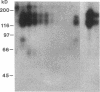
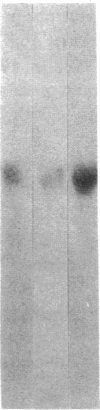
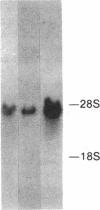
Abstract
Human monocytes adhere to fibronectin, but the receptor (or receptors) mediating this interaction has not been clearly identified. To examine the nature of this receptor, human monocytes were obtained by counter-current elutriation and were found to adhere to immobilized fibronectin but not to vitronectin or von Willebrand factor. Antibodies and peptides were used to determine whether monocyte adherence to immobilized fibronectin was mediated by a receptor similar to that which mediates the adherence of fibroblasts to fibronectin. Antibodies against both the alpha and beta subunits of the fibroblast fibronectin receptor (VLA-5) inhibited monocyte adherence to fibronectin. These antibodies immunoprecipitated two surface proteins from monocytes that migrated at 140 and 120 kD under nonreducing conditions. Surface proteins with identical apparent molecular weights were immunoprecipitated from surface-labeled MG-63 cells, a fibroblast-like cell line. A synthetic peptide containing the Arg-Gly-Asp-Ser (RGDS) sequence also inhibited monocyte adherence to fibronectin. cDNA hybridization experiments revealed the presence of mRNA for both the alpha and beta subunits of the fibroblast fibronectin receptor in human monocytes. We conclude that circulating monocytes express a fibronectin receptor that is structurally and functionally very similar, if not identical, to the fibronectin receptor in adherent fibroblasts, and that this receptor mediates monocyte adherence to immobilized fibronectin.
Full text
PDF

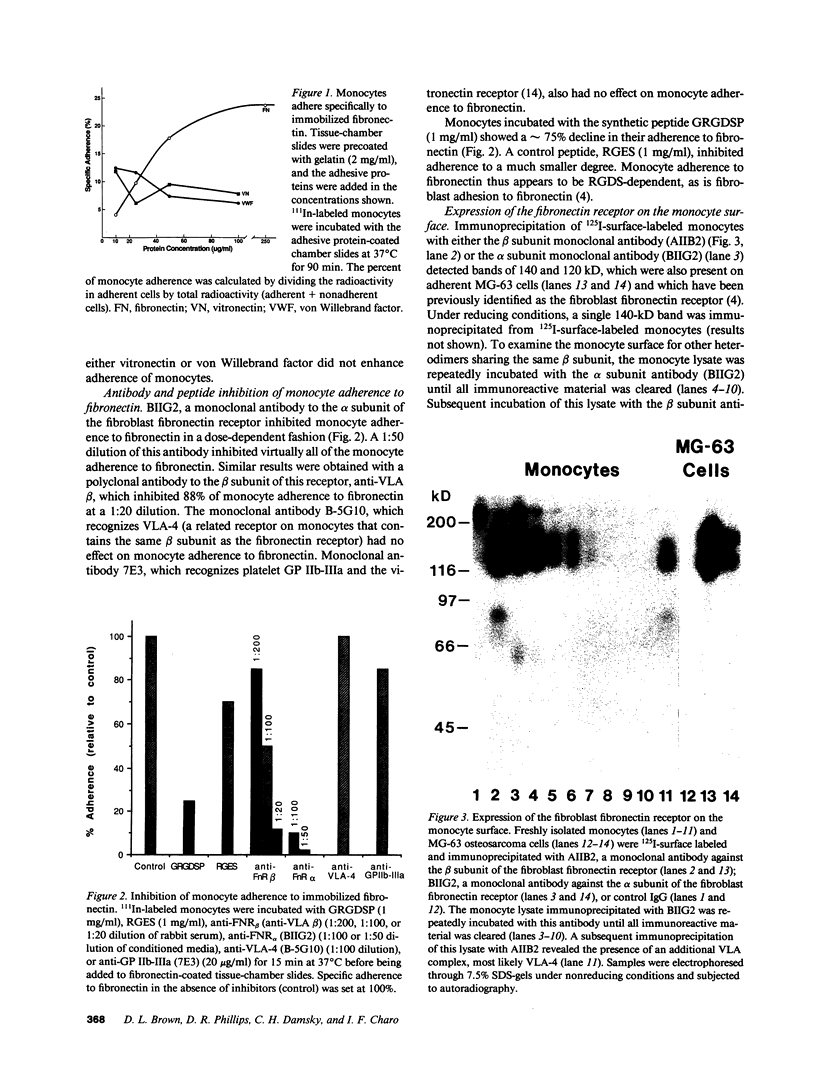


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. J., Goodwin J. L. Fibronectin receptors of phagocytes. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. J Exp Med. 1988 Mar 1;167(3):777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Charo I. F., Bekeart L. S., Phillips D. R. Platelet glycoprotein IIb-IIIa-like proteins mediate endothelial cell attachment to adhesive proteins and the extracellular matrix. J Biol Chem. 1987 Jul 25;262(21):9935–9938. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E., Miller E. J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J Exp Med. 1978 Jun 1;147(6):1584–1595. doi: 10.1084/jem.147.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Charo I. F., Phillips D. R. Human and bovine endothelial cells synthesize membrane proteins similar to human platelet glycoproteins IIb and IIIa. J Biol Chem. 1985 Sep 15;260(20):10893–10896. [PubMed] [Google Scholar]
- Fitzgerald L. A., Poncz M., Steiner B., Rall S. C., Jr, Bennett J. S., Phillips D. R. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987 Dec 15;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Seager J., Hokom M., Edwards P. A. Separation of and cholesterol synthesis by human lymphocytes and monocytes. J Lipid Res. 1979 Mar;20(3):379–388. [PubMed] [Google Scholar]
- Gebb C., Hayman E. G., Engvall E., Ruoslahti E. Interaction of vitronectin with collagen. J Biol Chem. 1986 Dec 15;261(35):16698–16703. [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Hemler M. E., Crouse C., Takada Y., Sonnenberg A. Multiple very late antigen (VLA) heterodimers on platelets. Evidence for distinct VLA-2, VLA-5 (fibronectin receptor), and VLA-6 structures. J Biol Chem. 1988 Jun 5;263(16):7660–7665. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hosein B., Bianco C. Monocyte receptors for fibronectin characterized by a monoclonal antibody that interferes with receptor activity. J Exp Med. 1985 Jul 1;162(1):157–170. doi: 10.1084/jem.162.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ginsberg M. H., Plow E. F., Ruoslahti E. Platelet membrane glycoprotein IIb/IIIa: member of a family of Arg-Gly-Asp--specific adhesion receptors. Science. 1986 Mar 28;231(4745):1559–1562. doi: 10.1126/science.2420006. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Recalde H. R. A simple method of obtaining monocytes in suspension. J Immunol Methods. 1984 Apr 13;69(1):71–77. doi: 10.1016/0022-1759(84)90278-3. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Scott D. M., Griffin B., Pepper D. S., Barnes M. J. The binding of purified factor VIII/von Willebrand factor to collagens of differing type and form. Thromb Res. 1981 Dec 1;24(5-6):467–472. doi: 10.1016/0049-3848(81)90080-3. [DOI] [PubMed] [Google Scholar]
- Switzer M. E., McKee P. A. Studies on human antihemophilic factor. Evidence for a covalently linked subunit structure. J Clin Invest. 1976 Apr;57(4):925–937. doi: 10.1172/JCI108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y., Huang C., Hemler M. E. Fibronectin receptor structures in the VLA family of heterodimers. Nature. 1987 Apr 9;326(6113):607–609. doi: 10.1038/326607a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias J. W., Bern M. M., Netland P. A., Zetter B. R. Monocyte adhesion to subendothelial components. Blood. 1987 Apr;69(4):1265–1268. [PubMed] [Google Scholar]