Abstract
Polyhydroxyalkanoate (PHA) is a biodegradable material with many potential biomedical applications, including medical implants and drug delivery. This study developed a system for screening production strains in order to optimize PHA production in Cupriavidus taiwanensis 184, 185, 186, 187, 204, 208, 209 and Pseudomona oleovorans ATCC 29347. In this study, Sudan black B staining, Infrared (IR) and Gas Chromatography (GC) analysis indicated that the best strain for PHA synthesis is C. taiwanensis 184, which obtains polyhydroxybutyrate (PHB). Cultivation of C. taiwanensis 184 under a pH of 7.0, at 30 °C, and at an agitation rate of 200 rpm, obtained a PHB content of 10% and PHB production of 0.14 g/L. The carbon and nitrogen types selected for analysis of PHB production by C. taiwanensis 184 were gluconic acid and NH4Cl, respectively. Optimal carbon/nitrogen ratio for PHB production was also determined. This study demonstrated a PHB content of 58.81% and a PHB production of 2.44 g/L when the carbon/nitrogen ratio of 8/1 was selected for C. taiwanensis 184. A two-stage fermentation strategy significantly enhanced PHB content and PHB production. Under a two-stage fermentation strategy with nutrient-limited conditions, C. taiwanensis 184 obtained a PHB content of 72% and a PHB concentration of 7 g/L. Finally, experimental results confirmed that optimizing the growth medium and fermentation conditions for cultivating the indigenous C. taiwanensis 184 strain substantially elevated PHB content from 10% to 72% and PHB production from 0.14 g/L to 7 g/L, respectively.
Keywords: PHB, Sudan black B staining, IR spectra, fermentation strategy, nutrient-limited conditions
1. Introduction
Rapid population growth in recent decades has resulted in severe environmental degradation. Because conventional plastics are not degradable by microorganisms, many companies have attempted to develop biodegradable alternatives. Polyhydroxyalkanoates (PHAs) are polyesters synthesized by various microorganisms, such as Ralstonia eutropha, Alcaligenes latus, Aeromonas hydrophila, Pseudomonas putida and Bacillus spp. [1–5]. Because of their good biodegradability and biocompatibility, PHAs have attracted interest in their use as an alternative to petroleum-based plastics including fine chemicals, plastics, printing materials, bio-fuel [6]. Furthermore, PHA properties for biomedical applications could also be varied, based on the co-monomer structures of the copolymers [7]. The PHA types, such as polyhydroxybutyrate (PHB), poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV), poly(hydroxybutyrate-co-hydroxyhexanoate) (PHBHHx) and polyhydroxyoctanoate (PHO) are frequently studied for biomedical applications including tissue regeneration devices, repair devices, repair patches and sutures [7–11]. In terms of drug delivery materials, PHAs which showed a faster drug release rate than that by ploylatic acid (PLA) are potential candidates [7]. Additionally, future studies would focus on other PHA types to reveal drug delivery properties depending on their copolymer structures [12–14]. The PHAs are energy storage materials that accumulate in bacteria under certain conditions, such as insufficient nitrogen, phosphorus, sulfur and oxygen, or excessive carbon [1–6].
The PHAs are classified by the number of carbon atoms in their monomers. In “short-chain length” PHAs, such as polyhydroxybutyrate (PHB) and polyhydroxyvalerate, carbon numbers of monomers are 3 to 5. Conversely, carbon numbers in medium chain-length PHA monomers range from 6 to 16. In addition to PHB, more than 140 different PHAs have been identified. The PHB identified in B. megaterium in 1926 by a French microbiologist was characterized by its large accumulations of PHB [7]. Because of its good biodegradability and biocompatibility, PHB has potential use in advanced drug delivery systems. However, PHA production strains are still needed. The lipophilic staining with Sudan Black B (SB staining) reportedly has high sensitivity in PHA screening [15]. After SB staining, bacteria containing PHAs exhibit dark granules. Therefore, Sudan Black B staining is a simple method of screening potential strains for PHAs.
The main fermentation strategies used to obtain bio-products are batch culture, fed-batch culture, continuous culture and two-stage fermentation. Two-stage fermentation is currently the most common method of producing PHAs [16]. In the first stage of the proposed process, biomass is increased to the level needed for PHB production. In the second stage, nutrients are limited in order to stimulate PHB synthesis by bacteria. This study also applied a two-stage fermentation strategy.
The aim of this study is to screen indigenous strains for PHB production. This study also evaluates the appropriate carbon and nitrogen types for PHB production and the feasibility of maximizing PHB production by optimizing growth conditions such as agitation rate, temperature and pH. Batch culture and two-stage fermentation strategies were also evaluated in terms of optimal carbon/nitrogen (C/N) ratios for PHB production.
2. Results and Discussions
2.1. The Selection of PHB Production Strain
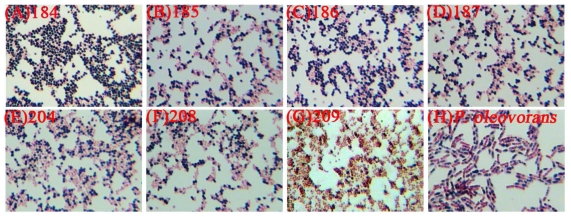
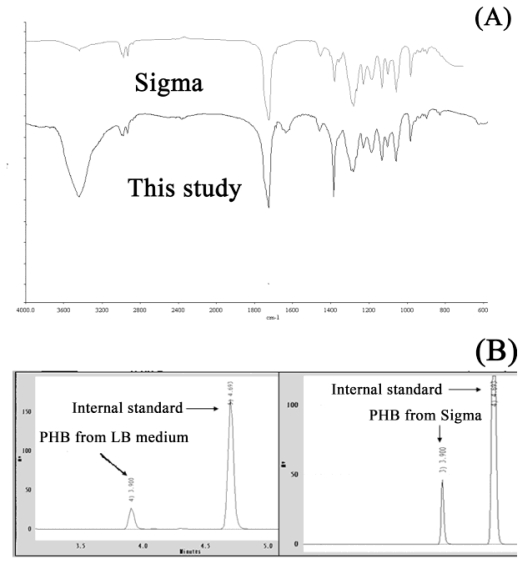
The local indigenous strains, i.e., C. taiwanensis 184, 185, 186, 187, 204, 208, 209 and Pseudomonas oleovorans ATCC 29347 were evaluated to select the PHA production strain. The PHA production and PHA type were verified by SB staining, Gas Chromatography (GC) and Infrared (IR), respectively. The PHA granules could exactly indicate the PHA production ability of microorganisms [15]. According to the results of SB staining, the C. taiwanensis 184 showed significant PHA granules (black section) over other stains (Figure 1). Furthermore, the PHA production of the local indigenous strains was further evaluated by GC. Among the eight strains, C. taiwanensis 184 also showed the highest PHA production of 0.14 g/L and PHA content of 10% more than the other strains (Table 1). According to the above mentioned results, this study suggested that C. taiwanensis 184 should be the production strain used. The purified PHA extracted from the culture broth of C. taiwanensis 184 was analyzed by IR and GC. The IR spectrum of the purified PHA product shows two mainly intense absorption peaks at 1720 cm −1 and 1280 cm −1 corresponding to C=O and C-O functional groups, respectively (Figure 2A). Hence, the purified PHA extracted from the culture broth of C. taiwanensis 184 should be polyester. Moreover, the GC spectrum of the purified PHA product also showed two major peaks occurred at retention time 3.9 minutes and 4.6 minutes, which corresponds to the internal standard (benzoic acid) and PHB standard purchased from Sigma, respectively (Figure 2B). These results (see Figure 2) also indicated that the strain, C. taiwanensis 184, was a potential strain for producing PHB and hence this strain was selected.
Figure 1.
Sudan black B stain of PHB granules (black section) on C. taiwanensis and P. oleovorans ATCC 29347 observed under 100X oil immersion objective.
Table 1.
Comparison of PHB content and PHB concentration among the eight strains (n = 3).
| Strains | PHB content (%) | PHB concentration (g/L) |
|---|---|---|
| C. taiwanensis 184 | 9.67 ± 0.11 | 0.15 ± 0.03 |
| C. taiwanensis 185 | 3.43 ± 0.09 | 0.07 ± 0.01 |
| C. taiwanensis 186 | 2.08 ± 0.02 | 0.09 ± 0.02 |
| C. taiwanensis 187 | 0.45 ± 0.06 | 0.03 ± 0.01 |
| C. taiwanensis 204 | 0.54 ± 0.08 | 0.04 ± 0.01 |
| C. taiwanensis 208 | 0.25 ± 0.03 | 0.01 ± 0.01 |
| C. taiwanensis 209 | 0.63 ± 0.06 | 0.02 ± 0.01 |
| P. oleovorans ATCC 29347 | 0.09 ± 0.01 | 0.03 ± 0.01 |
Figure 2.
Comparison of IR spectra of PHB standard obtained from Sigma with PHB from MM9 medium (A) and GC analysis of PHB standard obtained from Sigma and PHB from LB medium (B).
2.2. Effect of Growth Condition on PHB Production by C. taiwanensis 184
2.2.1. Effect of Initial Culture pH
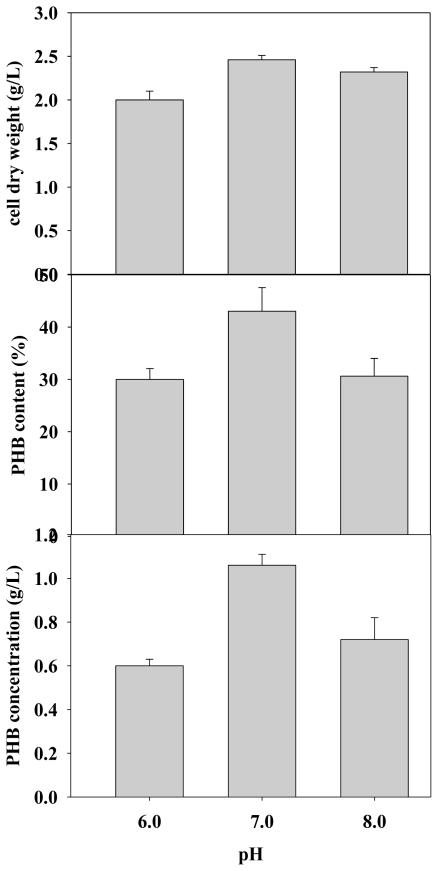
Typically, metabolic processes are highly susceptible to even slight changes in pH. Therefore, proper control of pH is critical. Figure 3 shows the effect of initial culture pH on cell dry weight (CDW) and PHB production. The experimental results showed that the initial pH, which was controlled with Defined M9 medium (DM9), could be adjusted by adding concentrated hydrochloric acid or sodium hydroxide. Figure 3 clearly shows that an initial pH of 7.0 obtained the highest PHB content (43.04%). These results were consistent with Palleroni and Palleroni [17], who recommended a pH range of 6.0 to 7.5 for microbial growth and PHB production. Although PHB production can be controlled by precisely manipulating pH, the experimental data indicated that pH values other than 7 affect PHB production. These results suggested that PHB production is sensitive to the pH of cultivation.
Figure 3.
Effects of various pH values on PHB production with C. taiwanensis 184 (n = 3).
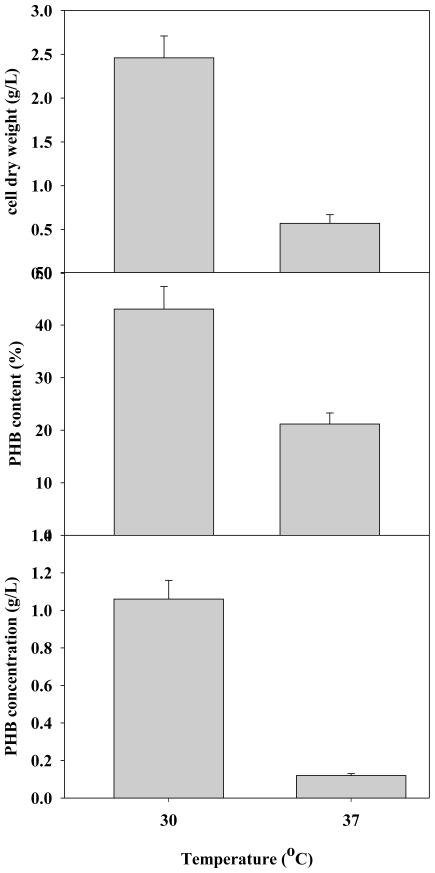
2.2.2. Effect of Culture Temperature
Because temperature, pH and agitation rate all affect dissolved oxygen levels and mass transfer efficiency, these environmental factors profoundly affect cellular growth and bioproduct production. Thus, the effects of temperature on CDW and PHB production by C. taiwanensis 184 were also evaluated. In this work, PHB production by C. taiwanensis 184 was examined at 30 °C and 37 °C. Figure 4 shows that the optimal temperature for cell growth and PHB production was 30 °C. The highest CDW of 1.06 g/L and PHB production of 1.06 g/L occurred at 30 °C.
Figure 4.
Effects of various temperatures on PHB production with C. taiwanensis 184 (n = 3).
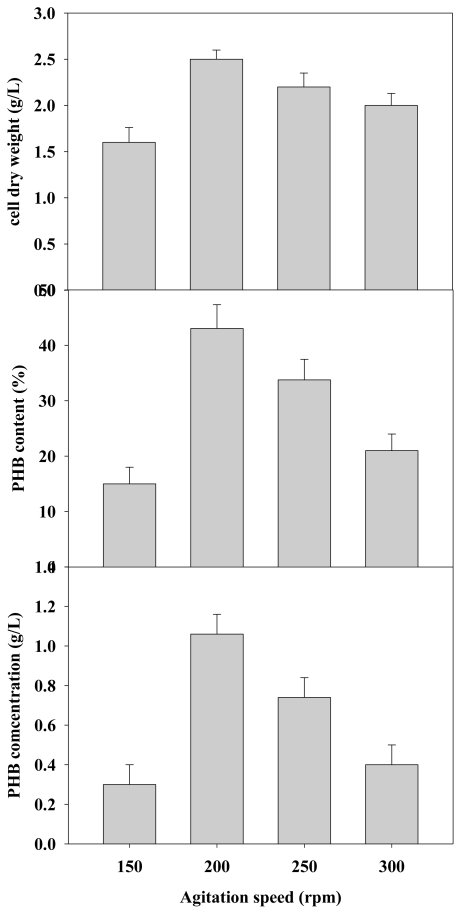
2.2.3. Effect of Agitation Rate
The effect of agitation rate on cell growth and PHB production was also analyzed. The experimental results showed that the CDW and PHB production in the batch culture increased, respectively, and agitation rate increased from 150 to 200 rpm. Therefore, an elevated agitation rate apparently enhanced both cell growth and PHB production from 0.2 g/L to 1.05 g/L (Figure 5). Limiting agitation rate to 200 rpm also slightly decreased PHB production from 1.05 g/L to 0.4 g/L, probably because of the excessive shear force produced at agitation speeds exceeding 250 rpm.
Figure 5.
Effects of various agitation rates on PHB production with C. taiwanensis 184 (n = 3).
2.3. Media Optimization
2.3.1. Effect of Carbon and Nitrogen Types on PHB Production by C. taiwanensis 184
Exactly how different carbon and nitrogen types affect PHB production was evaluated using various carbon sources (including carbohydrates, e.g., glucose, food-level glucose, lactose, raffinose, sucrose, galactose, dulcitol, and mannitol; and hydrocarbons, e.g., ethanol, gluconic acid, oxalic acid, malic acid, and galacturonic acid); and various nitrogen sources, e.g., NH4Cl, CH3COONH4, NH4NO3, (NH4)2SO4, and NH2CONH2. The carbohydrates were sterilized separately by filtration and added to defined M9 medium at a final concentration of 1% (w/v). Various carbon and nitrogen sources at concentrations of 4 g/L and 1 g/L, respectively, were used as the substrate to evaluate their effects on PHB accumulation in defined M9 media. The cultivation was performed at a temperature of 30 °C and an agitation rate of 200 rpm under aerobic conditions. The C. taiwanensis 184 strain was examined for PHB production in different carbon and nitrogen sources. Among these tests in this study, gluconic acid supported PHB synthesis. However, other than glucose, carbon sources did not enhance PHB synthesis. Conversely, although all nitrogen sources positively affected PHB synthesis, NH4Cl had the largest effect. Hence, Table 2 shows that the medium with gluconic acid and NH4Cl as the carbon and nitrogen source, respectively, apparently had a positive effect on PHB production. Thus, the final composition of the modified M9 medium (MM9 medium) was as follows: Gluconic acid: 4 g/L, NH4Cl: 1 g/L, Na2HPO4: 7 g/L, NaH2PO4: 3 g/L, 0.01 M CaCl2: 10 mL and 0.1 M MgSO4·7H2O: 10 mL.
Table 2.
Effects of various carbon and nitrogen sources on PHB synthesis (n = 3).
| Carbon types | Nitrogen types | ||||
|---|---|---|---|---|---|
| Carbohydrates | Hydrocarbons | Inorganic nitrogen | |||
| Carbon source | PHB production | Carbon source | PHB production (g/L) | Nitrogen source | PHB production (g/L) |
| Glucose | + | Ethanol | − | NH4Cl | ++ |
| Glucose (food) | + | Gluconic acid | ++ | CH3COONH2 | + |
| Lactose | − | Oxalic acid | − | NH4NO3 | + |
| Raffinose | − | Malic acid | − | (NH4)2SO4 | + |
| Sucrose | − | Galacturoni c acid | − | NH2CONH2 | + |
| Galactose | − | ||||
| Dulcitol | − | ||||
| Mannitol | − | ||||
2.3.2. Effect of Carbon/Nitrogen Ratio on PHB Production by C. taiwanensis 184
In living organisms, carbon (C) requirements are generally larger than nitrogen (N) requirements. The balance of these elements (C/N) determines how bacteria use an organic material [18]. To enhance PHB production, the C/N ratios of 1/1, 2/1, 4/1, 8/1 and 20/1 in MM9 medium were compared to determine the optimal ratio. Table 3 shows that a C/N ratio of 8/1 obtained the highest values for CDW (4.15 g/L), PHB production (2.44 g/L), and PHB content (58.81%). These results suggest that a C/N ratio of 8/1 is optimal for the accumulation of PHB by C. taiwanensis 184. However, at ratios above 8/1, PHB production consistently increased up to a ratio of 20/1, at which point the C/N ratio decreased. The PHB production by C. taiwanensis 184 was 0.89 g/L when PHB content was 52.77% (Table 3), and C/N ratio was 20/1. In addition, PHB production by C. taiwanensis 184 also decreased at C/N ratios higher or lower than 8/1. These results are attributable to high concentration of carbon source that involves the substrate, and C. taiwanensis 184 is inhibited, reducing CDW, PHB content and PHB production. Additionally, high C/N and low C/N ratios might affect the physiological conditions of the microorganisms, including cell proliferation and polymerization of PHB [18]. Therefore, the optimal C/N ratio of 8/1 was used in subsequent experiments.
Table 3.
Effects of carbon/nitrogen ratio on the synthesis of PHB (n = 3).
| C/N (mol/mol) | CDW (g/L) | PHB Concentration (g/L) | PHB Content (%) |
|---|---|---|---|
| 1/1 | 1.42 ± 0.28 | 0.42 ± 0.05 | 29.21 ± 2.33 |
| 2/1 | 2.42 ± 0.62 | 1.04 ± 0.12 | 43.10 ± 3.15 |
| 4/1 | 2.45 ± 0.57 | 1.20 ± 0.14 | 48.86 ± 2.19 |
| 8/1 | 4.15 ± 0.91 | 2.44 ± 0.36 | 58.81 ± 3.93 |
| 20/1 | 1.69 ± 0.43 | 0.89 ± 0.02 | 52.77 ± 2.62 |
2.4. Two-Stage Fermentation in 5 L Fermenter
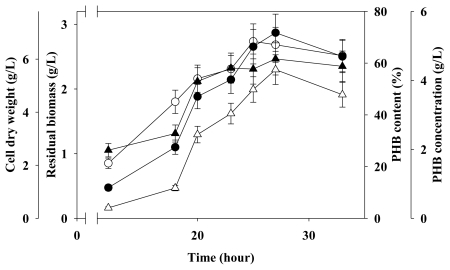
To enhance PHB accumulation, two-stage fermentation strategies were used. To date, few reports have specifically analyzed nutrient limitation conditions. In recent years, two-stage fermentation has become the preferred strategy for enhancing PHA production [16,19–21]. Therefore, this study also applied the two-stage fermentation strategy to evaluate PHB production in C. taiwanensis 184. Figure 6 shows the time course of PHB production under the above optimized conditions in a 5-liter fermenter with a 3-liter volume. Initially, PHB production was 3.8 g/L, PHB content was 62%, CDW was 7.2 g/L, and residual biomass was 3.2 g/L. By the end of the fermentation process, the results of PHB production, PHB content, CDW and residual biomass were 3.3 g/L, 59%, 6 g/L and 2.5 g/L, respectively. These experimental results indicate that the optimal duration of the second stage experiment was approximately 28 hours.
Figure 6.
Time course of cell growth and PHB production with C. taiwanensis 184 in a 5-liter fermenter (culture conditions: initial pH = 7, temperature = 30 °C, agitation rate = 200 rpm, carbon/nitrogen ratio = 8:1; (n = 3)). Closed circle: CDW; Open circle: Residual biomass; Closed triangle: PHB content; Open triangle: PHB concentration.
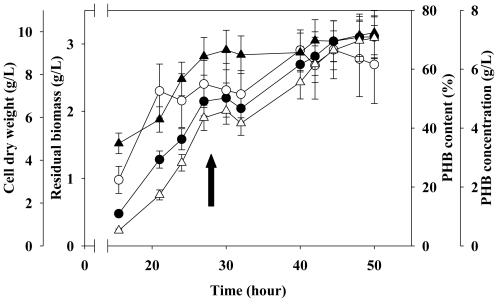
The two-stage fermentation strategy was performed under nutrient-limited conditions to stimulate PHB production by C. taiwanensis 184. This study indicated that increasing biomass is essential for PHB production in the first stage. The nutrient limitation conditions in the second stage then cause the bacteria to synthesize PHB. The optimal nutrient limitation strategy for enhancing PHB production, reportedly limits magnesium, phosphorous and sulfur [22–26]. This study also evaluated the effects of NaH2PO4 and MgSO4·7H2O. The best limitation condition for PHB production was half magnesium-limitation (0.05 M) rather than phosphate-limitation or sulfate-limitation at the second stage (data not shown). In all nutrient limitation conditions, CDW slightly increased when magnesium concentration was halved. Furthermore, the residual biomass maintained at a constant level (shown in Figure 7) and the PHB production increased, which was attributed to intracellular accumulation of PHB at the second stage. In the second stage, biomass on MM9 media gradually increased for up to 50 hours after cultivation under all limitation conditions. Interestingly, when magnesium was limited but not completely depleted, PHB content in the MM9 media was 72%, and PHB production was 7 g/L. The above results indicate that, when magnesium is limited, crude biomass increases from 7 g/L to 10 g/L in the first stage of fermentation, and PHB production increases during the second stage of fermentation. These results also suggest that magnesium may be crucial to the production of PHB by C. taiwanensis 184. The limitation on cellular growth in the second stage may prompt the C. taiwanensis 184 to store PHB as an energy source based on a physiological adaptation [24]. The medium that was used in the second stage might prompt C. taiwanensis 184 to store polymer during this period of physiological adaptation [24]. This observation is consistent with reports that limiting nitrogen sharply increases the PHB content of A. latus from 52% to 83% [23].
Figure 7.
Time course of PHB production, residual biomass and CDW by C. taiwanensis 184 using nutrient-limiting strategy (n = 3). Closed circle: CDW; Open circle: Residual biomass; Closed triangle: PHB content; Open triangle: PHB concentration. The arrow indicates the starting time for the second stage.
3. Experimental Section
3.1. Microorganisms Screening for PHB Production
Eight indigenous bacterial strains screened from Taiwan for PHB production were C. taiwanensis 184, C. taiwanensis 185, C. taiwanensis 186, C. taiwanensis 187, C. taiwanensis 204, C. taiwanensis 208 and C. taiwanensis 209 were identified by phylogenic analysis of the 16S rDNA and P. oleovorans ATCC 29347 purchased from Bioresource Collection and Research Center, Food Industrial Research and Development Institute, Taiwan. A loopful of each culture taken from a slant was inoculated into 2 mL growth medium containing beef extract (Sigma, St. Louis, MO): 1.0 g/L, peptone (Sigma): 5.0 g/L, yeast extract (Sigma): 2.0 g/L, NaCl (Sigma): 5.0 g/L, with a pH of 7.0. For the experiments, 2 mL of inoculum was inoculated into 98 mL liquid medium and cultivated at 30 °C and 200 rpm after 12-h cultivation and incubated for 24 hours in an aerobic environment. The cultures were aseptically centrifuged at 2000 × g for 30 min to separate the biomass. The biomass was then inoculated into flasks containing the production media described below and grown for 48 hours at 30 °C and at 200 rpm on a rotary shaker. To identify the best PHB production stain, Sudan black B staining was performed, and PHA synthesis was measured by GC and IR spectral (IFS28; Bruker, Bemen, Germany) analysis.
3.2. PHB Production Media and Conditions
In the basally-defined M9 medium (DM9 medium) used to experimentally cultivate the strains, the media content was as follows: 4 g/L goucose (Sigma), 1 g/L NH4Cl (Sigma), 7 g/L Na2HPO4 (Sigma), 3 g/L NaH2PO4 (Sigma), 10 mL 0.01 M CaCl2 (Sigma) and 10 mL 0.1 M MgSO4·7H2O (Sigma). Various carbon and nitrogen sources were also selected for evaluating PHB production. Exactly how carbon and nitrogen types affected PHB production was evaluated by analyzing various carbon sources (including carbohydrates, e.g., Glucose, Food-level glucose, Lactose, Raffinose, Sucrose, Galactose, Dulcitol, Mannitol), hydrocarbons, e.g., Ethanol, Gluconic acid, Oxalic acid, Malic acid and Galacturonic acid), and various nitrogen sources (e.g., NH4Cl (Sigma), CH3COONH4 (Sigma), NH4NO3 (Sigma), (NH4)2SO4 (Sigma) and NH2CONH2 (Sigma)). To investigate the effects of temperatures (30 °C and 37 °C), pHs (6–8) and agitation rates (150–300 rpm) on PHB production, the batch culture was performed, respectively. In these experiments, batch fermentation was performed in a 500 mL shake flask containing 100 mL of culture medium. Culture medium components and fermentation conditions varied according to the experimental design.
3.3. Quantifications of Cell Growth and PHB
Cell growth was monitored by turbidity at a 600 nm optimal density. Cell concentration defined as dry cell weight per liter of culture broth and PHB concentration, was determined as previously described [12]. Residual gluconic acid test was estimated by HPLC column (Aminex HPX-87H, BIO-RAD), Mobile phase: 5mM H2SO4 (Sigma), flow rate: 0.5 mL/min, RI detector: L-2490 (Hitachi, Tokyo, Japan).
3.4. Two-Stage Fermentation Strategy for PHB Production
The PHB synthesis was analyzed under varying fermentation conditions, including pH, temperature, and agitator speed. The PHB production in the 5 L fermenter was evaluated to determine the proper conditions for cultivating C. taiwanensis 184. Additionally, PHB production in the 5 L fermenter was evaluated when using a two-stage fermentation strategy. After increasing cell concentration in the first stage, a nutrient limitation strategy was used for PHB production in the second stage as described elsewhere [16,19–21].
3.5. Staining of Bacteria with Sudan Black B
Several drops of microbial broth were fixed on a glass slide by applying heat and then stained with a 3% Sudan Black B (w/v in 70% ethanol, Sigma) solution for 10 minutes. The slide was then immersed in xylene until completely decolonized. The sample was counterstained with safranin (5% w/v in distilled water, Sigma) for 10 seconds, washed again with distilled water, and dried. After adding several drops of immersion oil directly to the completely dry slide, the cells were examined by optical microscopy (Leica, Tokyo, Japan) (15).
3.6. Analytical Methods
The GC analysis was performed after methanolyzing the polymer in sulfuric acid and methanol asas described by Lutke et al. [27]. Internal standard benzoic acid was used to measure PHB concentration by comparing PHB peaks. The infrared (IR) spectra of polymer samples were analyzed according to Lutke et al. [27]. The purity of the standard PHB that was purchased from Sigma is defined as 100%. Based on this definition, the relative purity of the purified PHB, as used in the GC and IR analyses, was determined to be around 95%.
4. Conclusion
Screening of the indigenous strain Cupriavidus taiwanensis 184 by Sudan Black B staining confirmed its potent PHB production. Moreover, PHB production in MM9 medium was 4.15 g/L when measured under the following conditions: Temperature of 30 °C, pH of 7 and agitation speed of 200 rpm. To enhance PHB accumulation, a two-stage fermentation strategy was used. This study showed that such a strategy significantly enhanced PHB content and PHB production. Under a two-stage fermentation strategy with nutrient-limited conditions, C. taiwanensis 184 obtained a PHB content of 72% and a PHB concentration of 7 g/L. However, further research is needed to determine the commercial potential of C. taiwanensis 184 for producing long-chain biodegradable polymers. The findings of this study provide a reference for further research in the use of PHB for manufacturing biodegradable polymers.
Acknowledgements
The authors gratefully acknowledge the financial support by the National Science Council of the Republic of China under grant numbers NSC 96-2221-E-155-044-MY2, NSC 98-2221-E-155-026 and NSC 99-2622-E-155-001-CC2.
References
- 1.Chakraborty P, Gibbons W, Muthukumarappanl K. Conversion of volatile fatty acids into polyhydroxyalkanoate by Ralstonia eutropha. J. Appl. Microbiol. 2009;106:1996–2005. doi: 10.1111/j.1365-2672.2009.04158.x. [DOI] [PubMed] [Google Scholar]
- 2.Yu PH, Chua H, Huang AL, Ho KP. Conversion of industrial food wastes by Alcaligenes latus into polyhydroxyalkanoates. Appl Biochem Biotechnol. 1999;77–79:445–454. doi: 10.1385/abab:78:1-3:445. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HF, Ma L, Wang ZH, Chen GQ. Biosynthesis and characterization of 3-hydroxyalkanoate terpolyesters with adjustable properties by Aeromonas hydrophila. Biotechnol. Bioeng. 2009;104:582–589. doi: 10.1002/bit.22409. [DOI] [PubMed] [Google Scholar]
- 4.Ren Q, de Roo G, Witholt B, Zinn M, Thöny-Meyer L. Influence of growth stage on activities of polyhydroxyalkanoate (PHA) polymerase and PHA depolymerase in Pseudomonas putida U. BMC Microbiol. 2010;10:254. doi: 10.1186/1471-2180-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh M, Patel SK, Kalia VC. Bacillus subtilis as potential producer for polyhydroxyalkanoates. Microb. Cell Fact. 2009;8:38. doi: 10.1186/1475-2859-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chanprateep S. Current trends in biodegradable polyhydroxyalkanoates. J. Biosci. Bioeng. 2010;110:621–632. doi: 10.1016/j.jbiosc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009;38:2434–2446. doi: 10.1039/b812677c. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Li P, Chen CB, Wang ZH, Ma P, Chen GQ. The improvement of fibroblast growth on hydrophobic biopolyesters by coating with polyhydroxyalkanoate granule binding protein PhaP fused with cell adhesion motif RGD. Biomaterials. 2010;31:8921–8930. doi: 10.1016/j.biomaterials.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Cousley RR. A stent-guided mini-implant system. J. Clin. Orthod. 2009;43:403–407. [PubMed] [Google Scholar]
- 10.Xu XY, Li XT, Peng SW, Xiao JF, Liu C, Fang G, Chen KC, Chen GQ. The behaviour of neural stem cells on polyhydroxyalkanoate nanofiber scaffolds. Biomaterials. 2010;31:3967–3975. doi: 10.1016/j.biomaterials.2010.01.132. [DOI] [PubMed] [Google Scholar]
- 11.Han I, Shim KJ, Kim JY, Im SU, Sung YK, Kim M, Kang IK, Kim JC. Effect of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanofiber matrices cocultured with hair follicular epithelial and dermal cells for biological wound dressing. Artif Organs. 2007;31:801–808. doi: 10.1111/j.1525-1594.2007.00466.x. [DOI] [PubMed] [Google Scholar]
- 12.Yilgor P, Hasirci N, Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J. Biomed. Mater. Res A. 2010;93:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 13.Zhu XH, Wang CH, Tong YW. In vitro characterization of hepatocyte growth factor release from PHBV/PLGA microsphere scaffold. J. Biomed. Mater. Res A. 2009;89:411–423. doi: 10.1002/jbm.a.31978. [DOI] [PubMed] [Google Scholar]
- 14.Xiong YC, Yao YC, Zhan XY, Chen GQ. Application of polyhydroxyalkanoates nanoparticles as intracellular sustained drug-release vectors. J. Biomater. Sci. Polym. Ed. 2010;21:127–140. doi: 10.1163/156856209X410283. [DOI] [PubMed] [Google Scholar]
- 15.Burdon KL. Fatty materials in bacteria and fungi revealed by staining dried, fixed slide preparations. J. Bacteriol. 1946;52:665–678. doi: 10.1128/jb.52.6.665-678.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartmann R, Hany R, Witholt B, Zinn M. Simultaneous biosynthesis of two copolymers in Pseudomonas putida GPo1 using a two-stage continuous culture system. Biomacromolecules. 2010;11:1488–1493. doi: 10.1021/bm100118t. [DOI] [PubMed] [Google Scholar]
- 17.Palleroni NJ, Palleroni AV. Alcaligenes latus, a new species of hydrogen-utilizing bacteria. Int. J. Syst. Bacteriol. 1978;28:416–424. [Google Scholar]
- 18.Chanprateep S, Katakura Y, Visetkoop S, Shimizu H, Kulpreecha S, Shioya S. Characterization of new isolated Ralstonia eutropha strain A-04 and kinetic study of biodegradable copolyester poly(3-hydroxybutyrate-co-4-hydroxybutyrate) production. J. Ind. Microbiol. Biotechnol. 2008;35:1205–1215. doi: 10.1007/s10295-008-0427-5. [DOI] [PubMed] [Google Scholar]
- 19.Sun Z, Ramsay JA, Guay M, Ramsay B. Increasing the yield of MCL-PHA from nonanoic acid by co-feeding glucose during the PHA accumulation stage in two-stage fed-batch fermentations of Pseudomonas putida KT2440. J. Biotechnol. 2007;132:280–282. doi: 10.1016/j.jbiotec.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 20.Albuquerque MG, Eiroa M, Torres C, Nunes BR, Reis MA. Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses. J. Biotechnol. 2007;130:411–421. doi: 10.1016/j.jbiotec.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 21.Liu HY, Hall PV, Darby JL, Coats ER, Green PG, Thompson DE, Loge FJ. Production of polyhydroxyalkanoate during treatment of tomato cannery wastewater. Water Environ. Res. 2008;80:367–372. doi: 10.2175/106143007x221535. [DOI] [PubMed] [Google Scholar]
- 22.Lee SY, Wong HH, Choi J, Lee SH, Lee SC, Han CS. Production of medium-chainlength polyhydroxyalkanoates by high-cell-density cultivation of Pseudomonas putida under phosphorus limitation. Biotechnol. Bioeng. 2000;68:466–470. [PubMed] [Google Scholar]
- 23.Valappil SP, Rai R, Bucke C, Roy I. Polyhydroxyalkanoate biosynthesis in Bacillus cereus SPV under varied limiting conditions and an insight into the biosynthetic genes involved. J. Appl. Microbiol. 2008;104:1624–1635. doi: 10.1111/j.1365-2672.2007.03678.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Lee SY. Poly(3-hydroxybutyrate) production with high productivity and high polymer content by a Fed-batch culture of Alcaligenes latus under nitrogen limitation. Appl Environ Microbiol. 1997;63:3703–3706. doi: 10.1128/aem.63.9.3703-3706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albuquerque MG, Torres CA, Reis MA. Polyhydroxyalkanoate (PHA) production by a mixed microbial culture using sugar molasses: effect of the influent substrate concentration on culture selection. Water Res 2010. 44:3419–3433. doi: 10.1016/j.watres.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Shang L, Jiang M, Chang HN. Poly(3-hydroxybutyrate) synthesis in fed-batch culture of Ralstonia eutropha with phosphate limitation under different glucose concentrations. Biotechnol. Lett. 2003;25:1415–1419. doi: 10.1023/a:1025047410699. [DOI] [PubMed] [Google Scholar]
- 27.Lütke-Eversloh T, Bergander K, Luftmann H, Steinbüchel A. Identification of a new class of biopolymer: bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiology. 2001;147:11–19. doi: 10.1099/00221287-147-1-11. [DOI] [PubMed] [Google Scholar]