doi: 10.1006/dbio.1993.1032. [DOI] [PubMed] [Google Scholar]
- 5.Knudson W, Peterson RS. In: Chemistry and Biology of Hyaluronan. Garg HG, Hales CA, editors. Boston: Elsevier; 2004. pp. 83–123. [Google Scholar]
- 6.Jiang H, Knudson CB, Knudson W. Arthritis Rheum. 2001;44:2599–2619. doi: 10.1002/1529-0131(200111)44:11<2599::aid-art440>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, Peterson RS, Wang W, Bartnik E, Knudson CB, Knudson W. J. Biol. Chem. 2002;277:10531–10538. doi: 10.1074/jbc.M108654200. [DOI] [PubMed] [Google Scholar]
- 8.Knudson W, Chow G, Knudson CB. Matrix Biol. 2002;21:15–23. doi: 10.1016/s0945-053x(01)00186-x. [DOI] [PubMed] [Google Scholar]
- 9.Knudson W, Knudson C. Curr. Opin. Orthop. 2004;15:369–375. [Google Scholar]
- 10.Radeva G, Sharom FJ. Biochem. J. 2004;380:219–230. doi: 10.1042/BJ20031348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliferenko S, Paiha K, Harder T, Gerke V, Schwarzler C, Schwarz H, Beug H, Gunthert U, Huber LA. J. Cell Biol. 1999;146:843–854. doi: 10.1083/jcb.146.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foger N, Marhaba R, Zoller M. Eur. J. Immunol. 2000;30:2888–2899. doi: 10.1002/1521-4141(200010)30:10<2888::AID-IMMU2888>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 13.Bijlmakers MJ, Marsh M. Trends Cell Biol. 2003;13:32–42. doi: 10.1016/s0962-8924(02)00008-9. [DOI] [PubMed] [Google Scholar]
- 14.Liu D, Sy M-S. J. Exp. Med. 1996;183:1987–1994. doi: 10.1084/jem.183.5.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roy S, Plowman S, Rotblat B, Prior IA, Muncke C, Grainger S, Parton RG, Henis YI, Kloog Y, Hancock JF. Mol. Cell. Biol. 2005;25:6722–6733. doi: 10.1128/MCB.25.15.6722-6733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Percherancier Y, Planchenault T, Valenzuela-Fernandez A, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. J. Biol. Chem. 2001;276:31936–31944. doi: 10.1074/jbc.M104013200. [DOI] [PubMed] [Google Scholar]
- 17.Bonner WM, Laskey RA. Eur. J. Biochem. 1974;46:83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- 18.Hua Q, Knudson CB, Knudson W. J. Cell Sci. 1993;106:365–375. doi: 10.1242/jcs.106.1.365. [DOI] [PubMed] [Google Scholar]
- 19.Aguiar DJ, Knudson W, Knudson CB. Exp. Cell Res. 1999;252:292–302. doi: 10.1006/excr.1999.4641. [DOI] [PubMed] [Google Scholar]
- 20.Guo YJ, Ma J, Wong JH, Lin SC, Chang HC, Bigby M, Sy MS. Cell. Immunol. 1993;152:186–199. doi: 10.1006/cimm.1993.1278. [DOI] [PubMed] [Google Scholar]
- 21.Guo YJ, Lin SC, Wang JH, Bigby M, Sy MS. Int. Immunol. 1994;6:213–221. doi: 10.1093/intimm/6.2.213. [DOI] [PubMed] [Google Scholar]
- 22.Bourguignon LYW, Kalomiris EL, Lokeshwar VB. J. Biol. Chem. 1991;266:11761–11765. [PubMed] [Google Scholar]
- 23.Stickney JT, Bacon WC, Rojas M, Ratner N, Ip W. Cancer Res. 2004;64:2717–2724. doi: 10.1158/0008-5472.can-03-3798. [DOI] [PubMed] [Google Scholar]
- 24.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. J. Biol. Chem. 2004;279:26991–27007. doi: 10.1074/jbc.M311838200. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Yang C, Tong S, Weidmann A, Compans RW. J. Virol. 2002;76:11845–11852. doi: 10.1128/JVI.76.23.11845-11852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abrami L, Leppla SH, van der Goot FG. J. Cell Biol. 2006;172:309–320. doi: 10.1083/jcb.200507067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munshi UM, Clouser CL, Peegel H, Menon KM. Mol. Endocrinol. 2005;19:749–758. doi: 10.1210/me.2004-0335. [DOI] [PubMed] [Google Scholar]
- 28.Hicke L. Cell. 2001;106:527–530. doi: 10.1016/s0092-8674(01)00485-8. [DOI] [PubMed] [Google Scholar]
- 29.Roth AF, Davis NG. J. Biol. Chem. 2000;275:8143–8153. doi: 10.1074/jbc.275.11.8143. [DOI] [PubMed] [Google Scholar]
- 30.Haglund K, Di Fiore PP, Dikic I. Trends Biochem. Sci. 2003;28:598–603. doi: 10.1016/j.tibs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Marmor MD, Yarden Y. Oncogene. 2004;23:2057–2070. doi: 10.1038/sj.onc.1207390. [DOI] [PubMed] [Google Scholar]
- 32.Knudson W, Peterson RM. In: Hyaluronan: Structure, Metabolism, Biological Activities, Therapeutic Applications. Balazs EA, Hascall VC, editors. Vol. 1. Edgewater, NJ: Matrix Biology Institute; 2005. pp. 213–220. 2 vols. [Google Scholar]
- 33.Knudson CB, Knudson W. Clin. Orthop. 2004;427:152–162. [Google Scholar]
- 34.Resh MD. Biochim. Biophys. Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 35.Yeh DC, Duncan JA, Yamashita S, Michel T. J. Biol. Chem. 1999;274:33148–33154. doi: 10.1074/jbc.274.46.33148. [DOI] [PubMed] [Google Scholar]
- 36.Duncan JA, Gilman AG. J. Biol. Chem. 1998;273:15830–15837. doi: 10.1074/jbc.273.25.15830. [DOI] [PubMed] [Google Scholar]