Abstract
Insulin-like growth factors were discovered more than 50 years ago as mediators of growth hormone that effect growth and differentiation of bone and skeletal muscle. Interest of the role of insulin-like growth factors in cancer reached a peak in the 1990s, and then waned until the availability in the past 5 years of monoclonal antibodies and small molecules that block the insulin-like growth factor 1 receptor. In this article, we review the history of insulin-like growth factors and their role in growth, development, organism survival, and in cancer, both epithelial cancers and sarcomas. Recent developments regarding phase I to II clinical trials of such agents are discussed, as well as potential studies to consider in the future, given the lack of efficacy of one such monoclonal antibody in combination with cytotoxic chemotherapy in a first-line study in metastatic non–small-cell lung adenocarcinoma. Greater success with these agents clinically is expected when combining the agents with inhibitors of other cell signaling pathways in which cross-resistance has been observed.
INTRODUCTION
Insulin-like growth factors 1 and 2 (IGFs) are proteins produced by the liver in response to growth hormone produced by the pituitary. IGFs are responsible for the growth and development of somatic tissues, such as skeletal muscle and bone. After extensive research over the past two decades into the relationship of IGFs to cancer, systemic therapeutics that block IGF signaling are now available, with apparent clinical benefit in patients with carcinomas or sarcomas. The close relationship and interaction of IGF receptors to the insulin receptor (IR) couples effects on tumor metabolism with tumor cell survival.
In this perspective, I will review data regarding IGF signaling, cancer risk, and life span. Key findings regarding IGF receptor 1 (IGF1R) signaling and cancer are outlined in Table 1. The tight linkages to growth and metabolism of normal and tumor cells provide new possible therapeutic avenues to pursue for the treatment and prevention of both carcinomas and sarcomas, discussed below. The same linkages highlight possible mechanisms by which tumor cells could survive after blockade of IGF signaling. Further examination of these pathways in the context of specific cancer subtypes will be able to be leveraged into new therapeutic strategies, and over the long-term will have implications for cancer prevention and overall life span.
Table 1.
Key Motifs in IGF1R Signaling and Cancer
| Evolution of IGFR genes versus insulin genes |
| Growth (IGF) and metabolism (insulin), which are coupled pathways through one receptor in invertebrates, is separated into two separate but related signaling pathways (IGF1R and IR, respectively) |
| The activity of IGF1 and IGF2 is approximately 1% that of insulin with respect to glucose metabolism |
| IGF signaling cascade |
| Signaling through IGF1R uses several common mediators as EGFR, IR, and other RTKs |
| Heterodimers of RTKs (eg, HER2-IGF1R) are functional and represent one possible escape mechanism for IGF1R inhibition |
| IGF binding proteins can shuttle inside and outside the cell, and their role in signaling remains poorly understood |
| IGF1R can be found in the nucleus, potentially adding layers of complexity to gene regulation |
| IGF1R, organism growth, and cancer risk |
| Neither IGF1 nor IGF2 are needed for survival of vertebrates, but organisms that lack IGF1, IGF2, or both, are comparatively small |
| Small is beautiful: genetically engineered organisms lacking IGF1 or IGF2 survive longer than control animals |
| IGF1 signaling knockout animals also appear to have a lower risk of cancer than control littermates |
| Lower tonic signaling of IGF1 (or blockade of mTOR signaling) may be a way to both ameliorate poor glycemic control and the metabolic syndrome, and perhaps increase lifespan |
| IGF1R blockade in clinical trials |
| Single-agent IGF1R blockade is associated with (at best) a low response rate of uncertain durability in cancer patients |
| Synergy with EGFR and other RTK inhibitors (and with cytotoxic agents) may be the most effective way to use IGF1Rs |
| Dual mTOR and dual mTOR1-AKT have compelling mechanisms of action that will be interesting to pursue in future clinical trials |
| The integration of the understanding of metabolism and growth of cancer cells will impact clinical trials in cancer and those examining agents to increase human lifespan |
Abbreviations: IGF1R, insulin-like growth factor receptor 1; IR, insulin receptor; IGF, insulin-like growth factor; EGFR, epidermal growth factor receptor; RTK, receptor tyrosine kinases.
INSULIN AND RELATED GENES AND PROTEINS
The lack of structural variation of insulin and IGFs throughout phylogeny1–3 speaks to their importance in their control in growth and metabolism in multicellular organisms. In insects, insulin-related peptides are neurotransmitters, while in mollusks and other higher organisms insulin-like peptides mediate somatic growth (ie, connective tissue, muscle, bone).4 Analysis of the sequences of known insulin and related molecules5 yields four major families of proteins: insulin itself, IGFs, the bombyxins, and the relaxins. Bombyxins have roles in hormonal signaling (eg, ecdysone) that regulates different stages of insect development, and are found both in brain and gut (although IGF-like molecules play a role in these processes too)6–7; relaxins are involved in extracellular matrix remodeling and cell migration throughout phylogeny, properties a tumor cell could employ to its advantage.8 A key lesson learned from phylogenetic and physiology studies is that metabolism and growth are tightly coupled through a single signaling receptor in invertebrates, while vertebrates have uncoupled the process of growth and metabolism by separating the growth from metabolism into IGF and insulin signaling pathways, respectively.9
DISCOVERY OF IGFs
IGFs were found during the search for mediators of the activity of growth hormone (GH). In the original experiments by Salmon and Daughaday, 35S-labeled amino acid incorporation into cartilage explants was used as a surrogate for growth.10 The serum of normal rats induced 35S-amino acid incorporation into cartilage, but not serum from hypophysectomized rats, nor serum from hypophysectomized rats to which GH was added to the culture medium. However, serum from hypophysectomized rats treated with GH yielded serum that allowed for 35S-amino acid incorporation, indicating a second messenger was necessary for GH signaling. Furthermore, serum depleted of insulin using insulin-specific antisera still demonstrated anabolic insulin-like effects on muscle and fat, indicating insulin itself was not responsible.11 The proteins responsible for this activity were termed somatomedins A and C, and are now called IGF-1 and IGF-2, and are part of a feedback signaling loop between pituitary, liver, and GH-releasing hormone release by the hypothalamus.9
FUNCTIONS OF IGFs AND IGF BINDING PROTEINS
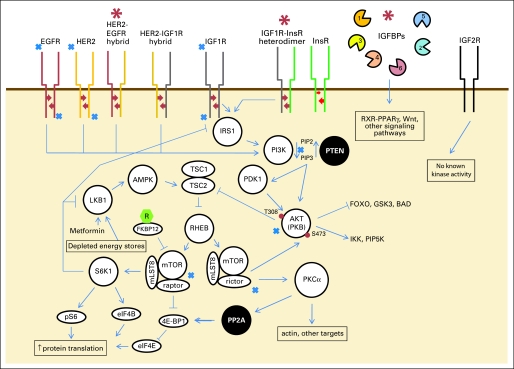
IGFs are present in high concentrations in serum, up to 1 ug/mL, and are mostly protein bound. Both IGF1 and IGF2 bind the IGF1R (CD221), the active signaling molecule, while only IGF2 binds to IGF 2 receptor (IGF2R, CD222), which is also a mannose-6-phosphate or scavenger receptor that does not appear to have the ability to signal intracellularly (Fig 1).
Fig 1.
Insulin-like growth factor receptor 1 (IGF1R) signaling pathway and potential mechanisms of resistance to IGF1R blockade (red asterisks). Heterodimers between IGF1R and related proteins are one mechanism of resistance to IGF1R inhibitors, and, conversely, signaling through IGF1R is one mechanism of resistance through human epidermal growth factor receptor 2 (HER2) and other epidermal growth factor receptor (EGFR) family members. White circles represent kinases, black circles phosphatases. Blue X indicates signaling points for which there are inhibitors in clinical trials or commercially available. Green hexagon with R represents sirolimus (rapamycin). Red arrows represent autophosphorylation of receptor tyrosine kinases (RTKs). Red dots represent phosphate groups. A list of clinical trials involving single-agent IGF1R inhibitors is included in Appendix Table A1 (online only). IGFBPs, insulin-like growth factor binding proteins; RXR, retinoid X receptor.
IGFs recapitulate many of the activities of insulin, such as increase in glucose metabolism in fat, increase in glucose transport, inhibition of lipolysis, and increasing lipid, glycogen, and protein synthesis, but with only 1% to 2% the potency of insulin. Similarly, while hypoglycemia is characteristic of insulin-producing islet cell tumors of the pancreas, it is only the rare patient with solitary fibrous tumor (SFT)/hemangiopericytoma (HPC), gastrointestinal stromal tumor (GIST), and other sarcomas who develop hypoglycemia, in whom production of a high molecular weight version of IGF2 signals through an IGF2R/IR heterodimer (Doege-Potter syndrome).12–15
IGFs also promote differentiation of myoblastic or osteoblastic tissues into muscle and bone.16 The assessment of signaling through IGF1R is made more complex by the formation of heterodimers between IR and IGF1R,17 suggesting a potential mechanism of escape from cell signaling blockade employing a small molecule IGF1R inhibitors; IR/IGF1R heterodimers appear to signal through IGF1R rather than IR.17 Conversely, monoclonal antibodies against IGF1R may neutralize both IGF1R homodimers and IGF1R/IR heterodimers. The striking possibility for direct effects of IGF1R on DNA or DNA binding proteins is suggested by the finding of IGF1R in the nucleus of carcinoma cells.18 A recent article by Sehat et al18a also notes identification of IGF1R in the cell nucleus.
IGF1R signaling is further regulated by six IGF binding proteins (IGFBPs). Some IGFBPs compete for activity of IGFs at the receptor level and antagonize IGF function. Others (eg, IGFBP2, IGFBP5) appear to amplify IGF signaling.19 Adding further to the complexity of IGFBP activity is the finding of proteolytic cleavage fragments of IGFBPs in serum; it is unclear if the fragments have biologic activity as well.20–22 It is clear that IGFBPs have functions independent of IGF1R. For example, IGFBP4 can bind Wnt receptors FRZ8 and LRP6 and inhibit Wnt3A binding.23 IGFBP6 both is secreted and found in the nucleus.24 IGFBP3 and IGFBP5 are ligands for the retinoid X receptor (RXR) alpha25,26 and interact with the retinoic acid receptor (RAR). The interaction of IGFBP3 with RAR/RXR appears to be due to the carboxy-terminus of IGFBP3, which has a nuclear localization signal and binding domains for RXR-alpha, thus disrupting RXR-retinoic acid receptor heterodimers.27–28 Steroid hormone receptors also interact with IGF1R, through steroid receptor coactivator 3 (gene NCOA3), which is amplified in breast and other carcinomas,29–31 in turn increasing levels of IGFBP3.32
Adding to the fascination of this group of molecules is the understanding that a molecule involved in adipocyte differentiation, PPAR-gamma, forms heterodimers with RXR, implicating IGF signaling with control of metabolism at yet another level.33–36 Transforming growth factor beta and retinoids appear to mediate carcinoma cell line growth inhibition by induction of IGFBP3 expression, a change that can be reversed with addition of IGF2.33,35,37 Finally, IGFBP3 levels may predict for outcomes for patients with GIST and Ewing sarcoma.38–39 These briefest glimpses of IGFBP physiology underscore the detail we will require to better understand this signaling pathway within a specific cellular context.
IGF1R SIGNALING, GROWTH, AND DEVELOPMENT
While IGFs themselves are not necessary for survival, mouse models and other studies in animals have provided striking data regarding the role of IGFs in the development of somatic tissues. IGF1−/− or IGF2−/− mice are viable, but approximately 40% smaller than littermates.40 Conversely, IGF1R signaling is necessary for viability in mice; IGF1R−/− mice die at birth and are approximately 55% smaller than littermates. Interestingly, IGF1-IGF2 doubly deficient mice remain viable, but 70% smaller than littermates.40 There have not been enough long-term studies on such animals to determine cancer risks in such animals compared with controls. Beyond rodents, the size of different breeds of dogs appears to be related to plasma IGF1 levels. Single nucleotide polymorphisms (SNPs) are found in the IGF1 gene between large and small breeds of dogs as well.41
Data implicate IGF1 signaling in variations in human growth and development. Rare IGF-1 deficient humans have been identified (Laron syndrome, in which GH receptor is mutated or otherwise inactive), who demonstrate short stature, delayed bone age, and low IGF1 levels in the setting of increased levels of GH.42 Variations in IGF1 levels are also at least weakly related to stature among different African tribes, perhaps not surprising given the role of pituitary GH production and gigantism or other variations in stature. Occasional infants are found with defects in IGF1R signaling, who demonstrate poor antenatal and postnatal growth.43
Conversely, acromegaly or gigantism can arise from a state of prolonged excess pituitary GH production, depending on its onset with respect to development. Beckwith-Wiedemann syndrome involves an excess of plasma IGF2 levels, in which subjects demonstrate exomphalos, macroglossia, gigantism, and hemihypertrophy. Beckwith-Wiedemann syndrome genetically maps to chromosome 11p15, near genes encoding IGF2 and insulin, and appears to arise from epigenetic changes in gene methylation of IGF2 as opposed to a mutation or SNP causing increased IGF2 production.44 These findings are reminiscent of data from insulin resistance syndromes in which other tissues proliferate (eg, polycystic ovary syndrome [PCO]), characterized by insulin resistance and insulin excess. Insulin excess in PCO is associated with an increased ratio of IGF1 to IGFBP1, and alterations in IGF1 and IGFBPs are observed in the metabolic syndrome.45–47 Metformin, by activating LKB1 downstream of IGF1R and IR (Fig 1), causes a decreased ratio of IGF1 to IGFBP1, decreased androgen production, and reversal of the irregular menses and other symptoms associated with PCO.48 However, in other syndromes associated with hypertrophy of tissues (eg, diffuse idiopathic skeletal hyperostosis), insulin resistance appears to be mediated through a different mechanism.49
IGFs AND LINKS TO LIFE SPAN
One of the surprising aspects of IGF signaling from a variety of animal models concerns effects on life span.50 Heterozygous IGF1R+/− mice live longer than IGF1R+/+ littermates.51 Furthermore, alterations in Klotho, a gene that modulates IGF1R signaling, affect animal life span as well. Klotho−/− mice show accelerated rates of aging, while reintroduction of Klotho reverses the effect.52 Other downstream cellular effectors of IGF1R signaling are also associated with variations in life span. Downstream signaling molecules IRS-1 and IRS-2 have been examined regarding animal survival, and only IRS1−/−, not IRS2−/− mice, live longer than littermates.53 This finding is also borne out in human studies, in which patients with SNPs in IGF1R or PI3KCB had lower free IGF1 levels and lived longer than people without these SNPs.54 These data are relevant given that sirtuin levels are also modulated in part by IGF1R.55 Sirtuins modulate life span in different organisms from yeast to flies to C elegans, experiments in which caloric restriction led to increased survival.56
The link between IGF1R signaling, sirtuin expression, and survival is provocative. A link between the metabolic syndrome, IR, and IGF1R signaling and overall survival make IGF1R signaling a possible target for altering overall all-cause mortality.57 Already there are data that blockade with sirolimus of a downstream effector of IGF1R signaling, the mammalian target of rapamycin 1 (mTOR1), leads to an overall survival advantage in comparison to control organisms.58 Furthermore, deletion of S6 kinase (S6K1), a downstream effector of IGF1R as well as adenosine monophosphate kinase (AMPK) is associated with increased life span in mice compared to littermate controls, further linking these signaling pathways with survival.59 As further demonstration of the connection between IR and IGF1R signaling, aged S6K1−/− mice show less insulin resistance than their littermate controls. While the deleterious effects of mTOR inhibitors on lipid balance in humans may limit their use in the clinic, there will likely be other means to decrease IGF1R signaling to attempt to increase life span. Data regarding stimulation of AMPK through metformin (Fig 1), exercise, or other modulators may provide the functional link between IGF1R signaling, AMPK, sirtuin levels, and salutary physiological effects.60–61
IGFs AND CANCER RISK
While there are large variations in IGF and IGFBP levels in the population, there appear to be at least some links between IGF1 serum levels and cancer risk. As an extreme version of such a study, 228 subjects with defective signaling in the GH pathway were compared to 338 unaffected relatives. The risk of cancer was 0% in the study population, and 9% to 24% in the control population.62 Other support for this hypothesis comes from analysis of large prospective population studies. The Nurses' and Physicians' Health Studies have demonstrated a reasonably consistent relationship between higher IGF1 levels, lower IGFBP3 levels, and an increased risk of prostate, breast, and colorectal cancer.63–66
However, other data contradict a relationship between levels of IGF expression and cancer outcomes. Subjects with acromegaly appear to have a modest increase in cancer risk, in particular an approximately two-fold increased risk of colorectal cancer,67–69 but it is less than one might expect given the surfeit in signaling from excess GH.70 Patients with acromegaly have competing risks over time, such as diabetes and cardiovascular disease, which may complicate such an analysis. Thus, the relationship between IGFs and cancer risk remains unclear.
As with studies of serum IGF and IGFBP levels, it is important to understand the frequency of a genetic polymorphism in a given population to assess its role in potential risk for a disorder. Thus, such data are highly heterogeneous as well, borne out by the variety of epidemiologic studies of cancer risk and genetic polymorphisms in IGF and related signaling pathway members. While polymorphisms are understandably linked to levels of one component of the IGF1R signaling pathway or another, it is not clear at present how many of these will be germane in terms of cancer risk.71–74
LINKING IGF SIGNALING TO CANCER
As with ERBB2/HER2, IGF1R does not appear to be mutated in any cancers, unlike other receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR) and KIT that are mutated and oncogenic in specific cancers. It is therefore not possible to equate IGF1R or its ligands as oncogenic per se. However, IGF signaling has other features that are important for the survival of cancer cells, such as the role of IGF1R in permitting anchorage-independent cell growth.75 The importance of IGF signaling to the development of cancer is highlighted by its role in Simian virus (SV40) T-antigen–induced carcinogenesis76 and in MYC-induced transformation of fibroblasts and other cells.77,78 While relatively low expression levels of MYC have been shown to be oncogenic (or permissive for cell survival), greater MYC overexpression itself is proapoptotic. Remarkably, specific growth factors such as platelet-derived growth factor (PDGF) or IGF1, but not others such as EGF or basic fibroblast growth factor, protect cells from the apoptotic signal of increased MYC expression and permit further cell growth.79 As result, IGF1R signaling appears to be necessary but not sufficient for cancer cell growth. By extension, that MYC overexpression engages apoptotic and p53/ARF tumor suppressive pathways implies a deep link between IGF1 signaling, proliferation, and cancer cell survival, deeply intertwining energy metabolism, cell stress, and cell proliferation.80–81 The September 10, 2010, issue of Journal of Clinical Oncology addresses in more detail the issues of host factors such as energy balance and the risk of cancer and cancer progression.
IGF SIGNALING AND CARCINOMAS
IGF1R signaling appears to be a key factor in the growth of carcinomas. First, carcinomas can demonstrate strong cell surface expression of IGF1R.82–85 IGF1R signaling is necessary for soft agar growth of fibroblasts transfected with a plasmid expressing EGFR under a strong promoter (SV40).86 Signaling through IGF1R appears to be important for survival of at least some forms of carcinoma. For example, IGF1R overexpression appears to be characteristic of colorectal carcinomas versus adenomas82 and is associated with resistance to HER2 inhibitors in breast cancer.87,88 Other data indicate that EGFR family member signaling through IGF1R (ie, crosstalk) is important in signaling in carcinoma growth and survival.89–93
Of perhaps greater relevance to cancer therapeutics, IGF1R expression appears to be activated in carcinomas that are resistant to HER2 or EGFR inhibitors.89–93 Heterodimers between IGF1R and HER2/ERBB2 have already been identified as one mechanism of resistance of breast cancer cell lines to trastuzumab.94 Akt signaling, blocked in sensitive tumors with use of EGFR or HER2 inhibitors, is increased again in cell lines and tumors resistant to such inhibitors.90,95,96 The reactivation of Akt signaling is abrogated with the addition of an IGF1R inhibitor, with associated increased apoptosis of the cell lines.90 Sensitivity to EGFR or HER2 inhibitors may thus be restored by the additional blockade of IGF1R (Fig 1), immediately suggesting combinations of EGF family inhibitors with IGF1R inhibitors for patients with carcinomas. Decreased signaling through IGF1R is also observed in a trastuzumab-resistant breast cancer cell line treated with lapatinib (blocking EGFR and HER2), indicating that decreased IGF1R signaling may be a useful readout for the efficacy of EGFR family members.96
Conversely, resistance to IGF1R inhibitors can be mediated by signaling through EGFR family members (Fig 1). In a panel of ovarian cancer cell lines, EGFR/HER1 or ERBB2/HER2 can mediate resistance to an IGF1R inhibitor.97 Notably, greater inhibition of signaling is achieved with combinations of pan-HER and IGF1R inhibitors in this cell line than with either compound separately, again suggesting combinations of receptor tyrosine kinase–specific agents that could be useful broadly in the clinic.97 The finding of the interaction of IGF1R and EGFR family members may have broader applicability to solid tumors given the known dependence of a subset of astrocytomas to EGFR signaling in some, but not all studies,98–99 and the finding of the utility of combinations of IGF1R and EGFR inhibitors in a glioma cell line.100 Preclinical data also support the use of a combination of IGF1R inhibitors and cytotoxic chemotherapy in the treatment of carcinomas.101,102 Data regarding recent clinical trials is given below after discussing the importance of IGF1R signaling in sarcomas.
IGFs AND PEDIATRIC SARCOMAS
Since IGF1R signaling is important in the development of connective tissues and growth of carcinomas, it is not surprising that IGF1R signaling is important in the development of sarcomas. There has been long-term interest from several laboratories in IGF1R signaling and its role in sarcomagenesis, an interest that is now maturing with the availability of antibodies and small molecules targeting IGF1R. While IGF1 levels may have at least a weak association with cancer risk, as noted above, there are not direct data regarding IGF1 levels and sarcoma risk.
As with carcinomas, while there have been no mutations seen in IGF1R in sarcomas, the biology of some of the most common pediatric sarcomas involves obligate signaling through IGF1R to maintain the neoplastic phenotype. For example, in alveolar rhabdomyosarcoma, a sarcoma that contains a characteristic chromosomal translocation t(1;13) or t(2;13), the PAX3/7-FOXOA1 fusion protein activates IGF1R promoter, and increases expression of IGF1R.103 The degree of signaling Akt activation, which appears to be at least in part a function of increased IGF1R signaling, correlates with poor survival in stage III patients in the study IRS-IV.104 Patients with stage III rhabdomyosarcoma have an approximately 50% survival rate. By separating patients into those with high or low levels of Akt phosphorylation (ie, higher or lower tonic IGF1R signaling), it is possible to identify those patients who will fare poorly or well, respectively, and adjusting adjuvant treatment accordingly.105
Other data highlight the importance of IGF1R as a potential therapeutic modality in alveolar rhabdomyosarcoma. Treatment of cell lines with mTOR1 inhibitor rapamycin leads to paradoxical phosphorylation in Akt at serine 473, activation that can be blocked by inhibition of IGF1R (Fig 1);105 similar findings have been observed in a variety of epithelial cancer cell lines;106 anti-IGF1R monoclonal antibodies are active against human rhabdomyosarcoma cell line xenografts.107 These data suggest that a combination of an mTOR inhibitor and an IGF1R inhibitor may be an effective means of treating rhabdomyosarcoma and potentially other cancers.107
Data are compelling with another translocation-associated pediatric sarcoma. Ewing sarcoma typically contains t(11;22) EWSR1-FLI1 or related translocations/gene fusions. IGF1R appears to be important to sarcomagenesis from the EWSR1-FLI1 fusion gene, since Ewing sarcoma cell lines only survive when IGF1R signaling is intact.108 As with the utility of IGF1R inhibitors with chemotherapy in non–small-cell lung cancer (NSCLC) below, small molecule inhibitors of IGF1R synergize with chemotherapy or kinase directed therapy in Ewing sarcoma cell lines.109 Most compelling are clinical responses to IGF1R inhibitors, discussed below. Osteogenic sarcoma110–112 and desmoplastic small round cell tumor113 may also be sensitive to IGF1R blockade. These (and clinical data on SFT/HPC below) are the first bone fide demonstrations of clinical responses to IGF1R-directed monotherapy, giving hope that combinations with other targeted agents or cytotoxic chemotherapy will be even more effective.
IGF1R AND SARCOMAS MORE COMMONLY OBSERVED IN ADULTS
Pediatric sarcomas only represent approximately 15% of sarcomas, leaving the other more than 50 sarcoma subtypes largely unexplored with respect to IGF1R signaling. Expression arrays and Western blots have identified adult sarcomas subtypes that have the greatest relative amount of IGFs at the mRNA or protein level.114–120 For example, as a surrogate for IGF1R signaling, those sarcomas with highest IGF2 expression by immunohistochemistry include SFT/HPC, malignant peripheral-nerve sheath tumor, GIST, and synovial sarcoma (Fig 2).120 Although consistent expression would not foreordain a good response to agents that block the target, subtypes can be eliminated for testing that lack expression of the target of interest, understanding that our screening tests may be insensitive for assessing expression of a relevant protein.121
Fig 2.
Relative expression of insulin growth factor 2 (IGF2) protein by immunohistochemistry in different sarcoma subtypes.120 The number of samples examined for each subtype is indicated in parentheses. SFT, solitary fibrous tumor; HPC, hemangiopericytoma; MPNST, malignant peripheral-nerve sheath tumor; HGUPS, high-grade undifferentiated pleomorphic sarcoma; MFH, malignant fibrous histiocytoma; PVNS, pigmented villonodular synovitis; TGCT, tenosynovial giant cell tumor.
Data from cell lines for many forms of sarcoma are lacking, except for GIST. The approximately 7% of GIST without KIT or PDGFRA mutations express IGF1R in excess of that seen in KIT or PDGFRA mutant GIST,122,123 and cell death is observed after application of IGF1R inhibitors to these cell lines.123 The finding of IGF1R expression in GIST without KIT or PDGFR mutations (often found in GISTs in patients younger than 25 years, so-called pediatric GISTs) has led to the suggestion to develop IGF1R inhibitors for patients with KIT/PDGFRA GIST without mutations. Whether combinations of IGF1R with KIT inhibitors could lead to clinical responses in KIT or PDGFRA mutant GIST patients with disease progressing on imatinib and sunitinib awaits future study as well.
RESULTS FROM CLINICAL TRIALS OF IGF1R INHIBITORS
Over the past year, clinical studies of IGF1R inhibitors have been published to get a sense of the role of IGF1R inhibitors (monoclonal antibodies) in clinical practice. In phase I studies these antibodies have been well-tolerated as single agents (Table 2).124–126 Toxicity has been generally National Cancer Institute grade 1 to 2, and includes elevations of liver function tests, asthenia, nausea, thrombocytopenia, and arthralgias. Modest single activity has been observed in Ewing sarcoma to date, with two objective responses of 16 patients in an expansion cohort for a phase I study (12% response rate). Anecdotes of patients with HPC/SFT are a potential exciting proof of principle of the utility of IGF1R blockade,127,128 given the consistent overexpression of IGF2 in HPC/SFT129 and the hypoglycemia of malignancy observed in some people with this tumor, as noted above.
Table 2.
Phase I to II Clinical Trials of IGF1R Inhibitory Monoclonal Antibodies, Toxicity, and Outcomes
| Agent | Type of Clinical Trial | No. of Subjects | Dose and Schedule | Observed Toxicity, Most Common AEs | Outcome |
|---|---|---|---|---|---|
| Single-agent studies | |||||
| Figitumumab (CP-751, 871) | Phase I/multiple myeloma (11 dose levels), with dexamethasone or sirolimus permitted after first dosing if no PR | 47 | Variable dose IV once; every 28 days dosing permitted | Asthenia; anemia (15%); increased ALT/AST; hyperglycemia (11%; all grade 1 to 2, in combination with dexamethasone) | Two PR in patients progressing on dexamethasone |
| Phase I/solid tumors (four dose levels) | 24 | Variable dose IV every 21 days | Hyperglycemia; elevated GGT, ALT/AST; nausea; fatigue (all grade 1 to 2 except one grade 3 fatigue) | MTD not reached; maximum feasible dose 20 mg/kg/dose; 10/15 with stable disease as best outcome; no RECIST responses | |
| Phase I cohort expansion: adrenocortical cancer | 14 | 20 mg/kg every 21 days | 1 grade 4 GGT elevation; grade 3 elevations in GGT, glucose, alk phos, ALT/AST | 8/14 with SD | |
| Phase I cohort expansion: sarcomas | 29 (16 Ewing sarcomas) | 20 mg/kg every 21 days | One grade 4 uric acid elevation; 1 grade 3 DVT | 1 CR, 1 PR Ewing sarcoma, 8/29 with SD more than 4 months | |
| AMG479 | Phase I | 53 | Variable dose (20 mg/kg IV maximum) every 14 days | Fatigue, thrombocytopenia, fever, rash, chills, anorexia (all grade 1 to 2); one DLT thrombocytopenia G3; eight episodes thrombocytopenia grade 3, one elevated ALT/AST grade 3 seen in expansion phase | 1 CR Ewing sarcoma; 1 unconfirmed PR Ewing sarcoma; 1 PR neuroendocrine tumor |
| Combination studies | |||||
| Figitumumab, carboplatin, paclitaxel | Phase I | 42 | Paclitaxel 200 mg/m2, carboplatin (AUC 6), figitumumab (0.05 to 20 mg/kg) every 3 weeks | SAEs: fatigue, diarrhea, hyperglycemia, GGT elevation, and thrombocytopenia (1 patient each) | MTD not identified; 13 PR, 2 CR (NSCLC, ovarian carcinoma) |
| Randomized phase II | 156 (2:1 randomization in favor of three-drug combination) | Paclitaxel 200 mg/m2, carboplatin (AUC 6), ± figitumumab (20 mg/kg) every 3 weeks; after 6 cycles, could continue figitumumab | 54% (three drugs) versus 42% (two drugs) RECIST RR; 14/18 randomly assigned patients with squamous cell cancer receiving three drugs had PR; HR for PFS for three drug combination versus 2 drug combination 0.56 to 0.8 |
Abbreviations: IGF1R, insulin-like growth factor receptor 1; AE, adverse event; PR, partial response; IV, intravenous; GGT, gamma glutamyl transpeptidase; MTD, minimal residual disease; RECIST, Response Evaluation Criteria for Solid Tumors; alk phos, alkaline phosphatase; SD, stable disease; DLT, dose-limiting toxicities; DVT, deep vein thrombosis; CR, complete response; AUC, area under the curve; SAE, serious adverse event; NSCLC, non–small-cell lung cancer; RR, response rate; HR, hazard ratio; PFS, progression-free survival.
In combination studies, figitumumab appeared promising with carboplatin and paclitaxel in phase I trial (mostly patients with NSCLC) and a randomized phase II study (NSCLC). However, a phase III study of carboplatin, paclitaxel, with or without figitumumab in first-line for metastatic NSCLC was stopped on December 29, 2009, owing to a recommendation from the data safety monitoring board that the study was unlikely to meet the primary end point of improving overall survival.130 The finding of only modest activity in Ewing sarcoma and lack of survival advantage in NSCLC in combination with cytotoxic agents has led to a rapid deceleration of clinical development of IGF1R antagonists. Given the data regarding resistance patterns to EGFR and HER2 inhibitors, it may well be that only in combination will better clinical results be obtained, except in very select situations. The finding of specific molecular cohorts of NSCLC that responded to figitumumab-based therapy lends hope that, as with crizotinib in anaplastic lymphoma receptor tyrosine kinase + NSCLC,131 appropriate patients for IGF1R therapy may be identified by prospective screening.132
FUTURE DIRECTIONS
The recent clinical findings are a clarion call for a better integrated understanding of IGF1R, mTOR, and ras-raf signaling, which should lead to new generations of clinical trials in cancer with agents that modulate this pathway (Table 3). A striking example of the integration of dietary intake, metabolism, and cancer growth is made plain with the demonstration of the sensitivity and resistance to dietary restriction of growth of different human carcinoma xenografts in mice.133 One mechanism of resistance to dietary restriction of tumor growth appears to be the loss of PTEN expression, directly implicating Akt andmTOR in this process. Additional hope of the use of IGF1R inhibitors in cancer therapy is highlighted with the demonstration of the reversal of a form of EGFR inhibitor tolerance by an IGF1R inhibitor (or a histone deacetylase inhibitor).134
Table 3.
Questions to Be Addressed Regarding IGF1R in Cancer Therapeutics and Prevention
| Cellular level |
| Which partners cross-talk with IGF1R at the cell membrane and inside the cell? |
| Does resistance develop to IGF1R via EGFR family or other structurally related molecules? |
| How do IGFBPs interact with IGF1R to amplify or dampen IGF1R signaling? |
| How do IGFBPs signal independently of IGF1R? |
| Do proteolytic fragments of IGFBPs have signaling activity? |
| Can the expression of IGF2R affect IGF2 levels to a significant degree to affect changes in IGF1R signaling? |
| Which form of Akt is most relevant for IGF1R signaling in a given cell? |
| Patient level |
| What is the role of IGF1R in angiogenesis? |
| Are IGF1R inhibitors safe and effective in combinations with other targeted agents or cytotoxic chemotherapy? |
| Will horizontal (multiple kinases) or vertical (several steps) be more important in combining targeted agents? |
| If effective, what is the best sequence of IGF1R inhibitor and cytotoxic chemotherapy? |
| Population level |
| Are IGF or IGFBP levels associated with cancer risk independent of other factors? |
| Will SNP analysis of IGF signaling pathway genes yield insight into cancer risk? |
| Can slowing IGF1R signaling decrease cancer risk? |
| Will decreasing IGF1R signaling increase lifespan? |
| Is the hyperlipidemia observed with mTOR inhibitors separable from its other potentially useful metabolic effects? |
Abbreviations: IGF1R, insulin-like growth factor receptor 1; EGFR, epidermal growth factor receptor; IGF, insulin-like growth factor; IGFBPs, insulin-like growth factor binding proteins; SNP, single nucleotide polymorphism.
At every step of IGF1R signaling we are learning of increasing complexity of signaling pathways, for example the role of IGF1R signaling in coordinated signaling with estrogen receptor,88,135 Fas/Fas ligand,136 or p53,137,138 some of which appear to be mediated by IGFBPs. Equally interesting and perhaps of greater societal relevance will be studies that link mTOR inhibition, downstream effectors of AMPK, IGF1R, p53, and sirtuins to endurance and life span.
It is difficult to overestimate the potential effects of understanding the relationship between metabolism, life span, and cancer will have on generations to come. A recent publication indicated that we need to prepare for a human life span of longer than 100 years for children born after 2000 in economically developed societies.139 An economics treatise from a generation ago, “Small is Beautiful: Economics as if People Mattered ” by E.F. Schumacher,140 foretold of the strain on the planet of an exponential increase in demand for natural resources on global economic development, and, converse to the idea of “bigger is better,” called for sustainable development on a local level.
Cancer cells have already solved the complex equation balancing the unbridled need for metabolic resources with survival of the tumor,141 perhaps best illustrated by the Gompertzian kinetics of tumor growth.142,143 Both in a literal sense (increased survival of smaller IGF knockout mice v littermates) and a figurative sense (the association of decreased tonic IGF1R signaling with lower cancer risk and increased life span), the lessons of “Small is Beautiful” appear to have credence in the energy balance of both the cancer cell and host. It is increasingly certain that we can modify IGF1R signaling in a normal host and affect life span and glucose tolerance. As of 2010, the effects of IGF1R inhibitors do not appear to be like insulin for diabetes, like platinum compounds for germ cell tumors, nor like imatinib for chronic myelogenous leukemia or GIST for the vast majority of patients with cancer. Nonetheless, further dissecting IGF1R signaling in both the cancer and in the host should allow for the development of new antineoplastic strategies that cut across different histologic tumor classifications.
Acknowledgment
I thank colleagues for useful discussions on these topics over the past year, in particular with Lee Helman, MD, David Thomas, MBBS, PhD, FRACP, Andy Wagner, MD, PhD, and Bill Tap, MD, constructive comments from Stephen Cannistra, MD, and two reviewers, and for the book recommendation from Larry Norton, MD.
Appendix
Table A1.
Inhibitors of Steps at/Downstream From IGF1R and a Link to Single-Agent Clinical Trials
| Target | Agent | Target Disease | Clinicaltrials.gov Study Identifier* |
|---|---|---|---|
| IGF1, IGF2 | MEDI-573 | Phase I | NCT00816361 (o); |
| IGF1R | Figitumumab (CP-751,871) | Colorectal cancer; Ewing sarcoma; breast cancer; phase I | NCT00560560 (c); NCT00560235 (c); NCT00635245 (o); NCT00474760 (c) |
| R1507 | Breast cancer; pediatric phase I; Ewing sarcoma, other sarcomas | NCT00882674 (o); NCT00560144 (c); NCT00609141 (c) | |
| BIIB022 | Phase I | NCT00555724 (c) | |
| AMG479 | Ovarian cancer; Ewing sarcoma, DSRCT; phase I | NCT00719212 (o); NCT00563680 (c); NCT00562380 (c) | |
| Dalotuzumab (MK-0646) | Breast cancer; | NCT00759785 (o) | |
| Cixutumumab (IMC-A12) | Pediatric cancer; thymoma, thymic carcinoma; phase I | NCT00831844 (o); NCT00965250 (o); NCT00785538 (c), NCT00785941 (c) | |
| SCH717454 | Colorectal cancer; osteosarcoma, Ewing sarcoma | NCT00551213 (c); NCT00617890 (o) | |
| IGF1R kinase (and other kinases) | XL228 | Phase I | NCT00526838 (o) |
| KW2450 | Phase I | NCT00921336 (o) | |
| PI3 kinase | SF1126 | Phase I | NCT00907205 (o) |
| GDC0941 | Phase I | NCT00876109 (o); NCT00876122 (o) | |
| PX866 | Phase I | NCT00726583 (o) | |
| BGT226 | Phase I | NCT00600275 (o); NCT00742105 (o) | |
| XL147 | Phase I | NCT01013324 (o); NCT00486135 (o) | |
| CAL101 | Hematologic malignancy phase I | NCT00710528 (o) | |
| PI3 kinase and mTOR | XL765 | Phase I | NCT00485719 (o) |
| BEZ235 | Phase I/II breast cancer | NCT00620594 (o) | |
| PF04691502 | Phase I | NCT00927823 (o) | |
| AKT | GSK2141795 | Phase I | NCT00920257 (o) |
| Triciribine | Phase I | NCT00363454 (o) | |
| Perifosine | Pediatric phase I; glioma; sarcomas; Waldenstrom macroglobulinemia; renal carcinoma; CLL/SLL | NCT00776867 (o); NCT00590954 (o); NCT00401388 (c); NCT00422656 (o); NCT00498966 (c); NCT00873457 (o) | |
| mTOR1 | Temsirolimus | FDA approved | |
| Everolimus | FDA approved | ||
| Sirolimus | FDA approved for transplant patients | ||
| Ridaforolimus (MK8669, AP23573) | Pediatric phase I; sarcomas; K-ras mutant non–small-cell lung cancer; endometrial cancer | NCT00704054 (o); NCT00538239 (c); NCT00818675 (o); NCT00770185 (o) | |
| mTOR1 + mTOR2 | AZD8055 | Phase I | NCT00731263 (o) |
Abbreviations: IGF1R, insulin-like growth factor receptor 1; IGF, insulin-like growth factor; (o), open; (c), closed to new patients; DSRCT, desmoplastic small round cell tumor; CLL/SLL, chronic lymphocytic leukemia/small lymphocytic lymphoma; FDA, US Food and Drug Administration.
Studies accessioned January 15, 2010. Not included are studies of FDA-approved agents, completed studies, or studies terminated by the sponsor.
Footnotes
Supported by Grants No. CA47179, CA148260, and CM62202 from the National Cancer Institute, National Cancer Institute American Society of Clinical Oncology Foundation/Cancer Therapy Evaluation Program Clinical Investigator Team Leadership Award, Cycle for Survival, and the Shuman Family Fund for GIST Research.
Author's disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHOR'S DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Robert G. Maki, Novartis, Bayer, Ziopharm Research Funding: Robert G. Maki, Hoffman La Roche, Pfizer Expert Testimony: None Other Remuneration: None
REFERENCES
- 1.Matsumoto S, Isogai A, Suzuki A. N-terminal amino acid sequence of an insect neurohormone, melanization and reddish coloration hormone (MRCH): Heterogeneity and sequence homology with human insulin-like growth factor II. FEBS Lett. 1985;189:115–118. doi: 10.1016/0014-5793(85)80853-x. [DOI] [PubMed] [Google Scholar]
- 2.Reinecke M, Collet C. The phylogeny of the insulin-like growth factors. Int Rev Cytol. 1998;183:1–94. doi: 10.1016/s0074-7696(08)60142-4. [DOI] [PubMed] [Google Scholar]
- 3.Emdin SO, Falkmer S. Phylogeny of insulin: Some evolutionary aspects of insulin production with particular regard to the biosynthesis of insulin in Myxine glutinosa. Acta Paediatr Scand. 1977;270(suppl):15–25. doi: 10.1111/j.1651-2227.1977.tb15117.x. [DOI] [PubMed] [Google Scholar]
- 4.Ebberink RHM, Smit AB, van Minnen J. The insulin family: Evolution of structure and function in vertebrates and invertebrates. Biol Bull. 1989;177:176–182. [Google Scholar]
- 5.Marchler-Bauer A, Anderson JB, Derbyshire MK, et al. CDD: A conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:D237–D240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iwami M. Bombyxin: An insect brain peptide that belongs to the insulin family. Zoolog Sci. 2000;17:1035–1044. doi: 10.2108/zsj.17.1035. [DOI] [PubMed] [Google Scholar]
- 7.Okamoto N, Yamanaka N, Satake H, et al. An ecdysteroid-inducible insulin-like growth factor-like peptide regulates adult development of the silkmoth Bombyx mori. FEBS J. 2009;276:1221–1232. doi: 10.1111/j.1742-4658.2008.06859.x. [DOI] [PubMed] [Google Scholar]
- 8.Klonisch T, Bialek J, Radestock Y, et al. Relaxin-like ligand-receptor systems are autocrine/paracrine effectors in tumor cells and modulate cancer progression and tissue invasiveness. Adv Exp Med Biol. 2007;612:104–118. doi: 10.1007/978-0-387-74672-2_8. [DOI] [PubMed] [Google Scholar]
- 9.Froesch ER, Schmid C, Schwander J, et al. Actions of insulin-like growth factors. Annu Rev Physiol. 1985;47:443–467. doi: 10.1146/annurev.ph.47.030185.002303. [DOI] [PubMed] [Google Scholar]
- 10.Salmon WD, Jr, Daughaday WH. A hormonally controlled serum factor which stimulates sulfate incorporation by cartilage in vitro. J Lab Clin Med. 1957;49:825–836. [PubMed] [Google Scholar]
- 11.Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769–2776. [PubMed] [Google Scholar]
- 12.Li Y, Chang Q, Rubin BP, et al. Insulin receptor activation in solitary fibrous tumours. J Pathol. 2007;211:550–554. doi: 10.1002/path.2136. [DOI] [PubMed] [Google Scholar]
- 13.Rikhof B, de Jong S, Suurmeijer AJ, et al. The insulin-like growth factor system and sarcomas. J Pathol. 2009;217:469–482. doi: 10.1002/path.2499. [DOI] [PubMed] [Google Scholar]
- 14.Zapf J. Role of insulin-like growth factor (IGF) II and IGF binding proteins in extrapancreatic tumour hypoglycaemia. J Intern Med. 1993;234:543–552. doi: 10.1111/j.1365-2796.1993.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 15.Daughaday WH, Emanuele MA, Brooks MH, et al. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988;319:1434–1440. doi: 10.1056/NEJM198812013192202. [DOI] [PubMed] [Google Scholar]
- 16.Schmid C, Steiner T, Froesch ER. Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett. 1983;161:117–121. doi: 10.1016/0014-5793(83)80742-x. [DOI] [PubMed] [Google Scholar]
- 17.Seely BL, Reichart DR, Takata Y, et al. A functional assessment of insulin/insulin-like growth factor-I hybrid receptors. Endocrinology. 1995;136:1635–1641. doi: 10.1210/endo.136.4.7895674. [DOI] [PubMed] [Google Scholar]
- 18.Aleksic T, Chitnis MM, Perestenko OV, et al. Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res. 2010;70:6412–6419. doi: 10.1158/0008-5472.CAN-10-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Sehat B, Tofigh A, Lin Y, et al. SUMOylation mediates the nuclear translocation and signaling of the IGF-1 receptor. Sci Signal. doi: 10.1126/scisignal.2000628. doi: 10.1126/scisignal.2000628. [DOI] [PubMed] [Google Scholar]
- 19.Grimberg A, Cohen P. Role of insulin-like growth factors and their binding proteins in growth control and carcinogenesis. J Cell Physiol. 2000;183:1–9. doi: 10.1002/(SICI)1097-4652(200004)183:1<1::AID-JCP1>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michael IP, Pampalakis G, Mikolajczyk SD, et al. Human tissue kallikrein 5 is a member of a proteolytic cascade pathway involved in seminal clot liquefaction and potentially in prostate cancer progression. J Biol Chem. 2006;281:12743–12750. doi: 10.1074/jbc.M600326200. [DOI] [PubMed] [Google Scholar]
- 21.Frost VJ, Macaulay VM, Wass JA, et al. Proteolytic modification of insulin-like growth factor-binding proteins: Comparison of conditioned media from human cell lines, circulating proteases and characterized enzymes. J Endocrinol. 1993;138:545–554. doi: 10.1677/joe.0.1380545. [DOI] [PubMed] [Google Scholar]
- 22.Schmid C, Rutishauser J, Schlapfer I, et al. Intact but not truncated insulin-like growth factor binding protein-3 (IGFBP-3) blocks IGF I-induced stimulation of osteoblasts: Control of IGF signalling to bone cells by IGFBP-3-specific proteolysis? Biochem Biophys Res Commun. 1991;179:579–585. doi: 10.1016/0006-291x(91)91410-e. [DOI] [PubMed] [Google Scholar]
- 23.Zhu W, Shiojima I, Ito Y, et al. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature. 2008;454:345–349. doi: 10.1038/nature07027. [DOI] [PubMed] [Google Scholar]
- 24.Iosef C, Gkourasas T, Jia CY, et al. A functional nuclear localization signal in insulin-like growth factor binding protein-6 mediates its nuclear import. Endocrinology. 2008;149:1214–1226. doi: 10.1210/en.2007-0959. [DOI] [PubMed] [Google Scholar]
- 25.Liu B, Lee HY, Weinzimer SA, et al. Direct functional interactions between insulin-like growth factor-binding protein-3 and retinoid X receptor-alpha regulate transcriptional signaling and apoptosis. J Biol Chem. 2000;275:33607–33613. doi: 10.1074/jbc.M002547200. [DOI] [PubMed] [Google Scholar]
- 26.Schedlich LJ, O'Han MK, Leong GM, et al. Insulin-like growth factor binding protein-3 prevents retinoid receptor heterodimerization: Implications for retinoic acid-sensitivity in human breast cancer cells. Biochem Biophys Res Commun. 2004;314:83–88. doi: 10.1016/j.bbrc.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 27.Schedlich LJ, Graham LD, O'Han MK, et al. Molecular basis of the interaction between IGFBP-3 and retinoid X receptor: Role in modulation of RAR-signaling. Arch Biochem Biophys. 2007;465:359–369. doi: 10.1016/j.abb.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Lee KW, Cohen P. Nuclear effects: Unexpected intracellular actions of insulin-like growth factor binding protein-3. J Endocrinol. 2002;175:33–40. doi: 10.1677/joe.0.1750033. [DOI] [PubMed] [Google Scholar]
- 29.Xu FP, Xie D, Wen JM, et al. SRC-3/AIB1 protein and gene amplification levels in human esophageal squamous cell carcinomas. Cancer Lett. 2007;245:69–74. doi: 10.1016/j.canlet.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 30.Hussein-Fikret S, Fuller PJ. Expression of nuclear receptor coregulators in ovarian stromal and epithelial tumours. Mol Cell Endocrinol. 2005;229:149–160. doi: 10.1016/j.mce.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Henke RT, Haddad BR, Kim SE, et al. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin Cancer Res. 2004;10:6134–6142. doi: 10.1158/1078-0432.CCR-04-0561. [DOI] [PubMed] [Google Scholar]
- 32.York B, Yu C, Sagen JV, et al. Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc Natl Acad Sci U S A. 2010;107:11122–11127. doi: 10.1073/pnas.1005262107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh Y, Muller HL, Ng L, et al. Transforming growth factor-beta-induced cell growth inhibition in human breast cancer cells is mediated through insulin-like growth factor-binding protein-3 action. J Biol Chem. 1995;270:13589–13592. doi: 10.1074/jbc.270.23.13589. [DOI] [PubMed] [Google Scholar]
- 34.Gucev ZS, Oh Y, Kelley KM, et al. Insulin-like growth factor binding protein 3 mediates retinoic acid- and transforming growth factor beta 2-induced growth inhibition in human breast cancer cells. Cancer Res. 1996;56:1545–1550. [PubMed] [Google Scholar]
- 35.Walker G, MacLeod K, Williams AR, et al. Insulin-like growth factor binding proteins IGFBP3, IGFBP4, and IGFBP5 predict endocrine responsiveness in patients with ovarian cancer. Clin Cancer Res. 2007;13:1438–1444. doi: 10.1158/1078-0432.CCR-06-2245. [DOI] [PubMed] [Google Scholar]
- 36.Sheen-Chen SM, Zhang H, Huang CC, et al. Insulin-like growth factor-binding protein-3 in breast cancer: Analysis with tissue microarray. Anticancer Res. 2009;29:1131–1135. [PubMed] [Google Scholar]
- 37.Munoz MT, Barrios V, Pozo J, et al. Insulin-like growth factor I, its binding proteins 1 and 3, and growth hormone-binding protein in children and adolescents with insulin-dependent diabetes mellitus: Clinical implications. Pediatr Res. 1996;39:992–998. doi: 10.1203/00006450-199606000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Toretsky JA, Steinberg SM, Thakar M, et al. Insulin-like growth factor type 1 (IGF-1) and IGF binding protein-3 in patients with Ewing sarcoma family of tumors. Cancer. 2001;92:2941–2947. doi: 10.1002/1097-0142(20011201)92:11<2941::aid-cncr10072>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 39.Trent JC, Ramdas L, Dupart J, et al. Early effects of imatinib mesylate on the expression of insulin-like growth factor binding protein-3 and positron emission tomography in patients with gastrointestinal stromal tumor. Cancer. 2006;107:1898–1908. doi: 10.1002/cncr.22214. [DOI] [PubMed] [Google Scholar]
- 40.Liu JP, Baker J, Perkins AS, et al. Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 41.Sutter NB, Bustamante CD, Chase K, et al. A single IGF1 allele is a major determinant of small size in dogs. Science. 2007;316:112–115. doi: 10.1126/science.1137045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savage MO, Attie KM, David A, et al. Endocrine assessment, molecular characterization and treatment of growth hormone insensitivity disorders. Nat Clin Pract Endocrinol Metab. 2006;2:395–407. doi: 10.1038/ncpendmet0195. [DOI] [PubMed] [Google Scholar]
- 43.Abuzzahab MJ, Schneider A, Goddard A, et al. IGF-I receptor mutations resulting in intrauterine and postnatal growth retardation. N Engl J Med. 2003;349:2211–2222. doi: 10.1056/NEJMoa010107. [DOI] [PubMed] [Google Scholar]
- 44.Sparago A, Cerrato F, Vernucci M, et al. Microdeletions in the human H19 DMR result in loss of IGF2 imprinting and Beckwith-Wiedemann syndrome. Nat Genet. 2004;36:958–960. doi: 10.1038/ng1410. [DOI] [PubMed] [Google Scholar]
- 45.Conway GS, Jacobs HS, Holly JM, et al. Effects of luteinizing hormone, insulin, insulin-like growth factor-I and insulin-like growth factor small binding protein 1 in the polycystic ovary syndrome. Clin Endocrinol (Oxf) 1990;33:593–603. doi: 10.1111/j.1365-2265.1990.tb03897.x. [DOI] [PubMed] [Google Scholar]
- 46.Lemne C, Brismar K. Insulin-like growth factor binding protein-1 as a marker of the metabolic syndrome–a study in borderline hypertension. Blood Press. 1998;7:89–95. [PubMed] [Google Scholar]
- 47.Yeap B, Chubb SA, Ho K, et al. Insulin-like growth factor-I and its binding proteins 3 and 1 are differentially associated with metabolic syndrome in older men. Eur J Endocrinol. 2010;162:249–257. doi: 10.1530/EJE-09-0852. [DOI] [PubMed] [Google Scholar]
- 48.De Leo V, La Marca A, Orvieto R, et al. Effect of metformin on insulin-like growth factor (IGF) I and IGF-binding protein I in polycystic ovary syndrome. J Clin Endocrinol Metab. 2000;85:1598–1600. doi: 10.1210/jcem.85.4.6560. [DOI] [PubMed] [Google Scholar]
- 49.Eckertova M, Krskova K, Penesova A, et al. Impaired insulin secretion and uptake in patients with diffuse idiopathic skeletal hyperostosis. Endocr Regul. 2009;43:149–155. [PubMed] [Google Scholar]
- 50.Piper MD, Selman C, McElwee JJ, et al. Separating cause from effect: How does insulin/IGF signalling control lifespan in worms, flies and mice? J Intern Med. 2008;263:179–191. doi: 10.1111/j.1365-2796.2007.01906.x. [DOI] [PubMed] [Google Scholar]
- 51.Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 52.Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selman C, Lingard S, Choudhury AI, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818. doi: 10.1096/fj.07-9261com. [DOI] [PubMed] [Google Scholar]
- 54.Bonafe M, Barbieri M, Marchegiani F, et al. Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: Cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab. 2003;88:3299–3304. doi: 10.1210/jc.2002-021810. [DOI] [PubMed] [Google Scholar]
- 55.Al-Regaiey KA, Masternak MM, Bonkowski M, et al. Long-lived growth hormone receptor knockout mice: Interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146:851–860. doi: 10.1210/en.2004-1120. [DOI] [PubMed] [Google Scholar]
- 56.Guarente L. Sirtuins in aging and disease. Cold Spring Harb Symp Quant Biol. 2007;72:483–488. doi: 10.1101/sqb.2007.72.024. [DOI] [PubMed] [Google Scholar]
- 57.Liang F, Kume S, Koya D. SIRT1 and insulin resistance. Nat Rev Endocrinol. 2009;5:367–373. doi: 10.1038/nrendo.2009.101. [DOI] [PubMed] [Google Scholar]
- 58.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144. doi: 10.1126/science.1177221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134:405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shevah O, Laron Z. Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: A preliminary report. Growth Horm IGF Res. 2007;17:54–57. doi: 10.1016/j.ghir.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor-I and prostate cancer risk: A prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 64.Chan JM, Stampfer MJ, Ma J, et al. Insulin-like growth factor-I (IGF-I) and IGF binding protein-3 as predictors of advanced-stage prostate cancer. J Natl Cancer Inst. 2002;94:1099–1106. doi: 10.1093/jnci/94.14.1099. [DOI] [PubMed] [Google Scholar]
- 65.Hankinson SE, Willett WC, Colditz GA, et al. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393–1396. doi: 10.1016/S0140-6736(97)10384-1. [DOI] [PubMed] [Google Scholar]
- 66.Ma J, Pollak MN, Giovannucci E, et al. Prospective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3. J Natl Cancer Inst. 1999;91:620–625. doi: 10.1093/jnci/91.7.620. [DOI] [PubMed] [Google Scholar]
- 67.Brunner JE, Johnson CC, Zafar S, et al. Colon cancer and polyps in acromegaly: Increased risk associated with family history of colon cancer. Clin Endocrinol (Oxf) 1990;32:65–71. doi: 10.1111/j.1365-2265.1990.tb03751.x. [DOI] [PubMed] [Google Scholar]
- 68.Pines A, Rozen P, Ron E, et al. Gastrointestinal tumors in acromegalic patients. Am J Gastroenterol. 1985;80:266–269. [PubMed] [Google Scholar]
- 69.Ron E, Gridley G, Hrubec Z, et al. Acromegaly and gastrointestinal cancer. Cancer. 1991;68:1673–1677. doi: 10.1002/1097-0142(19911015)68:8<1673::aid-cncr2820680802>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 70.Renehan AG, Brennan BM. Acromegaly, growth hormone and cancer risk. Best Pract Res Clin Endocrinol Metab. 2008;22:639–657. doi: 10.1016/j.beem.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 71.Chen W, Wang S, Tian T, et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: Evidence from 96 studies. Eur J Hum Genet. 2009;17:1668–1675. doi: 10.1038/ejhg.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harrela M, Koistinen H, Kaprio J, et al. Genetic and environmental components of interindividual variation in circulating levels of IGF-I, IGF-II, IGFBP-1, and IGFBP-3. J Clin Invest. 1996;98:2612–2615. doi: 10.1172/JCI119081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Marchand L, Donlon T, Seifried A, et al. Association of a common polymorphism in the human GH1 gene with colorectal neoplasia. J Natl Cancer Inst. 2002;94:454–460. doi: 10.1093/jnci/94.6.454. [DOI] [PubMed] [Google Scholar]
- 74.Patel AV, Cheng I, Canzian F, et al. IGF-1, IGFBP-1, and IGFBP-3 polymorphisms predict circulating IGF levels but not breast cancer risk: Findings from the Breast and Prostate Cancer Cohort Consortium (BPC3) PLoS One. 2008;3:e2578. doi: 10.1371/journal.pone.0002578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brodt P, Samani A, Navab R. Inhibition of the type I insulin-like growth factor receptor expression and signaling: Novel strategies for antimetastatic therapy. Biochem Pharmacol. 2000;60:1101–1107. doi: 10.1016/s0006-2952(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 76.Christofori G, Naik P, Hanahan D. Deregulation of both imprinted and expressed alleles of the insulin-like growth factor 2 gene during beta-cell tumorigenesis. Nat Genet. 1995;10:196–201. doi: 10.1038/ng0695-196. [DOI] [PubMed] [Google Scholar]
- 77.Evan GI, Wyllie AH, Gilbert CS, et al. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 78.Fanidi A, Harrington EA, Evan GI. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- 79.Harrington EA, Bennett MR, Fanidi A, et al. C-Myc-induced apoptosis in fibroblasts is inhibited by specific cytokines. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Murphy DJ, Junttila MR, Pouyet L, et al. Distinct thresholds govern Myc's biological output in vivo. Cancer Cell. 2008;14:447–457. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Levine AJ, Feng Z, Mak TW, et al. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 82.Hakam A, Yeatman TJ, Lu L, et al. Expression of insulin-like growth factor-1 receptor in human colorectal cancer. Hum Pathol. 1999;30:1128–1133. doi: 10.1016/s0046-8177(99)90027-8. [DOI] [PubMed] [Google Scholar]
- 83.Ouban A, Muraca P, Yeatman T, et al. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum Pathol. 2003;34:803–808. doi: 10.1016/s0046-8177(03)00291-0. [DOI] [PubMed] [Google Scholar]
- 84.Gualberto A, Melvin CL, Dean A, et al. Characterization of NSCLC patients responding to anti-IGF-IR therapy. J Clin Oncol. 2008;26(abstr 8000):424s. [Google Scholar]
- 85.Gong Y, Yao E, Shen R, et al. High expression levels of total IGF-1R and sensitivity of NSCLC cells in vitro to an anti-IGF-1R antibody (R1507) PLoS One. 2009;4:e7273. doi: 10.1371/journal.pone.0007273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coppola D, Ferber A, Miura M, et al. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol. 1994;14:4588–4595. doi: 10.1128/mcb.14.7.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riedemann J, Sohail M, Macaulay VM. Dual silencing of the EGF and type 1 IGF receptors suggests dominance of IGF signaling in human breast cancer cells. Biochem Biophys Res Commun. 2007;355:700–706. doi: 10.1016/j.bbrc.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 88.Chakraborty AK, Welsh A, Digiovanna MP. Co-targeting the insulin-like growth factor I receptor enhances growth-inhibitory and pro-apoptotic effects of anti-estrogens in human breast cancer cell lines. Breast Cancer Res Treat. 2009;120:327–335. doi: 10.1007/s10549-009-0382-5. [DOI] [PubMed] [Google Scholar]
- 89.Bender LM, Nahta R. Her2 cross talk and therapeutic resistance in breast cancer. Front Biosci. 2008;13:3906–3912. doi: 10.2741/2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chakraborty AK, Liang K, DiGiovanna MP. Co-targeting insulin-like growth factor I receptor and HER2: Dramatic effects of HER2 inhibitors on nonoverexpressing breast cancer. Cancer Res. 2008;68:1538–1545. doi: 10.1158/0008-5472.CAN-07-5935. [DOI] [PubMed] [Google Scholar]
- 91.Lu D, Zhang H, Koo H, et al. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280:19665–19672. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 92.Lu Y, Zi X, Zhao Y, et al. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 93.Ueda S, Hatsuse K, Tsuda H, et al. Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol. 2006;19:788–796. doi: 10.1038/modpathol.3800582. [DOI] [PubMed] [Google Scholar]
- 94.Nahta R, Yuan LX, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 95.Esparis-Ogando A, Ocana A, Rodriguez-Barrueco R, et al. Synergic antitumoral effect of an IGF-IR inhibitor and trastuzumab on HER2-overexpressing breast cancer cells. Ann Oncol. 2008;19:1860–1869. doi: 10.1093/annonc/mdn406. [DOI] [PubMed] [Google Scholar]
- 96.Nahta R, Yuan LX, Du Y, et al. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 97.Haluska P, Carboni JM, TenEyck C, et al. HER receptor signaling confers resistance to the insulin-like growth factor-I receptor inhibitor, BMS-536924. Mol Cancer Ther. 2008;7:2589–2598. doi: 10.1158/1535-7163.MCT-08-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Etienne MC, Formento JL, Lebrun-Frenay C, et al. Epidermal growth factor receptor and labeling index are independent prognostic factors in glial tumor outcome. Clin Cancer Res. 1998;4:2383–2390. [PubMed] [Google Scholar]
- 99.Waha A, Baumann A, Wolf HK, et al. Lack of prognostic relevance of alterations in the epidermal growth factor receptor-transforming growth factor-alpha pathway in human astrocytic gliomas. J Neurosurg. 1996;85:634–641. doi: 10.3171/jns.1996.85.4.0634. [DOI] [PubMed] [Google Scholar]
- 100.Steinbach JP, Eisenmann C, Klumpp A, et al. Co-inhibition of epidermal growth factor receptor and type 1 insulin-like growth factor receptor synergistically sensitizes human malignant glioma cells to CD95L-induced apoptosis. Biochem Biophys Res Commun. 2004;321:524–530. doi: 10.1016/j.bbrc.2004.06.175. [DOI] [PubMed] [Google Scholar]
- 101.Warshamana-Greene GS, Litz J, Buchdunger E, et al. The insulin-like growth factor-I receptor kinase inhibitor, NVP-ADW742, sensitizes small cell lung cancer cell lines to the effects of chemotherapy. Clin Cancer Res. 2005;11:1563–1571. doi: 10.1158/1078-0432.CCR-04-1544. [DOI] [PubMed] [Google Scholar]
- 102.Sun HZ, Wu SF, Tu ZH. Blockage of IGF-1R signaling sensitizes urinary bladder cancer cells to mitomycin-mediated cytotoxicity. Cell Res. 2001;11:107–115. doi: 10.1038/sj.cr.7290075. [DOI] [PubMed] [Google Scholar]
- 103.Ayalon D, Glaser T, Werner H. Transcriptional regulation of IGF-I receptor gene expression by the PAX3-FKHR oncoprotein. Growth Horm IGF Res. 2001;11:289–297. doi: 10.1054/ghir.2001.0244. [DOI] [PubMed] [Google Scholar]
- 104.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: Results for patients with nonmetastatic disease. J Clin Oncol. 2001;19:3091–3102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 105.Petricoin EF, III, Espina V, Araujo RP, et al. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer Res. 2007;67:3431–3440. doi: 10.1158/0008-5472.CAN-06-1344. [DOI] [PubMed] [Google Scholar]
- 106.O'Reilly KE, Rojo F, She QB, et al. MTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cao L, Yu Y, Darko I, et al. Addiction to elevated insulin-like growth factor I receptor and initial modulation of the AKT pathway define the responsiveness of rhabdomyosarcoma to the targeting antibody. Cancer Res. 2008;68:8039–8048. doi: 10.1158/0008-5472.CAN-08-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yee D, Favoni RE, Lebovic GS, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation: A potential autocrine growth factor. J Clin Invest. 1990;86:1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Martins AS, Mackintosh C, Martin DH, et al. Insulin-like growth factor I receptor pathway inhibition by ADW742, alone or in combination with imatinib, doxorubicin, or vincristine, is a novel therapeutic approach in Ewing tumor. Clin Cancer Res. 2006;12:3532–3540. doi: 10.1158/1078-0432.CCR-05-1778. [DOI] [PubMed] [Google Scholar]
- 110.Kappel CC, Velez-Yanguas MC, Hirschfeld S, et al. Human osteosarcoma cell lines are dependent on insulin-like growth factor I for in vitro growth. Cancer Res. 1994;54:2803–2807. [PubMed] [Google Scholar]
- 111.Scotlandi K, Manara MC, Nicoletti G, et al. Antitumor activity of the insulin-like growth factor-I receptor kinase inhibitor NVP-AEW541 in musculoskeletal tumors. Cancer Res. 2005;65:3868–3876. doi: 10.1158/0008-5472.CAN-04-3192. [DOI] [PubMed] [Google Scholar]
- 112.Kolb EA, Gorlick R. Development of IGF-IR inhibitors in pediatric sarcomas. Curr Oncol Rep. 2009;11:307–313. doi: 10.1007/s11912-009-0043-1. [DOI] [PubMed] [Google Scholar]
- 113.Karnieli E, Werner H, Rauscher FJ, III, et al. The IGF-I receptor gene promoter is a molecular target for the Ewing's sarcoma-Wilms' tumor 1 fusion protein. J Biol Chem. 1996;271:19304–19309. doi: 10.1074/jbc.271.32.19304. [DOI] [PubMed] [Google Scholar]
- 114.Chang Q, Li Y, White MF, et al. Constitutive activation of insulin receptor substrate 1 is a frequent event in human tumors: Therapeutic implications. Cancer Res. 2002;62:6035–6038. [PubMed] [Google Scholar]
- 115.Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293–1298. doi: 10.1093/annonc/mdn040. [DOI] [PubMed] [Google Scholar]
- 116.Francis P, Namlos HM, Muller C, et al. Diagnostic and prognostic gene expression signatures in 177 soft tissue sarcomas: Hypoxia-induced transcription profile signifies metastatic potential. BMC Genomics. 2007;8:73. doi: 10.1186/1471-2164-8-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Friedrichs N, Kuchler J, Endl E, et al. Insulin-like growth factor-1 receptor acts as a growth regulator in synovial sarcoma. J Pathol. 2008;216:428–439. doi: 10.1002/path.2438. [DOI] [PubMed] [Google Scholar]
- 118.Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560. [DOI] [PubMed] [Google Scholar]
- 119.Makawita S, Ho M, Durbin AD, et al. Expression of insulin-like growth factor pathway proteins in rhabdomyosarcoma: IGF-2 expression is associated with translocation-negative tumors. Pediatr Dev Pathol. 2009;12:127–135. doi: 10.2350/08-05-0477.1. [DOI] [PubMed] [Google Scholar]
- 120.Steigen SE, Schaeffer DF, West RB, et al. Expression of insulin-like growth factor 2 in mesenchymal neoplasms. Mod Pathol. 2009;22:914–921. doi: 10.1038/modpathol.2009.48. [DOI] [PubMed] [Google Scholar]
- 121.Chung KY, Shia J, Kemeny NE, et al. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803–1810. doi: 10.1200/JCO.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 122.Agaram NP, Laquaglia MP, Ustun B, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tarn C, Rink L, Merkel E, et al. Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A. 2008;105:8387–8392. doi: 10.1073/pnas.0803383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haluska P, Shaw HM, Batzel GN, et al. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13:5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 125.Lacy MQ, Alsina M, Fonseca R, et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J Clin Oncol. 2008;26:3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 126.Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 127.Stacchiotti S, Negri T, Palassini E, et al. Sunitinib malate and figitumumab in solitary fibrous tumor: Patterns and molecular bases of tumor response. Mol Cancer Ther. 2010;9:1286–1297. doi: 10.1158/1535-7163.MCT-09-1205. [DOI] [PubMed] [Google Scholar]
- 128.Quek RH, Morgan JA, Shapiro G, et al. Combination mTOR+IGF-IR inhibition: Phase I trial of everolimus and CP-751871 in patients with advanced sarcomas and other solid tumors. J Clin Oncol. 2010;28(abstr 10002):698s. doi: 10.1158/1078-0432.CCR-10-2621. [DOI] [PubMed] [Google Scholar]
- 129.Hajdu M, Singer S, Maki RG, et al. IGF2 over-expression in solitary fibrous tumours is independent of anatomical location and is related to loss of imprinting. J Pathol. 2010;221:300–307. doi: 10.1002/path.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pfizer Inc. Pfizer discontinues a phase 3 trial of figitumumab in non-small cell lung cancer (NSCLC) for futility 2009. http://www.pfizer.com/news/press_releases/pfizer_press_release_archive.jsp.
- 131.Kwak EL, Camidge DR, Clark J, et al. Clinical activity observed in a phase I dose escalation trial of an oral c-met and ALK inhibitor, PF-02341066. J Clin Oncol. 2009;27(abstr 3509):148s. [Google Scholar]
- 132.Gualberto A, Dolled-Filhart M, Gustavson M, et al. Molecular analysis of non-small cell lung cancer (NSCLC) identifies subsets with different sensitivity to insulin like growth factor I receptor (IGF-IR) inhibition. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-10-0089. [DOI] [PMC free article] [PubMed] [Retracted]
- 133.Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141:69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ueda S, Tsuda H, Sato K, et al. Alternative tyrosine phosphorylation of signaling kinases according to hormone receptor status in breast cancer overexpressing the insulin-like growth factor receptor type 1. Cancer Sci. 2006;97:597–604. doi: 10.1111/j.1349-7006.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Himpe E, Degaillier C, Coppens A, et al. Insulin-like growth factor-1 delays Fas-mediated apoptosis in human neutrophils through the phosphatidylinositol-3 kinase pathway. J Endocrinol. 2008;199:69–80. doi: 10.1677/JOE-08-0028. [DOI] [PubMed] [Google Scholar]
- 137.Froment P, Dupont J, Christophe-Marine J. Mdm2 exerts pro-apoptotic activities by antagonizing insulin-like growth factor-I-mediated survival. Cell Cycle. 2008;7:3098–3103. doi: 10.4161/cc.7.19.6807. [DOI] [PubMed] [Google Scholar]
- 138.Samowitz WS, Wolff RK, Ma KN, et al. Polymorphisms in insulin-related genes predispose to specific KRAS2 and TP53 mutations in colon cancer. Mutat Res. 2006;595:117–124. doi: 10.1016/j.mrfmmm.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 139.Christensen K, Doblhammer G, Rau R, et al. Ageing populations: The challenges ahead. The Lancet. 2009;374:1196–1208. doi: 10.1016/S0140-6736(09)61460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schumacher EF. London, United Kingdom: Blond & Briggs; 1973. Small is Beautiful: Economics as if People Mattered. [Google Scholar]
- 141.Lessnick SL, Dei Tos AP, Sorensen PH, et al. Small round cell sarcomas. Semin Oncol. 2009;36:338–346. doi: 10.1053/j.seminoncol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 142.Norton L, Simon R, Brereton HD, et al. Predicting the course of Gompertzian growth. Nature. 1976;264:542–545. doi: 10.1038/264542a0. [DOI] [PubMed] [Google Scholar]
- 143.Norton L. Evolving concepts in the systemic drug therapy of breast cancer. Semin Oncol. 1997;24:S10-3–S10-10. [PubMed] [Google Scholar]