Abstract
Future therapeutic intervention that could effectively decelerate the rate of degeneration within the substantia nigra pars compacta (SNc) could add years of mobility and reduce morbidity associated with Parkinson’s disease (PD). Neurodegenerative decline associated with PD is distinguished by extensive damage to SNc dopaminergic (DAergic) neurons and decay of the striatal tract. While genetic mutations or environmental toxins can precipitate pathology, progressive degenerative succession involves a gradual decline in DA neurotransmission/synaptic uptake, impaired oxidative glucose consumption, a rise in striatal lactate and chronic inflammation. Nutraceuticals play a fundamental role in energy metabolism and signaling transduction pathways that control neurotransmission and inflammation. However, the use of nutritional supplements to slow the progression of PD has met with considerable challenge and has thus far proven unsuccessful. This review re-examines precipitating factors and insults involved in PD and how nutraceuticals can affect each of these biological targets. Discussed are disease dynamics (Sections 1 and 2) and natural substances, vitamins and minerals that could impact disease processes (Section 3). Topics include nutritional influences on α-synuclein aggregation, ubiquitin proteasome function, mTOR signaling/lysosomal-autophagy, energy failure, faulty catecholamine trafficking, DA oxidation, synthesis of toxic DA-quinones, o-semiquinones, benzothiazolines, hyperhomocyseinemia, methylation, inflammation and irreversible oxidation of neuromelanin. In summary, it is clear that future research will be required to consider the multi-faceted nature of this disease and re-examine how and why the use of nutritional multi-vitamin-mineral and plant-based combinations could be used to slow the progression of PD, if possible.
Keywords: Parkinson’s disease, neuroprotective, neuromelanin, nutrition, vitamins
1. Introduction
1.1. Pathology
The pathology of Parkinson’s disease (PD) involves chronic degeneration of dopaminergic (DAergic) neurons in the substantia nigra pars compacta (SNc). Subsequent decay of the nigrostriatal tract manifests itself clinically by symptomatic rigidity, bradykinesia, postural instability and resting tremor. Prominent pathological manifestations associated with degeneration of SNc DAergic neurons include observations describing mitochondrial abnormalities [1–4], excessive cytosolic dopamine (DA) oxidation, α-synuclein aggregates, autophagolysosome dysfunction, defects in the ubiquitin-proteasome system (UPS), oxidative stress, nitrosative stress, iron released from bound storage and a gradual loss of neuromelanin (NM) [5–7]. These pathological insults are self reinforcing and can advance in cyclical fashion, often intensified by decaying levels of glutathione (GSH), which render greater oxidative damage (via O2, H2O2, OH) [8,9] or lipid/protein nitration (via ONOO−) and accumulation of 3-nitrotyrosine, protein carbonyls, 8-hydroxyguanosine, malondialdehyde and hydroxynonenol in degenerating neurons [10–12]. Neurological degeneration can also be aggravated by chronic central nervous system (CNS) inflammation which can involve recruitment of activated microglial cells, release of cytotoxic molecules, free radicals and glutamate, which can provoke neuritic beading, excitotoxic, apoptotic and necrotic degeneration [13]. While gradual loss of DAergic SNc pigmented cells occur as a natural process of aging—early diagnosis of PD is associated with a 30%/60% reduction of DAergic neurons/striatal DA which is attributable to degeneration of striatal axon terminals [14]. Though the etiology circumscribing the selective loss of SNc DAergic neurons in PD is not fully understood, we do know that reportedly ~5–10% of PD patients display mutations in genes such as DJ-1, PTEN-induced kinase 1 (PINK-I), leucine-rich repeat kinase 2 (LRRK2) G2019S, park-1/Synuclein (SNCA), ubiquitin-carboxy-terminal-hydrolase L1, parkin (Del3-5, T240R, Q311X) [15–18], ATP13A2 (Park 9), β-glucocerebrosidase and mitochondrial proteins such as park 13 Omi/Htra2, Complex I [19–22]. The larger majority of PD cases result from a fusion of natural aging and/or environmental exposures to pesticides, a history of depression, viral/bacterial infections, metals, antipsychotic/antidepressant drugs, rural/farm living or lack of habitual cigarette smoking/tobacco use or consumption of caffeine [23–27]. All of these studies provide partial insight as to the precipitating factors involved with PD onset and progression. Moreover, the greatest commonality appears to be either genetic mutations or environmental triggers that lead to direct/indirect accumulation of malfunctional mitochondria, which precede selective DAergic SNc degeneration.
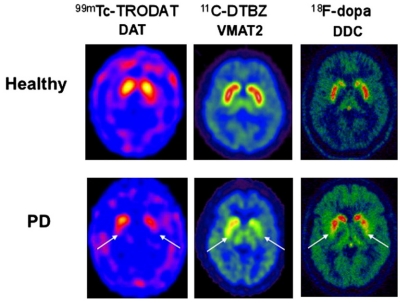
The extent of DAergic SNc losses in human PD can be imaged using positron emission tomography (PET) or single photon emission computerized tomography (SPECT). Radioactive tracers in PD patients have been used to substantiate (1) compromised integrity of pre-synpatic nigrostriatal projections, i.e., [18F]LDOPA (which monitors DA uptake, metabolism, DOPA decarbarboxylase (DDC), DA storage within intact nerve terminals); (2) faulty DA transporters (DAT) i.e., CFT, C-RTI-32, FP-CIT ligands, [11C]methylphenidate (MP)/99mTc-TRODAT-1 or (3) abnormal type-2-vesicular monoamine transporter (VMAT2) function using tracers such as [11C]-dihydrotetrabenazine (DTBZ) which measures cytoplastic DA uptake into synaptic vesicles [28–30]. Chronic SNc DAergic degeneration parallels a reduction of [18]F-DOPA uptake and DAT binding which are foundational events to faulty circuitry in the basal ganglia that ultimately triggers locomotive disability [31].
1.2. Treatment
In order to counteract the loss of SNc DAergic neurons, medical treatments are aimed at modulating neurotransmitter (NT) function. Prescription medicines allow for fluid voluntary movement, reduction of tremors and a sustained quality of life. Routine adjunct therapies often combine levodopa/dopa-decarboxylase inhibitors Sinemet® and Madopar® with DA receptor agonists, catechol-o-methyltransferase inhibitors, monoamine oxidase (MAO) inhibitors, anti-cholinergics and surgical treatments [32]. While prescription drugs ameliorate the symptoms, they do not necessarily address the central etiology of degeneration and therefore a number of alternative approaches have been considered to slow the progression of this disease.
1.3. Previous Studies on Therapeutic Agents to Slow Progression of PD
Innovative strategies to slow the progression have met with partial success in experimental models, and to a less significant extent in clinical trials. Most neuroprotective strategies seem to fall under the general classes of anti-inflammatory, anti-apoptotic, anti-oxidants, enzyme inhibitors, growth factors, alternative medicine or receptor antagonists/agonists. Experimental trials elucidating efficacy of neuroprotective agents are rapidly expanding, and have thus far included superoxide dismutase (SOD)/catalase/peroxidase mimetics [33], anti-apoptotic MAO inhibitors, rasagiline [34–38], (−)-epigallocatechin-3-gallate, iron chelator/antioxidant/anti-inflammatory combinations [39–41], celastrol, nitric oxide synthase (NOS) inhibitors [42,43], COX, c-jun N-terminal kinase (JNK) inhibitors [44–47], alpha-tocopherol, coenzyme Q10, lipoic acid [48–52], creatine [53,54] melatonin, catalpol from root of Rehmannia glutinosa Libosch [55,56], N-acetyl-l-cysteine (NAC), thiol antioxidants [57], nerve growth factors [58,59], dehydroepiandrosterone [60], estrogen receptor agonists [61], adenosine A2 receptor antagonists [62–68], S-allylcysteine [66], mGlu2/3 metabotropic [67], acupuncture [68] traditional Chinese medicine Zhen-Wu-Tang [69] angiotensin-converting enzyme inhibitors [70], nicotine, ginseng, ginkgo biloba, caffeine and cannabis [71].
Despite the success using a vast range of therapeutic agents in preliminary experiments, there is a general failure of clinical trials to substantiate therapeutic effects that slow disease progression, in particular for antioxidants. This may be attributable to limitations in the current animal or in vitro models that make extrapolation of information for human PD difficult. Further, the pathology is very complex and may not be effectively antagonized with just single therapy antioxidant, ergogenic, anti-inflammatory regimens.
The use of nutritional supplements to slow the progression of PD has also not been fully substantiated by evidenced-based studies. The aim of this review is to re-visit the pathology of PD, and in light of pathological processes further discuss the rationale behind potential use of vitamin/mineral nutraceutical neuroprotective agents. In this review, the details of pathology are presented in Sections 1 and 2, and further discussed relevant to nutrient interactions in Section 3. Discussion includes the role of vitamins and minerals in the established United States recommended daily allowances, as well as macronutrients and plant based constituents that modulate processes with specific relevance to PD. The review is a combination of past literature and proposed theory based on known molecules that affect known biological targets which range from mitochondrial malfunction, inflammation, DA oxidation and defective UPS/lysosomal autophagy processes. Moreover, some of the compounds proposed in this review have not yet been evaluated.
2. Review
2.1. Energy Failure—Loss of OXPHOS, Rise in Anaerobic Glycolysis & Lactate, ATP Depletion
We first review the most prominent issue underlying the loss of DAergic neurons, which is a fundamental failure in glucose metabolism due to aberration of mitochondrial respiration. It is important to note that mitochondrial malfunction could initially occur due to toxic effects of α-synuclein, endogenous neurotoxins or exogenous environmental factors. However, experimental models often employ use of mitochondrial toxins such as 1-methyl-4-phenylpyridinium (MPP+), rotenone or endogenous isoquinolines to selectively target neuropathological damage similar to, but not identical to PD degenerative effects mainly in the SNc and the locus coeruelus (LC) [72–74].
Loss of mitochondrial function leads to immediate failure of DA neurotransmission and acceleration of glycolysis to overcome the loss of oxidative phosphorylation (OXPHOS) through substrate level phosphorylation (SLP) [75–77]. The impact of mitochondrial toxins on these energy processes is almost always observed. In vivo, administration of 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) generates an immediate rise in glucose utilization (detected with [2–14C]deoxyglucose), a drop in ATP, a rise in lactate production, reduction in striatal DAT/DA and loss of tyrosine hydroxylase immunoreactivity, effects which are exacerbated by α-synuclein [78–83]. The drop in ATP suggests that energy deficiency is clearly involved with the process of initiating degenerative decline [84]. Moreover, there is ample information to substantiate that a drop in energy corresponds to a rise in glycolysis to drive SLP, an indicator of metabolic stress. The use of proton magnetic resonance spectroscopy (1H-MRS) has been used to confirm sharp spikes in striatal lactate, which occur within 2 h of MPTP injection in C57BL/6 mice [85]. In primates, a long term study utilizing infusion of MPTP over 14 ± 5 months resulted in loss of DA pre-synaptic re-uptake, parallel to a 23-fold increase in striatal lactate production, which was sustained for up to 10 months post final administration of MPTP [86]. While the CNS is most often studied with respect to biochemical effects induced by systemic injection of MPTP, damage in peripheral tissue such as skeletal muscle also involves heightened anaerobic glycolytic function, elevation of lactic acid dehydrogenase and concomitant decrease of mitochondrial Complex IV, with no changes in mitochondrial Complex I [87]. These shifts toward anaerobic metabolism occur in tissues with capability to uptake MPP+ where similar patterns observed include loss of ATP, loss of OXPHOS, a rise in glycolysis, heightened production in lactate and neurotoxic effects which can be blocked by providing abundant glucose to growth media in order to sustain ATP production through glycolysis [77,88–93]. The reliance of damaged neurons on greater anaerobic glycolytic function is not exclusive to PD, as head trauma, seizure or ischemia can equally provoke a rise in brain/CSF lactate and loss of ATP parallel to neurological damages [94–98]. In Section 3, we discuss nutrients involved with propelling anaerobic function.
In the human brain, in vivo functional imaging strategies to assess glucose metabolism in the brain include PET with a [18F]2-fluoro-2-deoxy-d-glucose ([18F]FDG) tracer. This tracer is used to quantify elevated rates of glycolysis/glucose transport relative to surrounding tissue. Some limitations for this method involve the non-specific manner by which [18F]FDG accumulates in the brain. [18F]FDG enters through the glycolytic cycle prior to conversion of pyruvate, therefore its measurement does not differentiate between aerobic (OXPHOS) and anaerobic (SLP) metabolism. Further, uptake is not selective to cell type and therefore false positives (or heightened metabolic activities) are likely to occur in particular for diseases involving active inflammatory tissue, where metabolic rate of glucose is extremely high [99]. This technique however, has been used in sliced striatal tissue to corroborate that regional exposure to MPP+ can evokes a sharp rise in cerebral glucose metabolic rate (CMRglc) [93]. FDG PET studies could also be beneficial in terms of evaluating patterns in non-diseased, non-inflammatory models. FDG PET imaging techniques clearly show that the process of aging in monkeys, corresponds to loss in both regional cerebral blood flow/[15O] H2O and rCMRglc in many areas of the brain including the cerebellum, hippocampus, striatum, occipital cortex, temporal cortex, and frontal cortex [100]. Age associated hypometabolism in the human brain is also believed to precipitate increased risk for many age associated CNS neurodegenerative diseases [101]. Future research is now considering preventative implementation with nutrients that could assist in minimizing metabolic losses [101].
In brief, the loss of ATP in the SNc is detrimental because this single event can initiate a range of downstream collapse on energy requiring systems that can then lead to (1) catecholamine oxidation and formation of DA neurotoxins/free radicals (2) excitotoxic and programmed cell death (3) mitochondrial transition pore opening, matrix swelling, release of mitochondrial proteins into the cytosol, apoptosis [102–107] and microtubular/cell structure collapse [108].
2.2. Loss of DA Regulation and Trafficking—VMAT2
One of the first events brought about by reduction of ATP is a loss of energy requiring systems that drive DA trafficking. Section 3, also refers to a large number of nutraceuticals that can block these processes. Failure of DA trafficking, not only occurs due to decline in ATP, but can also result from genetic mutations in SNCA (A53T and A30P) [109], mitochondrial insufficiency or oxidative damage by ROS, all which trigger excessive DA release from SNc nerve terminals [110,111]. With regards to energy, a lack of ATP diminishes the capability of intracellular ATPase pumps to sequester DA into synaptic vesicles (where DA is stable due to slightly acidic pH), which is a pivotal factor in initiating a cascade of neurotoxic events [112,113]. Failure of vesicle monoamine transporter 2 (VMAT2) results in immediate leaked DA into the cytosolic compartment (easily subject to oxidative breakdown at neutral pH) where it readily oxidizes to form neurotoxic DA-quinones, o-semiquinones, dopaminergic poisons and related free radicals [114–116] which can then contribute to eventual decay of the striatal tract [117].
Further, functional loss of VMAT2 can occur due to age-associated losses in VMAT2 mRNA expression, which create vulnerability to extensive neurological damage in the presence of mitochondrial toxins such as MPTP in vivo or MPP+ in vitro [118–123]. MPP+ can cause further insult due to its ability to bind directly to VMAT2, gain entrance into synaptic vesicles and initiate extrusion of DA back into the cytoplasmic compartment [124,125].
2.3. Dopamine Oxidation
Inadequate function or expression of VMAT2 mRNA has also been reported in association with PD [126], which could precipitate three main routes by which the oxidation of DA can become pathological. These include (1) the enzymatic oxidation of DA via tyrosinase, phospholipase A2 (PLA2)/prostaglandin H synthase (COX), lipoxygenase and xanthine oxidase to form DA-quinone en route to neuromelanin synthesis (2) non-enzymatic autoxidation of DA by the presence of oxygen, H2O2, or metals and (3) the enzymatic oxidation of DA by MAO which can lead to H2O2 production and synthesis of DA-aldehydes. The heavy oxidation of DA (be it non-enzymatic or enzymatic) seems to initiate neurodegenerative pathogenesis, a depletion of glutathione, oxidation of available ascorbate and subsequent oxidative stress in the SNc area [127]. In Section 3, we provide information on nutraceuticals that may be able to antagonize each of the major routes of DA oxidation.
2.3.1. Enzymatic Oxidation of DA, the Neuromelanin Pathway & DA-Quinones
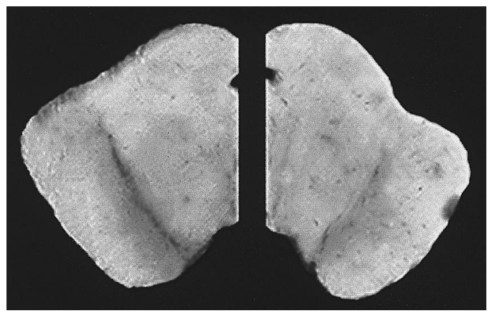
Understanding the role of target enzymes and how they exacerbate DA oxidation could be beneficial in directing future investigation or design of nutraceutical combinations. First, the enzymatic oxidation of DA occurs through heightened activity of tyrosinase, COX, lipoxygenase and xanthine oxidase which converts DA to DA-quinone en route to neuromelanin (NM) [128]. The neuromelanin pathway if intensified can produce deleterious DA-quinone neurotoxic metabolites such as o-semiquinones or benzothiazolines, which are potent inhibitors of mitochondrial pyruvate dehydrogenase (i.e., complex I/Krebs cycle) and initiators of α-synuclein fibrillization [129–132]. Oxidized DA can further react with thiols producing DA-cysteine adducts such as 5-S-cysteinyldopamine which mediate metal catalyzed oxidation of proteins, which lead to protein misfolding and aggregation [131]. While gradual accumulation of NM in SNc tissue occurs as a natural process of aging [133], an intense heightened dark melanized pigment (hyperpigmentation) appears in the SNc preceding not only neuronal degeneration but also α-synuclein aggregation, inflammation, oxidative stress, apoptosis, Lewy body formation, depletion of GSH, functional loss of DAT and the loss of tyrosine hydroxylase positive neurons [10,128,134,135]. With PD, a biphasic but final loss of NM occurs gradually due to massive oxidation, cell death and release of NM from dying cells [136]. The loss of melanized nigral DAergic neurons is evident in PD brains (Right) when compared to healthy controls (Left) as shown in Figure 3 and is a major part of the pathology [137]. Ultimately, the loss of NM renders failure of its natural protective function, which is to sequester iron, free radicals and toxic quinones [138].
Figure 3.
Melanized dopaminergic neurons of the substantia nigra from post mortem human brain. Brain sections taken through the mid- brain of a normal (left) and a Parkinson’s disease patient (right). The Parkinson’s diseased hemisphere on the right shows a loss of the melanized neurons in the substantia nigra in the ventral midbrain [137].
The generation of DA oxidative toxins also includes enzymatic conversion of dopaminochrome to 5,6,-dihydroxyindole by DT diaphorase, the free radical initiated conversion of o-hydroquinones (protective) to o-semiquinones (toxic) [132] and transglutaminases which incorporate sulfur amino acids into DA-cysteine conjugate toxic precursors to neuromelanin [139]. And, recent studies suggest that transglutaminase inhibitors could be useful to prevent cross-linking reactions that lead to neurodegenerative aggregated proteins [140]. Animal models deficient in enzymes capable of catalytically oxidizing DA to DA-quinone (i.e., absent of PLA2 COX2), show a resistance to DAergic neurotoxicity after administration of MPTP. This is also corroborated where knockout models for SOD/GSH Px show extensive damage with MPTP [140–144], and protective effects are observed with COX/PLA2 inhibitors [145–148].
2.3.2. Non Enzymatic Oxidation of DA, 6-OHDA, Release of Iron & Oxidative Stress
A second route for DA oxidation is non-enzymatically by reactive oxygen species (ROS) and metals (Fe2+, Cu2+, and Mn2+) [149,150]. The autoxidation of DA can render formation of 6-OHDA (a potent neurotoxin) and O2 −. If superoxide reacts with nitric oxide (NO) the formation of ONOO− is evident. Peroxynitrite in turn can cyclically re-oxidize DA, deplete available reduced glutathione/ascorbate (vitamin C), incur a substantial loss of endogenous GSH-peroxidase and destroy the natural ability of GSH to act as an antioxidant [129,151]. While PD patients display depletion of GSH within the SNc [152], the reduction of GSH (i.e., γ-glutamylcysteine synthetase inhibitor) in experimental models also renders the SNc vulnerable to the toxic effects of MPTP and 6-OHDA [8]. For this reason, thiol based dietary antioxidants could be considered for clinical trials, as some have reported they prevent MPTP induced toxicity in mice [57,153], attenuate pathological effects of 6-OHDA, ONOO− and block the formation of DA o-semiquinone neurotoxic radicals [154].
Once 6-OHDA is formed, its presence can trigger neurodegeneration through reduction of striatal zinc and metallothione (otherwise antioxidant/metal detoxification agents) and initiate selective release of free iron from ferritin, where pro-oxidant effects predominate [152,155–160]. This could be perilous given the already high concentrations of iron dispersed throughout the substantia nigra, globus pallidus, red nucleus and locus cerulus [161–163]. Heightened free iron deposits are found in the vicinity of neurodegenerative regions, located in microglia, astrocytes and oligodendrocytes in conjunction with a rise in heme oxygenase (HO-1) (an enzyme which yields free Fe2+ iron from heme) and disappearance of NM (loss of high affinity binding polymer for Fe2+) [164–168]. In PD patients, iron deposition near degenerating neurons [169], could be intensified by hereditary mutations in iron regulatory binding proteins [170,171], iron storage/transport proteins such as ferritin (L/H subunits stabilization: storage/ferroxidase mediated uptake and utilization), caeruloplasmin, iron regulatory protein 2, lactoferrin/melanotransferrin receptors or the divalent metal transporter-1 [172,173]. Further, the accumulation of iron could be heightened by HO-1 which is significantly expressed in SNc dopaminergic neurons, the nigral neuropil, reactive astrocytes and Lewy bodies [174].
To summarize, the dynamics of PD pathogenesis is believed to evolve in part from mitochondrial energy failure, and through a series of events—DA oxidation can perpetuate a cyclical generation of DAergic toxins and precipitate high levels of free iron released throughout the basal ganglia. These events initiate a forward cycle of self-perpetuated DA oxidation, loss of NM and Fe2+ mediated damage which can indirectly fuel this loop by additional damage to the 26S proteasome prompting accelerated α-synuclein protein aggregation [175] or production of OH radicals which can oxidize lipids/proteins and DNA [8]. This degenerative cascade could be worsened by genetic mutations in iron transport proteins such as divalent metal transporter 1 (DMT1/natural resistance associated macrophage protein 2/solute carrier family 11, member 2) as noted in the SN of PD patients [176].
In Section 3, we discuss nutraceuticals that could impact each of these processes, in particular focusing on the important role that iron may play in PD pathology [177–179], the removal of which with iron chelators (i.e., EGCG, VK-28, clioquincol) is protective in a number of experimental models [40,178,179]. We also discuss the importance of using nutrient based combinations that contain chelators as just one module, since equally destructive forces are contributed by energy compromise, DA oxidation, concentration of free Fe2+ and the subsequent down stream metal catalyzed aggregation of insoluble proteins [180].
2.3.3. Enzymatic DA Oxidation by MAO-DA Aldehydes & H2O2
The third major route for oxidation of DA is through routine deamination by MAO A or B. MAO activity increases with the natural process of aging and can yield toxic products such as hydrogen peroxide (H2O2), ammonia, aldehydes, reactive oxygen species [180–182], 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde. The latter two have been reported to condense with H2O2 to form neurotoxic OH radicals [183,184]. And, in catecholamine neurons, DA can directly react with H2O2 leading to formation of 6-OHDA (neurotoxin) or further condense with acetaldehyde to produce toxic endogenous precursors such as 1,2,3,4-tetrahydroisoquinoline, 1,2,3,4-tetrahydro-β-carboline and R-salsolinol which are then subject to methylation [185–190] by either nicotinamide/salsolinol or phenylethanolamine N-methyltransferases forming toxic N-methylated pyridines with structure similar to MPTP/MPP+ [191,186,187,191].
2.4. Excitotoxicity
Mitochondrial energy dysfunction not only leads to collapse of DAergic function, but also instability of neurons to maintain voltage at the plasma membrane. Depolarization can cause over-activation of NMDA receptors throughout the brain, where glycine binds to NR1 and glutamate to the NR2 initiating fast inward Ca2+ currents to the cytoplasm. The general theory of excitotoxicity has remained consistent throughout the years and has been described to involve depolarization of the plasma membrane creating excitability in part due to (1) release of Mg+ as a voltage dependent N-methyl-d-aspartate (NMDA) block at presynaptic receptors (2) greater susceptibility to excitatory postsynaptic inward Ca2+ currents in response to glutamate activation on ionotropic NMDA/AMPA/kainate receptors and (3) a loss of inhibitory GABA metabotropic-inward ion currents upon receptor activation.
Mitochondrial toxins such as rotenone can worsen the heightened amplitude of inward ionic currents, effects known to be are reversible by addition of ATP [192]. In terms of circuitry, a deficit of magnesium (Mg) or ATP can lead to failed regulatory control of intracellular Ca2+ systems through changes not only at the NMDA receptor but also intracellularly through influences on inositol 1,4,5-trisphosphate and ryanodine receptors [193,194]. In vivo, studies show that dietary deficiency of Mg lowers NMDA receptor activation threshold and correlates to the overexcitability of glutaminergic neurons [195]. In Section 3, we discuss the importance of dietary Mg, in this and many other processes involved with PD pathology.
In PD, the over-excitability of the NMDA receptor may contribute to neurodegeneration because Ca2+ activation of neuronal nNOS can lead to nitrosative stress—a known primary elemental monomer modification leading to toxic mis-folded and aggregated proteins [196,197]. In reciprocal fashion, the accumulation of α-synuclein can stimulate nNOS, caspase-3 and initiate poly(ADP-ribose) polymerase (PARP-1) cleavage, all events which contribute toward neurotoxicity [198]. The toxic effects of α-synuclein on activation of nNOS are corroborated by studies that demonstrate that effects are blocked in the presence of NMDA receptor antagonists such as MK-801 and APV [199].
In addition, mitochondrial toxicity (i.e., MPTP) also leads to accumulation of glutamate in the SNc to an extent parallel to degenerative lesion [200]. The rise in glutamate stimulates increase influx of Ca2+ calpain activation in the cytosolic compartment, and these toxic effects are reversed by administration of NMDA antagonists, calpain inhibitors or antioxidants [201–203]. While the role of the NMDA receptor as it relates to PD is continually debated, it is noteworthy to mention that there is a very delicate balance between preventing over-activation or under-activation of glutaminergic receptors. The function of glutamate in neurotransmission is required for synaptic plasticity. And, as such, some studies also show that NMDA agonists such as D-cycloserine are protective against MPTP induced DAergic degeneration and microglial activation in the brain [204]. So clearly, this topic is very complex.
2.5. Inflammation
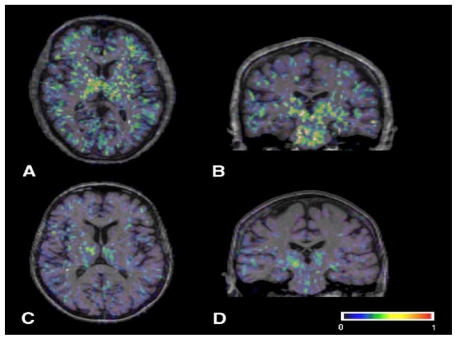
Both dying neurons and aggregated α-synuclein can trigger local gliosis, microglial activation, T cell infiltration and elevated expression/release of immunological participants [205]. These include major histocompatibility antigens, adhesion molecules, COX-2, IL-1b, IL-2, IL-4, IL-6, TNF-alpha, prostaglandins, glutamate, ROS, iNOS, MPO, NO and O2 − the latter two of which can react forming the neurotoxic molecule ONOO− [205–215]. Many of the inflammatory indicators are found in post-mortem tissue obtained form PD patients, particularly in regions of the SNc, striatum, LC and spinal fluid [216]. Major regulators of this response involve tyrosine kinase, phosphatidylinositiol 3-kinase (PI3K)/Akt, and the mitogen activated protein kinase (MAPK) signaling pathways such as c-Jun NH2-terminal Kinase (JNK), extracellular signal-regulated kinases (ERK) ½ p38 MAPK [46,217–222]. MAPK’s are evoked by cytokines or inflammatory stimuli, regulated by protein kinase A/cAMP and ultimately direct gene transcription by phosphorylating nuclear factor-kappa B (NF-κB) [223–226]. PET imaging using PK11195, [1-(2-chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinoline carboxamide] show active microglia occurring around neurodegenerative lesions in idiopathic PD patients vs. controls [227].
The use of anti-inflammatory agents may attenuate DAergic damage and antagonize global effects through targeting a number of signaling routes such as MAPKs, NF-kappaB activation/nuclear translocation or its association with the CREB-binding protein, IkappaB kinase (IKK), activating protein-1 (AP-1) and/or preventing IkappaB degradation or phosphorylation of c-jun N-terminal kinase (JNK) [228–231]. Substances that can inhibit any one of these mechanistic controls should block pro-inflammatory processes and antagonize the formation of iNOS, COX-2, PGE (2) or HO-1, thereby preventing DAergic loss induced by MPTP [232–237]. The co-expression of iNOS and COX-2 could be detrimental because the product formed by NO and O2 − (ONOO−) plays a highly relevant role in the pathological processes involved with PD. Removal of one or both of O2 − and NO/ONOO can prevent the deleterious effects of PD model toxins, as reported in transgenic mice deficient in iNOS, nNOS, NADPH oxidase or with overexpression of Mn/SOD conferring resistance to the toxicological effects of MPTP or intrastriatal injection of 6-OHDA [238–243]. Likewise, administration of specific nNOS/iNOS inhibitors or SOD mimetics can protect against the neurotoxicity of MPTP [33,42,244–246]. In the next section, we review nutraceuticals with multi-capabilities on anti-inflammatory signaling processes.
3. Nutraceuticals
3.1. Energy—Biochemistry and Metabolism
Considering the cascade of degeneration in PD as it involves mutations affecting protein degradation and folding, inadequate energy production in the SNc, DAergic malfunction, degenerative oxidative damage, excitotoxicity and inflammation, the question remains if any nutraceuticals or dietary practices as a lifetime habit can prevent or block one or more of these pathways and as a result slow the progression of PD. The next section provides a review of previous studies and future directives based on mechanisms as discussed above.
3.1.1. Pyruvic Acid
To overcome the loss of ATP, due to single or multiple hits directed at the mitochondria within neurons of the SNc, the first question to arise is to elucidate if it is possible to enhance anaerobic capability of the human brain, when physiological control over glucose concentrations are highly regulated. In order to optimize anaerobic glycolysis within the brain, dietary compounds must pass through the blood brain barrier (BBB) and likely work to promote key glycolytic regulators of substrate level phosphorylation including phosphoglycerate kinase, pyruvate kinase or lactic acid dehydrogenase (LDH), which would propel production of ATP [74,247,248]. Nutrient offsets associated with these enzymes are clearly altered during CNS neurological injury as evidenced by significant elevation in the ratios of lactate/pyruvate, NAD+/NADH and NADP+/NADPH [249]. These nutrient offsets suggest “metabolic stress” and accelerated use of nicotinamide reducing equivalents to drive survival processes to produce ATP through anaerobic shifts, in particular ischemia. The anaerobic process is fueled by the metabolite pyruvate (PY), which is a substrate for the LDH enzyme. Our investigations of the molecule PY, have indicated it to be the most powerful antioxidant of the glycolytic metabolites, also having capability of protect neuroblastoma against MPP+/6-OHDA and H2O2 toxicities in vitro [247–250]. It is possible that oral administration of PY could be capable of entering the brain, serving as a substrate for glycolysis, the Krebs cycle and the GABA shunt [251] and for this reason its use has been effective in preventing neurological damage associated with ischemic stroke [252]. Recently, other attributes of PY include an ability to block NMDA excitotoxicity in hippocampal neurons [253]. Although future research will be required to corroborate this, it would seem logical to combine oral administration of pyruvate, w/Mg (a required cofactor for pyruvate kinase) and niacin (precursor of reducing equivalents) which could help to restore nutrient offsets that occur due to accelerated anaerobic glycolysis in the CNS, when oxygen or mitochondrial function is insufficient.
3.1.2. Niacin
Furthermore, a deficiency of niacin is known to increase the risk for DAergic neurons to degenerate [191]. Likewise, the toxicity of MPTP is associated with a depletion of niacin, likely due to high demand for NAD+ in several biochemical processes including glycolysis and apoptotic over activation of PARP-1 [254–256]. The administration of niacin has shown protective against MPTP induced SNc cell loss and striatal DA depletion in vivo, effects which are possibly due to preventing drop in ATP by fueling glycolysis and preventing PARP-I NAD+ depletion [257–259]. Further upstream, PARP-1 is under regulatory control of tumor suppressor protein p53, a transcription factor that controls programmed cell death and cell cycle arrest. For this reason, it is not surprising that administration of either niacin, PARP-1/p53 inhibitors or PARP-1 knockout mice all show a resistance to MPTP mediated DAergic toxicity [256,257,260–262]. Therefore, the use of niacin as a therapeutic agent could be explored further, given its vast biochemical benefits including additional contribution to the pentose phosphate pathway, which regulates endogenous removal of H2O2 (a major contributing factor to PD pathology) through the GSH-Px system [191]. And, in our previous work, we have also found NADH to be a powerful antioxidant, alone capable of protecting against peroxide induced toxicity in neuroblastoma [247].
With regard to niacin, it is important to make note that a bit of controversy surrounds its use particularly as it relates to PD. Concern has been expressed that its administration could lead to synthesis of endogenous N-methylated nicotinamide, a compound with structural similarity to MPP+ [263]. Nicotinamide N-methyltransferase (NNMT) is the enzyme that can readily convert pyridines to toxic substances very similar to the PD toxic metabolite MPP+ [191]. While future research will be required to investigate these concerns, it may be possible to combine administration of niacin with natural compounds known to down regulate NNMT such as plant derived isoquinoline alkaloids, caffeine ± precursors (i.e., xanthosine—green tea or cocoa tea), which reportedly compete for methyl groups otherwise donated by s-adenosyl-l-methionine to drive NNMT enzyme activity [264–266]. The positive effects of caffeine are consistently reported as both administration of caffeine in animal models is therapeutic against MPTP and most importantly human epidemiological studies show that coffee consumption is associated with a decreased risk for developing PD [267,268]. As a side note, magnesium may also be a downregulator of NNMT [269].
3.1.3. Magnesium
Dietary magnesium (Mg) has a vast role in integrated human metabolism and is critically involved with production/utilization of ATP in the human brain. A number of studies suggest that a dietary deficiency of Mg is associated with greater loss of DAergic neurons [270]. And. low Mg brain tissue concentrations are evident in human PD patients [271]. With regards to the review on the pathology of PD above, Mg plays an indispensable role in proper DA uptake and vesicular storage and transport [272]. Heightened levels of Mg can attenuate effects of Ca2+ overload [273,274], augment the function of VMAT2 for sequestration of DA and provide a voltage dependent non-competitive block of the NMDA receptor otherwise responsible for excitability of neurons [199,275,276]. Ample Mg+ in the diet could be critical for PD patients, because of its diversity in energy related functions, energy storage processes (phosphocreatine), and its ability to thwart Ca2+ mediated neurotoxicity [276,277]. Mg also plays a critical functional role in activation of CuZn-SOD, and could thereby attenuate formation of ONOO−, involved with α-synuclein aggregation [278]. As a note, oral administration of Mg could benefit when co-administered with vitamin B6 and vitamin D. both which assist to maximize its adsorption and utilization.
3.1.4. B Vitamins and Regulation of Physiological Homocysteine
Another role for vitamin B6 pertaining to the pathology of PD is its conjunction with vitamin B12 and folate which regulate homocysteine by aiding its breakdown to methionine and tetrahydrofolate. These effects may attenuate neurotoxicity associated with hyperhomocysteinemia—a condition that is not only associated with PD pathology, but also the toxicity of MPTP in experimental models and as a side-effect of L-DOPA [279–281]. High levels of homocysteine could greater the severity of PD because it mediates toxicity by acting on NMDA receptors to precipitate oxidative stress, Ca2+ overload and apoptosis [282]. Vitamin B6 could also help to antagonize the hyperhomocysteinemic effects of nicotinamide via enhanced methylation [279]. Folate is another critical nutrient, where deficiencies in human PD patients have been observed in association with greater levels of plasma homocysteine [283,284]. In experimental models, the effects of hyperhomocysteinemia are known to potentiate the neurotoxic effects of MPTP [285]. For this reason, folate, B12 and vitamin B6 could be combined with a nutraceutical such as betaine and/or serine, which reduce homocysteine levels through aiding in its regulatory conversion to methionine or cysteine, respectively [286,287]. Garlic is another natural compound which can prevent the build up of homocysteine given its ability to stimulate cystathionine β-synthase and inhibit N5,N10-methylenehydrofolate reductase [288]. Further research will be required to explore the combined use of these particular B-vitamins with some of these nutraceuticals as it relates to homocysteine accumulation and neurotoxicity in human PD.
3.1.5. B Complex Vitamins, Riboflavin and Mitochondrial Disorders
There is also rationale to substantiate use of B-Complex vitamins for PD patients, due to the critical role these nutrients play in glucose metabolism and mitochondrial respiration. Vitamin B2 derivatives such as flavin adenine dinucleotide (FAD)/flavin mononucleotide (FMN) regulate aerobic mitochondrial metabolism by mediating redox reactions through the electron transport chain [74,289]. Interestingly, the use of oral riboflavin supplements in humans can reverse clinical symptoms associated with mitochondrial myopathy/pathologies (involving complex I–II), where reduction of lactate and restored mitochondrial function are associated with clinical improvements [290–294]. And, use of coenzyme Q10 (which plays a role in complex I–II function) for treatment of PD has been of considerable interest, although clinical trials have not yet confirmed therapeutic effects [295,258]. The B-Complex vitamins such as thiamin (vitamin B1), lipoic acid, biotin, vitamin B6, B12 folate, and pantothenate work together symbiotically to drive pyruvate dehydrogenase complex, gluconeogenesis and blood, glucose, oxygen delivery to the brain. The B-complex vitamins each play a unique role of equal importance but work collectively to optimize mitochondrial function, in particular when challenged with toxins such as rotenone [296]. Clinical trials for multi-vitamin supplements therefore could be considered.
3.1.6. Creatine, Chromium
Nutraceuticals that optimize ATP storage reserves may further strengthen the capacity of energy requiring systems. Known disturbances in choline/creatine have been observed in PD patients [297], and creatine supplements have been shown to protect against MPP+/MPTP, 6-OHDA and glucose deprivation [53,54,298]. However, preliminary studies in our lab have failed to show protective effects by creatine against MPTP induced DA degeneration in the mouse model, a topic under current investigation (unpublished). While creatine could be beneficial in augmenting ATP storage, chromium salts would be equally important in maintaining physiological glucose, glucose tolerance, insulin sensitivity [299] and glycemic functions [300]. Adequate chromium in the diet seems fitting given its role in optimizing systemic glucose metabolism, despite a lack of evidence to suggest chromium aberrations in cerebral spinal fluid of PD patients [301].
3.2. Plant Polyphenols—Attenuation of DA Oxidation
The use of vitamins to support energy function could further be combined with plant derived polyphenolic compounds (PDPC) that specifically target downstream toxic effects as a direct result to the loss of ATP. These include collapse of DA trafficking, DA oxidation, generation of ROS, fenton reactions, DAergic neurotoxins, loss of NM and CNS glial inflammation. A number of food-based molecules have previously been reported in the literature as being effective in antagonizing specific events within these processes.
3.2.1. Tyrosinase Inhibitors
As stated previously, the initial oxidation of DA to DA-quinone, or from DA quinone to its toxic metabolites are believed to contribute toward DAergic degeneration. Although future research will be required to substantiate this, these processes could be blocked by nutraceuticals such as polyphenolic inhibitors of tyrosinase, COX, lipoxygenase, PLA2, xanthine oxidase or antioxidants/metal chelators.
The first to review is tyrosinase/polyphenol oxidase (PPO) which is a copper requiring metalloenzyme that catalyzes formation of o-quinones. A heightened enzyme activity of tyrosinase could be associated with elevated risk for PD [302] and skin hyperpigmentation disorders [303] both which involve heightened oxidation of L-DOPA to form dopachrome [304,305]. These same processes are often researched in the field of food chemistry, due to food browning reactions occurring through PPO enzymes in vegetables such as potato or mushroom. Creatively, it has been proposed that such as model could serve practical for the investigation or screening of nutraceuticals against DA oxidation processes as it relates to PD [306]. Future research could be done to consider analysis of established nutraceuticals known to inhibit tyrosinase, some of which include the following:
3.2.2. COX Inhibitors
Natural inhibitors of COX could also block the initial step of enzymatic DA oxidation to DA quinone through PGH2 synthase. A review of the literature shows a number of promising plant derived polyphenolic compounds (PDPCs) as effective COX inhibitors such as:
3.2.3. Lipoxygenase Inhibitors
PDPC’s that may be able to block the initial step of enzymatic DA oxidation to DA-quinone through inhibition of lipoxygenase (5-LOX, 12-LOX) and include the following:
3.2.4. Phospholipase A2 Inhibitors
While PLA2 inhibitors attenuate DA oxidation reactions, they may serve dual function in PD pathology because they also block formation of arachidonic acid as a substrate for prostaglandins. PLA2 inhibitors could be combined with administration of omega-3 fatty acids (i.e., canola/fish oil), thereby reducing PGE2 (a pro-inflammatory prostaglandin specifically associated with PD pathology) [377]. Co-administration of vitamin E may enhance absorption of omega-3 fatty acids and prevent fatty acid oxidation. Future research could consider analysis of plant-derived compounds that are known to inhibit PLA2 in experimental models of SNc DAergic damage, some of which are known to include:
3.2.5. Xanthine Oxidase Inhibitors
The initial step of enzymatic DA oxidation to DA quinone could be attenuated by xanthine oxidase inhibitors, some of which are known to include the following:
3.2.6. Xanthine Oxidase and Superoxide Scavengers
Combined xanthine oxidase/superoxide scavengers may reduce oxidative stress, prevent formation of ONOO and attenuate the degenerative process, some of which are known to include:
3.3. Histidine, Quercetin and Zinc
Other polyphenolic compounds that may block the initial step of enzymatic DA oxidation include substances which down regulate DT diaphorase or mono-oxygenases such as EGCG [432], flavones [433] baicalin, oroxylin-A glucoronides [434], quercetin [435] or histidine [436]. While we mention the protective properties of EGCG and quercetin on PD related processes throughout this review, noted effects of histidine may also include its ability to augment the uptake and transport of zinc into the brain, where zinc can counteract the pro-oxidant effects of iron [437], ischemia-reperfusion [438,439] or mitochondrial toxins such as MPP+ [440]. See Section 3.8.
3.4. N Acetyl Cysteine
Thiol based compounds are believed to help slow non-enzymatic autoxidation of DA in the presence of ROS and metals (Fe2+, Cu2+, and Mn2+) [149,150]. Autoxidation of DA to 6-OHDA (a potent neurotoxin) and O2 − can be lethal in the presence of NO, forming ONOO−. Peroxynitrite can then re-oxidize DA and deplete available reduced glutathione and ascorbate [129,151]. Possible dietary counter intervention could include thiol antioxidants such as NAC which in experimental models blocks the autoxidation of DA, prevents MPTP induced toxicity in mice [57,153] attenuates pathological effects of 6-OHDA, ONOO− and blocks the formation of DA o-semiquinone neurotoxic radicals [154].
3.5. Hydrogen Peroxide Scavengers
The third route of DA oxidation is through deamination by MAO A or B which yields H2O2, ammonia [180–182], 3,4-dihydroxyphenylacetaldehyde and 3,4-dihydroxyphenylglycolaldehyde. The latter two condense with H2O2 to form OH radicals [183,184] and DA reacts with H2O2 leading to form 6-OHDA or condenses with acetaldehyde to produce toxic precursors subject to methylation [185–190]. Due to the importance of MAO activity and the initial condensation reaction between catecholamines and aldehydes that create precursors subject to methylation, future research could investigate therapeutic food based compounds that work as (1) MAO inhibitors (2) compounds that potentiate aldehyde dehydrogenase such as GSH, NAD+ (3) down regulate nicotinate/phenylethanolamine N-methyltransferases such as caffeine or 4) scavenge H2O2.
Removing hydrogen peroxide generated by MAO or DA autoxidation could be very beneficial in slowing the rate of progression in PD. Hydrogen peroxide, if present in high quantities can oxidize DA to 6-OHDA, which in turn can then react with 6-OHDA to propagate OH radicals, contributing to the formation of α-synuclein-Fe aggregates and insoluble filaments [35,441]. The generation of H2O2 in DAergic neurons initiates multiple degenerative processes such as improper degradation of oxidized proteins through the ubiquitin proteasome pathway, formation of dopachrome and toxic DA quinones [132,442,443]. The role for peroxide in PD pathogenesis is evidenced by the fact that its removal via potentiation of catalase/SOD prevents injury in MPTP models of injury. Transgenic mice that over express cytosolic CuZn-SO/GSH-Px or applied administration of SOD/catalase mimetics (which both dismutase O2 −, and convert subsequent H2O2 to water) provide protection against MPTP, paraquat and 6-OHDA in vivo models of injury [33,243,444–446]. In contrast, reduction in GSH-Px/CuZn SOD (i.e., knockout mice) leaves the SNc area vulnerable to oxidative stress and MPTP injury [143,447]. For these reasons, beneficial nutritional substances could include those that upregulate endogenous glutathione peroxidase and/or catalase, such as NAC, GSH, selenium, vitamin E, NADPH and curcumin [448]. Co-administration of niacin (which provides NADPH to drive GSH-Px) along with substances that augment function of GSH-PX could provide synergy in protecting SNc neurons from oxidative stress [57,153]. Other useful nutritional substances could include those that aid in SOD such as methionine, manganese, copper, zinc and propolis [449] and H2O2 scavengers which are known to include the following:
3.6. Iron Chelators
6-OHDA generated during DA oxidation, reduces metallothione and causes release of free iron from ferritin [152,155,160]. Natural substances that antagonize 6-OHDA toxicity such as NAC, GSH, cysteine, pyruvic acid, [455] and zingerone [456] or are integral constituents of metallothioneine such as serine, lysine and cysteine [155] could be further researched. The accumulation of free iron is deleterious because it is associated with degenerating SNc neurons, surrounding glial cells and found after administration of MPTP/6-OHDA in animals [164–169]. Faulty iron homeostasis in the basal ganglia could lead to a number of oxidative reactions, the acceleration of α-synuclein protein aggregation [175] and formation of OH radicals which can damage neuronal lipid/protein and DNA [8]. It is reported that the use of iron chelators protect against MPTP and 6-OHDA models of PD toxicity [40,178,179]. A number of natural substances are capable of reducing/chelating complex iron including the following:
3.7. Heme Oxygenase Inhibitors
The accumulation of iron can also occur due to overactivity of the HO-1 enzyme, which can convert heme to free Fe2+, and carbon monoxide, this also being significantly expressed in SNc dopaminergic neurons, the nigral neuropil, surrounding reactive astrocytes and Lewy bodies [174]. Up regulation of HO-1 occurs as a natural response to oxidative stress and correlates to iron deposition in the nigral area with degenerative SNc lesions. For this reason, potentially helpful nutritional substances may include those that can inhibit HO-1 directly such as cysteine, resevatrol, vitamin C, sulfur compounds (i.e., NAC, GSH) [471], apigenin [472], quercetin and kaempferol [473].
3.8. Zinc and Selenium
While reactive iron contributes to the degeneration in SNc, the administration of zinc (Zn) and selenium (Se) could strengthen combination nutraceutical strategies [474–476]. Dietary intake of Se, Zn are required for the function/expression of endogenous antioxidant enzymes and ample amounts can attenuate iron-induced, MPTP and 6-OHDA induced DAergic degeneration [150,475,477]. Furthermore, chronic inflammation can bring about a Zn deficiency due to the use of Zn-dependent transcription factors that regulate DNA/nucleic acid synthesis in response to cytokine activation in immunocompetant cells (i.e., hypozincemia) [478,479]. A Zn deficiency can also evoke a shift in the ratio of Cu/Zn rending less than normal function of the CuZn SOD, turning it from an antioxidant to a pro-oxidant enzyme [479]. A requirement for zinc in the body could be justified with PD patients, since Zn mediates (a) downregulation of glutamate release, inhibition of NMDA/mGlu-R receptors, protection against NMDA neurotoxicity (b) renders a positive modulation on GABA release (c) stimulates endogenous antioxidant enzymes and nerve growth factors (d) inhibits nNOS, endonucleases, pro-apoptotic cascades (e) augments synaptic plasticity and (f) is known to prevent age related deterioration of learning and memory [437].
Both zinc and selenium contribute to anti-inflammatory effects through downregulation of MAPK p38, JNK and NF-κB DNA binding/AP-1 c Jun activation, where the therapeutic effects of Se also involve a rise in glutathione peroxidase/reduction of lipid peroxidation, increased glucose uptake, ATP production through glycolysis and an anti-apoptotic effects [474,480].
3.9. Anti-Inflammatory Nutraceuticals
The CNS inflammatory response is under the ultimate control of kinases such as tyrosine kinase [217], PI3K/Akt, and mitogen activated protein kinase signaling pathways such as JNK, ERK ½ p38 MAPK [46,218–222]. The topic of inflammatory is far too large for this review and therefore is summarized as follows. Briefly, MAPK’s are evoked by cytokines or inflammatory stimuli, regulated by protein kinase A/cAMP and ultimately control gene transcription by phosphorylating NF-κB which then binds to the promoter region of genes to initiate transcription for a range of pro-inflammatory proteins [223–226]. Anti-inflammatory agents can antagonize global effects through targeting a number of these signaling routes such as MAPKs, NF-κB activation/nuclear translocation or its association with the CREB-binding protein, IkappaB kinase (IKK), activating protein-1 (AP-1) and/or preventing IkappaB degradation or phosphorylation of JNK [228–231]. In brief summary, natural substances that may provide protection include those that can inactivate phosphorylated MAPK’s such as ERK ½ kinase, p38 MAPK, JNK, inhibit IkappaB kinase, IkappaB degradation, NF-κB, AP-1 activation, antagonize COX-2/PGE2/iNOS and reduce expression of TNF-alpha and other pro-inflammatory proteins in immuno-competent cells some of which are listed as follows:
Additionally, phosphodiesterase (PDE) inhibitors, in particular PDE 1 and IV through altering cAMP can downregulate iNOS [511] and protect against MPTP toxicity [512]. Food based compounds known to inhibit PDE include the following:
3.10. Toxic Protein Aggregates
In this section we briefly discuss a potential for targeted nutraceutical therapies which would prevent accumulation of α-synuclein, augment the ubiquinone-proteasome system (UPS) or inhibit mammalian target of rapamycin (mTOR) signaling to upregulate autophagy, which may in the long term slow the progression of this disease.
3.10.1. Nutraceuticals—Reduction of aggregated α-SYNUCLEIN (PARK1)
In brief, the kinetics of α-synuclein aggregation involves a number of progressive stages some of which could be altered by nutraceuticals. A higher propensity for α-synuclein aggregation can occur due to missense mutations (A30P, A53T, E46K) in human PD [524]. The general kinetics of aggregation involves three stages: (1) a protein monomer must undergo a modification; (2) modified monomers can then readily interact with each other to form small aggregates and (3) aggregates after reaching a certain size, referred to as a “nucleus”, can undergo irreversible rapid volume expansion called elongation which result in the formation of fibrils, then taking up residence as toxic entities in neurons and Lewy bodies. The initial protein modifications can occur due to phosphorylation of α-Synuclein at Ser 129 (p-Ser 129), nitration at tyrosine residues and C-terminal truncation- all of which can lead to nucleation where aggregation becomes probable [525,526]. The initial protein modification stage can also occur due to neurotoxic insults including but not limited to DA oxidative products, NO, ROS and high concentration of metals [527–529]. In turn, α-synuclein can directly initiate increased membrane ion permeability, vesicle leakage of DA and decreased mitochondrial respiration [530,531], which in turn can generate compounds that lead to α-synuclein modification. In essence, α-synuclein can lead to toxicity, and neurotoxicity can lead to α-synuclein aggregation. In this review, we have covered information on nutraceuticals that indirectly attenuate events known to evoke the initial stages of propagative α-synuclein misfolding, such as iNOS, nNOS, DA oxidative products and the enzyme pathways by which DA quinones are produced (Sections 3.1–3.9). In addition, nutraceuticals that inhibit enzymes that otherwise phosphorylate α-synuclein such as polo-like kinases (i.e., thymoquinone–black cumin) [532], casein kinase II (i.e., ellagic acid) [533,534], Gprk2GRK2/5 [535] or proteases such as calpains, calcium-dependent non-lysosomal cysteine proteases may prevent a tendency for α-synuclein to aggregate or result in truncated toxins of α-synuclein. The use of any nutri-therapy which can prevent likelihood of aggregation, should lessen cell burden of accumulated insoluble proteins which otherwise has affinity for lipids, presynaptic vesicles, membranes and can cause considerable damage to organelles including mitochondria [536].
3.10.2. (Parkin) E3 ubiquitin ligase and Proteosomal Dysfunction
Once α-synuclein aggregates are formed, a second vulnerability for continued accumulation would be improper recognition and ubiquitination of specific target proteins for degradation by the proteasome. This can occur in part due to genetic defects in parkin-E3 ubiquitin ligase or its associate SCF complex (Skp1-Cullin-F-box protein complex) [537,538]. While nutritional constituents may not be able to halt faulty processes in ubiquitination, it may be possible to optimize the function of the proteasomal complex with dietary agents.
The proteasomal complex consists of a 20S proteolytic core with two 19S regulatory caps, responsible for recognition, proteolysis, unfolding and transport of proteins into the core lumen for processing. Inhibiting the function of the proteasome with lactacystin, PSI or MG-132 can effectively mimic PD pathology including selective SNc degeneration, α-synuclein positive inclusion like granules and activation of glial cells [539,540]. Nutraceutical substances such as iron chelators can protect against the adverse effects of such proteasomal inhibitors with capability to prevent lactacystin-induced DA neurodegeneration in vivo [541]. These protective effects are likely because the proteasome can also be adversely affected or inhibited by DA oxidative metabolites, DA quinones or ROS, effects that are also blocked by antioxidants such as GSH, ascorbic acid, vitamin E, SOD or catalase [542]. In this aspect nutraceuticals could serve useful to protect against further insult to an already vulnerable proteosomal complex, not only due to mutations in parkin, but also due to lack of endogenous proteasome activator PA28 expression in the SNc, concomitant to reduced function of α-subunit of the 20S proteasome in the SNc of sporadic PD patients [543,544].
3.10.3. Nutraceuticals, Autophagy and mTOR signaling
A second degradation route for eliminating α-synuclein aggregates and malfunctional mitochondria is through the process of autophagy. The removal of depolarized damaged mitochondria is mediated through a process called mitochondrial fission which is regulated by membrane constriction through dynamin-related protein (Drp1) mitochondrial fission 1 and GTP hydrolysis [545] in preparation for clearance through autolysosomes. Notable mutations in PINK1 can adversely affect this process, by preventing both Drp1-dependent fragmentation and phosphorylation/relocation of Parkin to mitochondria where it then fails to catalyze mitochondrial ubiquitination, recruitment of ubiquitin-binding autophagic components, HDAC6 and p62, and subsequent mitochondrial clearance [546]. Together, genetic mutations in both PINK1 and Parkin lead not only to failure of mitochondria, but also a lack of mitochondrial quality control for proper degradation of mitochondria that are no longer functional. While nutritional constituents may not be able to reverse protein defects associated with function of Park and PINK1 mutations, dietary factors can largely influence and activate autophagy-lysosomal function.
Autophagy is described as the means by which cells degrade oxidized and damaged membranes, organelles and mis-folded proteins. This process is initiated by formation of a phagophore which expands and engulfs portions of the cytoplasm then forming a autophagosome [547]. The initiation stages of autophagosome formation is under control of signaling by class III phosphoinositide 3-kinase and Atg 6 (Beclin-1), which regulates phosphorylation of microtubule-associated protein 1 light chain 3LC3 [548]. These phosphorylated LC3 marked vesicles are then trafficked along microtubules in a dynein reliant fashion and eventually fuse with lysosomes (autolysosomes), where contents are degraded by acidic lysosomal hydrolases. Lysosomes can also reach out on their own in a process called microautophagy where they directly engulf cytoplasm by invagination or septation. And, once inside the lysosome, cathepsin D becomes the main lysosomal enzyme involved in the degradation of α-synuclein [549].
The process of UPS and the autophagy-lysosomal systems are under direct control of mammalian target of rapamycin (mTOR) signaling. Stimuli that lead to upregulation of mTOR serve to block autophagy-lysosomal function and its contribution toward accumulated oxidized and damaged organelles/proteins. Signals that upregulate mTOR include those registering as high nutrient energy status signals, such as glucose, insulin, a high ratio of ATP/AMP ratio, leucine, oxidative stress [550], arginine [551] and high levels of amino acids [552]. The rise in mTOR and reduction in the autophagy-lysosome pathway can be chemically induced by 3-methyladenine or chloroquine, effects which lead to accumulation of Ser-129-phosphorylated α-synuclein [553]. This is opposite to the effects of rapamycin which through inhibition of mTOR activate clearance of aggregate-prone proteins, including α-synuclein as well as faulty mitochondria and prevent the toxic effects of proteosomal inhibitors on DAergic systems [554]. A number of substances in the diet are known to upregulate autophagy lysosomal function by downregulation of mTOR, some of which include resveratrol, spermidine, curcumin, piperine, caffeine, epigallocatechin gallate, garlic, S-allylcysteine [555–557], anthocyanins [558], selenium [559], eicosapentaenoic acid and lycopene [560]. Also, it is likely that nutraceuticals that could selectively inhibit IMPase, IP3, adenylate cyclase [561] or Akt signaling may downregulate mTOR and induce autophagosomal clearance [562].
4. Conclusion
In conclusion, this review provides information on nutritional biochemistry as it relates to pathological processes inherent to PD. PD pathology involves both regional and systemic nutrient offsets that are largely related to heightened anaerobic glycolysis, homocysteine metabolism, faulty aerobic energy metabolism, metabolic stress, iron deposition and catecholamine mediated oxidative stress. These offsets could be aggravated by blood -tissue nutrient deficiencies as commonly reported in human PD patients or the process of aging itself, both which could exacerbate protein mis-folding/aggregation, disruption of proteasomal processes or losses in DAergic neurotransmission. Future research will be needed to investigate a strategic means to employ combined nutraceuticals that work effectively and collectively to alter metabolism or pathological processes in such a way as to slow the progression of PD in humans. Any therapeutic strategy that can effectively do so, will afford extended quality of life to human PD patients.
Figure 1.
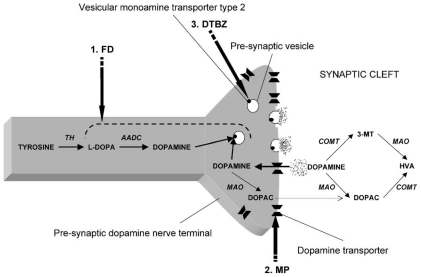
PET Imaging Tools Used in PD. Schematic representation of dopamine synthesis and metabolism, including sites of action of pre-synaptic dopaminergic PET ligands. (1) FD reflects uptake of l-dopa, the AADC activity, and the storage of dopamine in pre-synaptic vesicles; (2) MP binds to the dopamine transporter, which is specific for the gradient-determined re-uptake of dopamine; and (3) DTBZ binds to vesicular monoamine transporter type 2, which is responsible for the uptake of monoamines into pre-synaptic vesicles. In the striatum, more than 95% of the monoaminergic nerve terminals are dopaminergic. (AADC: aromatic amino acid decarboxylase; COMT: catechol-O-methyltransferase; DOPAC: 3,4-dihydroxyphenylacetic acid; DTBZ: [11C]-dihydrotetrabenazine; FD: 6-[18F]-fluoro-ldopa; HVA: homovanillic acid; l-DOPA: l-3,4-dihydroxyphenylalanine; MAO: monoamine oxidase; MP: [11C]-d-threomethylphenidate; 3-MT: 3- methoxytyramine; TH: tyrosine hydroxylase) [30].
Figure 2.
Imaging dopamine terminal function in healthy controls and early Parkinson’s disease (Modified from [28]).
Figure 4.
In the PD patient (A and B), binding is increased in the basal ganglia, pons and frontal regions, while the healthy control person (C and D) only shows constitutive [11C] (R)-PK11195 binding in the thalamus and pons. The color bar denotes binding potential values from 0 to 1 [227].
Table 1.
Tyrosinase Inhibitors.
| Tyrosinase Inhibitors | Reference |
|---|---|
| Tetrahydroxychalcones, Butein | [307,308] |
| Prenylated flavonoids, Sanggenon D | [309] |
| Sophoraflavanone G, Kuraridin, Kurarinone, Norkurarinol | [310,311] |
| Cinnamic acid, Aloin, Sophorcarpidine | [312,313] |
| Glabrene/Licorice, licuraside, isoliquiritin and licochalcone | [314,315] |
| Quercetin, Galangin, Morin, Fisetin, Luteolin, Apigenin, | [316] |
| Esculetin | [317] |
| Hexylresorcinol, Dodecylresorcinol | [318] |
| Oxyresveratrol | [319] |
| Gnetol | [320] |
| (−)-Epigallocatechin-3-gallate, Hinokitiol (beta-thujaplicin), Kojic acid | [321,322] |
| Reduced glutathione, cysteine, thiol compounds, ascorbic acid, acetic acid | [323–326] |
| Dimethylsulfide | [327] |
| Phytic acid | [328] |
| Tannic acid | [329] |
| Nobiletin | [330] |
| Kaempferol | [331,332] |
| Extract of hibiscus, carex pumila, and garcinia subelliptica | [333] |
| Wine phenolics | [334] |
| Green tea | [335] |
| Procyanidins, Grape seed extract | [336,337] |
| Gallic acid derivatives | [338] |
| Safflower | [339] |
| Aisic acid | [340] |
| Olive oil constituents | [341] |
Table 2.
Cycloxygenase I/II Inhibitors.
| Cycloxygenase I/II Inhibitors | Reference |
|---|---|
| Quercetin, Kampferol, Chrysin and Galangin | [342,343] |
| Anthocyanins, Delphinidin, Cyanidin, Malvidin | [344,345] |
| Galangin, Morin, Apigenin, Rutin, Catechin, EGCG, Quercetin, Chrysin, Flavones, Luteolin, Tectorigenin, Bilobetin, Nobiletin, Fisetin, Naringenin, Quercetin, Lonchocarpol, Tomentosanol and Wogonin | [346–349] |
| Quercetin, Quercetin 3-glucuronide, Quercetin 3′-sulfate 3′-methylquercetin 3-glucuronide | [350,351] |
| Ursolic acid, Eugenol, Pyrogallol and Cinnamaldehyde | [352] |
| Ipriflavone, Resveratrol, MSV-60, Amentoflavone, Ruscus extract | [353,354] |
| Notoginseng Prenylated flavonoids, Morusin, Kuwanon C, Sanggenon, Kazinol, Kuraridin, Kurarinone, Sophoraflavanone G | [355] |
| Butein and 7,3′,4′-trihydroxy flavone | [356] |
| Coumarins, Bergapten | [357] |
| Amentoflavone | [358] |
| Oroxylin A | [359] |
| Caffeic acid Phenethyl Ester and Propolis | [360] |
Table 3.
Lipoxygenase Inhibitors.
| Lipoxygenase Inhibitors | Reference |
|---|---|
| Luteolin, Baicalein, Fisetin, Quercetin, Eugenol, Curcumin, Cinnamaldehyde, Piperine, Capsaicin, Allyl sulfide, Oroxylin A, Wogonin | [361–364] |
| Morin, Galangin, Kaempherol, Taxifolin, EGCG, Esculetin, Propyl gallate | [365–367] |
| Coumarin, 7-hydroxy-derivative, Fraxetin, Daphnetin, Coumarin derivatives | [368] |
| Amentoflavone | [369] |
| Kuraridin, Sophoroflavonone G, Kenusanone A, Psoralidin | [370] |
| 3,5,6,7,3′,4′-hexamethoxyflavone, Sinensetin, Nobiletin, Tangeretin, Rhamnetin Tetramethylscutellarein, 6,7,8,3′,4′-heptamethoxyflavone, Hesperidin, Ferulic acid | [371] |
| Sophoraflavanone G, Quercetin, Kenusanone A | [372] |
| Circiliol, Hypolatein, Sideritloflavone | [373] |
| Silymarin | [374] |
| Bean (Phaseolus vulgaris L.) hulls | [375] |
| Cirsiliol, Hypolaetin, Hypolaetin-8-O-beta-d-glucoside, Gossypetin, Gossypin, Hibifolin, Leucocyanidol | [376,377] |
| Oroxylin A, Baicalein, Wogonin | [378] |
| Procyanidins | [379] |
| Quercetin glycosides | [380] |
| Entaureidin and 5,3′-dihydroxy-4′-methoxy-7-carbomethoxyflavonol | [381] |
Table 4.
Phospholipase A2 Inhibitors.
| Phospholipase A2 Inhibitors | Reference |
|---|---|
| Quercetin. Kaempferol, Myrecetin, Kaempferole-3-galactoside, Scutellarein, Ochnaflavone, Amentoflavone, Ginkgetin, Isoginkgetin, Morelloflavone, Bilobetin, Prenylated flavonoids | [342] |
| Ginkolide | [378] |
| Amentoflavone, Ginkgetin | [379] |
| Fish oil, Evening primrose oil | [380,381] |
| 2′,4′,7-trimethoxyflavone | [382] |
| Nobiletin | [383] |
| Rosmarinic acid | [384] |
| Omega-3 fatty acids | [385] |
Table 5.
Xanthine Oxidase Inhibitors.
| Xanthine Oxidase Inhibitors | Reference |
|---|---|
| Skull Cap (Scutellaria baicalensis (SbE)), Grape seed proanthocyanidins | [386] |
| Hesperitin, Theaflavin-3,3′-digallate, Cranberry juice | [387–389] |
| Chrysin, Phloretin, Luteolin, Kaempferol, Quercetin, Myrecetin, Galagin, Apigenin, Morin, Isorhamnetin, Fisetin, Rutin | [390–395] |
| EGCG, 4-t-butylcatechol, Catechin, Fisetin, Luteolin, Raxifolin | [395,396] |
| Quercetin glycosides | [397] |
| Apigenin, Quercetin, Isovitexin | [398] |
| Hydroxyl or Methyl Chalcones (i.e., 3,3,4,4-tetrahydroxychalcone), Esculetin, 4-methylumbelliferone | [399] |
| Propolis, Caffeic acid phenetyl ester, Chrysin, Galangin | [400,401] |
| 5,7,4′-Trihydroxy-6-methoxyflavone p-coumaric acid derivatives drupanin, 4-acetyl-3,5-diprenylcinnamic acid, trans-ferulic acid O-hexan-3-onyl-ether | [402] |
| Baicalein, Wogonin, Baicalin | [403–405] |
| Pycnogenol, Silymarin, Silybin, Silybin flavones, Purpurogallin | [406,407] |
| Black Tea | [408] |
| Procyanidins, Pygnogenol | [409–412] |
| Anthocyanins, Cyanidin, Cyanidin 3-O-beta-d-glucoside | [413] |
| Myricetin Glycosides | [414] |
Table 6.
Xanthine Oxidase and Superoxide Scavengers.
| Xanthine Oxidase and Superoxide Scavengers | Reference |
|---|---|
| EGCG, EGC, Pyrogallol, Catechin, Luteolin, Myrecetin, Rutin, Apigenin, Quercetin, Hesperitin, Naringenin, Biochanin, Retinol, Daidzein, Genestein, 4-t-butylcatechol, Taxifolin, Fisetin, Kaempferol, 5,7,4′-trihydroxy-6-methoxyflavone | [395,402,415–417] |
| Caffeic acid, Rosmarinic acid, Salvianolic acid, Sage | [418] |
| Apigenin, Quercetin, Diosmin | [419] |
| Green tea polyphenolics, Theaflavin, EGCG | [388,389,420,421] |
| Scutellarin | [422] |
| Oligomeric proanthocyanidins, EGCG, Delphinidin, Myrecetin, Gallic acid, Caffeic acid, Fisetin, Quercetin, Catechin, Epicatechin | [423] |
| Galangin/Caffeic acid phenethyl ester, Propolis, Caffeic, Chlorogenic acid, Gallic acid | [401,424,425] |
| Baicalein, Baicalin, Morin | [404,426,427] |
| Uric acid | [428] |
| Chrysoeriol ± glycoside | [429] |
| Anacardiaceae spice | [430] |
| Myrecetin, Fisetin, Quercetin | [431] |
Table 7.
Peroxide Scavengers.
| Peroxide Scavengers | Reference |
|---|---|
| Acacetin, Dihydrorobinetin, Fisetin, Isorhamnetin, Robinetin, Myricitrin, Hyperoside | [450] |
| Resveratrol, Catechin, Gallocatechin | [451,452] |
| Pygnogenol, Pyrogallol, Gallic acid, Anthocyanidins | [452,453] |
| Gallic acid, Trolox, Kaempferol | [454] |
| Vanillic/Caffeic acids | [450] |
| Baicalein | [448] |
| Hydroxytyrosol | [442] |
Table 8.
Iron Reducing/Chelating Compounds.
| Iron Reducing/Chelating Compounds | Reference |
|---|---|
| Rutin, Morin, Rosemary, Sage, Oregano | [457] |
| Phytic acid, Brown rice bran, Tannic acid | [449] |
| Apigenin, Diosmin, Phloretin, Fisetin, Taxifolin, Naringenin | [458] |
| Quercetin, Rutin, Myrecetin, Luteolin, Epicatechin Caffeic acid, Catechin, Kaempferol, Naringenin, Baicilein | [459–464] |
| Theaflavin, Theaflavin Digallate | [455,465,466] |
| Vitamin E, Zinc | [467] |
| Gallic Acid | [468] |
| Silymarin, Silybin | [469] |
| Rutin | [470] |
Table 9.
MAPK/NF-κB/iNOS/COX-2 (−).
| MAPK/NF-κB/iNOS/COX-2 (−) | Reference |
|---|---|
| Selenium, Zinc | [429,435] |
| Chrysin, Quercetin, Galangin, Propolis or its derivatives | [481–486] |
| Apigenin | [487,488] |
| Luteolin | [487,489,490] |
| Diosmetin, 3-hydroxyflavone, Pillion,4′,7′-dihydroxyflavone, Ayanin, Luteolin, Tectochrysin, 3′,4′-dihydroxyflavone, Tamarixetin, Genestein, Kaempferol, Izalpinin, Ombuine, Biochanin, Tectorigenin, Daidzein, 7-hydroxyflavone, Rhamnetin, flavone, EGCG, Mearnsetin, Liquiritigenin, Myrecetin | [491] |
| Hydroxychalcones | [228,492,493] |
| EGCG/Green tea | [494–496] |
| Butein | [497] |
| Anthocyanins | [344,498] |
| 5,6,3′,5′-tetramethoxy 7,4′-hydroxyflavone, Artemisia Absinthium, Wormwood, Blackwalnut | [235] |
| Scutellarin | [499] |
| Isovitexin | [500] |
| Naringin, Hesperitin and Naringenin | [501–503] |
| Baicalein | [504,505] |
| Silibinin, Silymarin | [225,506,507] |
| Amentoflavone | [508] |
| Licorice | [509] |
| Wogonin | [510] |
| Curcumin, Luteolin, Wognonin, Kaempferol, Nobiletin, Bilobetin | [342] |
Table 10.
Phosphodiesterase Inhibitors.
| Phosphodiesterase Inhibitors | Reference |
|---|---|
| Butein | [513] |
| Cirsimarin | [514] |
| Grape Skins, Anthocyanin, Malvidin | [515] |
| Diosmetin, Luteolin, Apigenin, Quercetin, Myrecetin | [516] |
| (+)-Catechin, Caffeic acid | [517] |
| Gingko Biloba | [518] |
| Biochanin A, Tyrphostin, Diadzein | [519] |
| Theophylline | [520] |
| Amentoflavone, Bilobetin, Sequoiaflavone, Ginkgetin, Isoginkgetin | [521] |
| Scutellarein, Phloretin, Naringenin | [522,523] |
Acknowledgment
This research was supported by a grant from NIH NCRR RCMI program (G12RR 03020).
Abbreviations
- 6-OHDA
6 Hydroxydopamine
- AADC
Aromatic amino acid decarboxylase
- AMPA
α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- AP-1
Activator protein 1
- ATP
Adenosine triphosphate
- BBB
Blood brain barrier
- CBF
Cerebral blood flow
- CMRG
Cerebral metabolic rate of glucose
- CMRG
Cerebral metabolic rate of glucose
- COMT
Catechol-O-methyltransferase
- COX
Cyclooxygenase
- CSF
Cerebral spinal fluid
- DA
Dopamine
- DAergic
Dopaminergic
- DAT
Dopamine transporter
- DOPAC
3,4-dihydroxyphenylacetic acid
- DTBZ
[11C]-dihydrotetrabenazine
- ERK
Extracellular signal-regulated kinases
- FAD
Flavin adenine dinucleotide
- FD
[18F]-fluoro-l dopa
- FDG
[18F]-Fluoro-deoxyglucose
- FMN
Flavin mononucleotide
- GABA
Γ-Aminobutyric acid
- GSHPx
Glutathione peroxidase
- GSH
Reduced Glutathione
- H2O2
Hydrogen Peroxide
- HO-1
Heme oxygenase 1
- HVA
Homovanillic acid
- IKK
I kappaB kinase
- IL
Interleukin
- INOS
Inducible NOS
- mRNA
Messenger ribonucleic acid
- mTOR
Mammalian target of rapamycin
- NA
Nucleus accumbens,
- NAC
N acetyl L cysteine
- NAD+
Nicotinamide adenine dinucleotide
- NADH
Nicotinamide adenine dinucleotide reduced
- NADPH
Nicotinamide adenine dinucleotide phosphate reduced
- NF-κB
Nuclear factor-kappa B
- NM
Neuromelanin
- NMDA
N-methyl-D-aspartate
- NNMT
Nicotinamide N-methyl transferase
- NOS
Nitric oxide synthase
- NT
Neurotransmitter
- O2−
Superoxide
- OXPHOS
Oxidative Phosphorylation
- PARP-1
Poly [ADP-ribose] polymerase 1
- PD
Parkinson’s disease
- PDE
Phosphodiesterase
- PDPC
Plant derived polyphenolic compounds
- PET
Positron emission tomography
- PGE2
Prostaglandin E2
- PGH2
Prostaglandin H2 synthase
- PI3K
Phosphoinositide 3 kinase
- PINK-1
PTEN-induced putative kinase 1
- PK
Pyruvate kinase
- PLA2
Phospholipase A2
- PPO
Polyphenol Oxidase
- PY
Pyruvate
- ROS
Reactive Oxygen Species
- Se
Selenium
- SLP
Substrate level phosphorylation
- JNK
c-jun N-terminal kinase
- LC
Locus coeruleus
- LDH
Lactate Dehydrogenase
- l-DOPA
l-3,4-dihydroxyphenylalanine
- LOX
Lipoxygenase
- MAO
Monoamine oxidase
- MAPK
Mitogen-activated protein kinase
- MP
11C-methylphenidate
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- SN
Substantia nigra
- SNc
Substantia nigra pars compacta
- SNCA
α-synuclein
- SOD
Superoxide dismutase
- SPECT
Single photon emission computed tomography
- TH
Tyrosine hydroxylase
- TNF
Tumor necrosis factor
- UPS
Ubiquitin-proteasome system
- VMAT2
Type-2-vesicular monoamine transporter
- Zn
Zinc
References
- 1.Burch D, Sheerin F. Parkinson’s disease. Lancet. 2005;365:622–627. doi: 10.1016/S0140-6736(05)17915-X. [DOI] [PubMed] [Google Scholar]
- 2.Lin TK, Liou CW, Chen SD, Chuang YC, Tiao MM, Wang PW, Chen JB, Chuang JH. Mitochondrial dysfunction and biogenesis in the pathogenesis of Parkinson’s disease. Chang Gung Med. J. 2009;32:589–599. [PubMed] [Google Scholar]
- 3.Dagda RK, Chu CT. Mitochondrial quality control: insights on how Parkinson’s disease related genes PINK1, parkin, and Omi/HtrA2 interact to maintain mitochondrial homeostasis. J. Bioenerg. Biomembr. 2009;41:473–479. doi: 10.1007/s10863-009-9255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishioka K, Vilariño-Güell C, Cobb SA, Kachergus JM, Ross OA, Hentati E, Hentati F, Farrer MJ. Genetic variation of the mitochondrial complex I subunit NDUFV2 and Parkinson’s disease. Parkinsonism Relat. Disord. 2010;10:686–687. doi: 10.1016/j.parkreldis.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy OA, Malagelada C, Greene LA. Cell death pathways in Parkinson’s disease: Proximal triggers, distal effectors, and final steps. Apoptosis. 2009;14:478–500. doi: 10.1007/s10495-008-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagatsu T. Parkinson’s disease: changes in apoptosis-related factors suggesting possible gene therapy. J. Neural. Transm. 2002;109:731–745. doi: 10.1007/s007020200061. [DOI] [PubMed] [Google Scholar]
- 7.Tofaris GK, Spillantini MG. Alpha-synuclein dysfunction in Lewy body diseases. Mov. Disord. 2005;20:S37–S44. doi: 10.1002/mds.20538. [DOI] [PubMed] [Google Scholar]
- 8.Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson’s disease. Biochem. Pharmacol. 2002;64:1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MD, Yu LR, Conrads TP, Kinoshita Y, Uo T, McBee JK, Veenstra TD, Morrison RS. The proteomics of neurodegeneration. Am. J Pharmacogenomics. 2005;5:259–270. doi: 10.2165/00129785-200505040-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hald A, Lotharius J. Oxidative stress and inflammation in Parkinson’s disease: is there a causal link? Exp. Neurol. 2005;193:279–290. doi: 10.1016/j.expneurol.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Sato S, Mizuno Y, Hattori N. Urinary 8-hydroxydeoxyguanosine levels as a biomarker for progression of Parkinson disease. Neurology. 2005;64:1081–1083. doi: 10.1212/01.WNL.0000154597.24838.6B. [DOI] [PubMed] [Google Scholar]
- 12.Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o′-dityrosine in brain tissue of 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson’s disease. J. Biol. Chem. 1999;274:34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A. Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J. Biol. Chem. 2005;280:10444–10454. doi: 10.1074/jbc.M413863200. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HC, Ulane CM, Burke RE. Clinical progression in Parkinson disease and the neurobiology of axons. Ann. Neurol. 2010;67:715–725. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fahn S, Sulzer D. Neurodegeneration and neuroprotection in Parkinson disease. NeuroRx. 2004;1:139–154. doi: 10.1602/neurorx.1.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bertram L, Tanzi RE. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 2005;115:1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyun DH, Lee M, Halliwell B, Jenner P. Effect of overexpression of wild-type or mutant parkin on the cellular response induced by toxic insults. J. Neurosci. Res. 2005;82:232–244. doi: 10.1002/jnr.20638. [DOI] [PubMed] [Google Scholar]
- 18.Mortiboys H, Johansen KK, Aasly JO, Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- 19.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008;4:600–609. doi: 10.1038/ncpneuro0924. [DOI] [PubMed] [Google Scholar]
- 20.Arvanitakis Z, Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and progression of rigidity and gait disturbance in older persons. Neurology. 2004;63:996–1001. doi: 10.1212/01.wnl.0000138432.16676.4b. [DOI] [PubMed] [Google Scholar]
- 21.Klein RC, de Jong BM, de Vries JJ, Leenders KL. Direct comparison between regional cerebral metabolism in progressive supranuclear palsy and Parkinson’s disease. Mov. Disord. 2005;20:1021–1030. doi: 10.1002/mds.20493. [DOI] [PubMed] [Google Scholar]
- 22.Lesage S, Brice A. Parkinson’s disease: from monogenic forms to genetic susceptibility factors. Hum. Mol. Genet. 2009;18:R48–R59. doi: 10.1093/hmg/ddp012. [DOI] [PubMed] [Google Scholar]
- 23.Tanner CM, Ross GW, Jewell SA, Hauser RA, Jankovic J, Factor SA, Bressman S, Deligtisch A, Marras C, Lyons KE, Bhudhikanok GS, Roucoux DF, Meng C, Abbott RD, Langston JW. Occupation and risk of parkinsonism: a multicenter case-control study. Arch. Neurol. 2009;66:1106–1113. doi: 10.1001/archneurol.2009.195. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen N, Pradel V, Micallef J, Montastruc JL, Blin O. Drug-induced Parkinson syndromes. Therapie. 2004;59:105–112. doi: 10.2515/therapie:2004021. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal J, Chakraborty DP, Sarkar B, Banerjee TK, Mukherjee SC, Ray BC, Rao VR. Environmental and familial risk factors of Parkinsons disease: case-control study. Can. J. Neurol. Sci. 2010;37:637–642. doi: 10.1017/s0317167100010829. [DOI] [PubMed] [Google Scholar]
- 26.Allam MF, Del Castillo AS, Navajas RF. Parkinson’s disease risk factors: genetic, environmental, or both? Neurol. Res. 2005;27:206–208. doi: 10.1179/016164105X22057. [DOI] [PubMed] [Google Scholar]
- 27.Logroscino G. The role of early life environmental risk factors in Parkinson disease: what is the evidence? Environ. Health Perspect. 2005;113:1234–1238. doi: 10.1289/ehp.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pavese N, Brooks DJ. Imaging neurodegeneration in Parkinson’s disease. Biochim. Biophys Acta. 2009;1792:722–729. doi: 10.1016/j.bbadis.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Thobois S, Guillouet S, Broussolle E. Contributions of PET and SPECT to the understanding of the pathophysiology of Parkinson’s disease. Neurophysiol. Clin. 2001;31:321–340. doi: 10.1016/s0987-7053(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 30.Au WL, Adams JR, Troiano AR, Stoessl AJ. Parkinson’s disease: in vivo assessment of disease progression using positron emission tomography. Brain Res. Mol. Brain Res. 2005;134:24–33. doi: 10.1016/j.molbrainres.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Galvan A, Wichmann T. Pathophysiology of parkinsonism. Clin. Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pal PK, Netravathi M. Management of neurodegenerative disorders: Parkinson’s disease and Alzheimer’s disease. J. Indian Med. Assoc. 2005;103:168–170. [PubMed] [Google Scholar]
- 33.Samai M, Sharpe MA, Gard PR, Chatterjee PK. Comparison of the effects of the superoxide dismutase mimetics EUK-134 and tempol on paraquat-induced nephrotoxicity. Free Radic. Biol. Med. 2007;43:528–534. doi: 10.1016/j.freeradbiomed.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Weinreb O, Amit T, Bar-Am O, Youdim MB. Rasagiline: a novel anti-Parkinsonian monoamine oxidase-B inhibitor with neuroprotective activity. Prog. Neurobiol. 2010;92:330–344. doi: 10.1016/j.pneurobio.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Lew MF, Hauser RA, Hurtig HI, Ondo WG, Wojcieszek J, Goren T, Fitzer-Attas CJ. Long-term efficacy of rasagiline in early Parkinson’s disease. Int. J. Neurosci. 2010;120:404–408. doi: 10.3109/00207451003778744. [DOI] [PubMed] [Google Scholar]
- 36.Weinreb O, Amit T, Bar-Am O, Chillag-Talmor O, Youdim MB. Novel neuroprotective mechanism of action of rasagiline is associated with its propargyl moiety: interaction of Bcl-2 family members with PKC pathway. Ann. N. Y. Acad. Sci. 2005;1053:348–355. doi: 10.1196/annals.1344.030. [DOI] [PubMed] [Google Scholar]
- 37.Zheng H, Gal S, Weiner LM, Bar-Am O, Warshawsky A, Fridkin M, Youdim MB. Novel multifunctional neuroprotective iron chelator-monoamine oxidase inhibitor drugs for neurodegenerative diseases: in vitro studies on antioxidant activity, prevention of lipid peroxide formation and monoamine oxidase inhibition. J. Neurochem. 2005;95:68–78. doi: 10.1111/j.1471-4159.2005.03340.x. [DOI] [PubMed] [Google Scholar]
- 38.Youdim MB, Fridkin M, Zheng H. Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases. Mech. Ageing Dev. 2005;126:317–326. doi: 10.1016/j.mad.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 39.Mandel SA, Avramovich-Tirosh Y, Reznichenko L, Zheng H, Weinreb O, Amit T, Youdim MB. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- 40.Mandel S, Maor G, Youdim MB. Iron and alpha-synuclein in the substantia nigra of MPTP-treated mice: effect of neuroprotective drugs R-apomorphine and green tea polyphenol (−)-epigallocatechin-3-gallate. J. Mol. Neurosci. 2004;24:401–416. doi: 10.1385/JMN:24:3:401. [DOI] [PubMed] [Google Scholar]
- 41.Cleren C, Calingasan NY, Chen J, Beal MF. Celastrol protects against MPTP- and 3-nitropropionic acid-induced neurotoxicity. J. Neurochem. 2005;94:995–1004. doi: 10.1111/j.1471-4159.2005.03253.x. [DOI] [PubMed] [Google Scholar]
- 42.Klivenyi P, Andreassen OA, Ferrante RJ, Lancelot E, Reif D, Beal MF. Inhibition of neuronal nitric oxide synthase protects against MPTP toxicity. Neuroreport. 2000;11:1265–1268. doi: 10.1097/00001756-200004270-00024. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe H, Muramatsu Y, Kurosaki R, Michimata M, Matsubara M, Imai Y, Araki T. Protective effects of neuronal nitric oxide synthase inhibitor in mouse brain against MPTP neurotoxicity: an immunohistological study. Eur. Neuropsychopharmacol. 2004;14:93–104. doi: 10.1016/S0924-977X(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 44.Wang W, Shi L, Xie Y, Ma C, Li W, Su X, Huang S, Chen R, Zhu Z, Mao Z, Han Y, Li M. SP600125, a new JNK inhibitor, protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Neurosci. Res. 2004;48:195–202. doi: 10.1016/j.neures.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 45.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuan CY, Burke RE. Targeting the JNK signaling pathway for stroke and Parkinson’s diseases therapy. Curr. Drug Targets CNS Neurol. Disord. 2005;4:63–67. doi: 10.2174/1568007053005145. [DOI] [PubMed] [Google Scholar]
- 47.Silva RM, Kuan CY, Rakic P, Burke RE. Mixed lineage kinase-c-jun N-terminal kinase signaling pathway: a new therapeutic target in Parkinson’s disease. Mov. Disord. 2005;20:653–664. doi: 10.1002/mds.20390. [DOI] [PubMed] [Google Scholar]
- 48.Testa CM, Sherer TB, Greenamyre JT. Rotenone induces oxidative stress and dopaminergic neuron damage in organotypic substantia nigra cultures. Brain Res. Mol. Brain Res. 2005;134:109–118. doi: 10.1016/j.molbrainres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Virmani A, Gaetani F, Binienda Z. Effects of metabolic modifiers such as carnitines, coenzyme Q10, and PUFAs against different forms of neurotoxic insults: metabolic inhibitors, MPTP, and methamphetamine. Ann. N. Y. Acad. Sci. 2005;1053:183–191. doi: 10.1196/annals.1344.016. [DOI] [PubMed] [Google Scholar]
- 50.Bhat V, Weiner WJ. Parkinson’s disease. Diagnosis and the initiation of therapy. Minerva Med. 2005;96:145–154. [PubMed] [Google Scholar]
- 51.Shults CW. Therapeutic role of coenzyme Q(10) in Parkinson’s disease. Pharmacol. Ther. 2005;107:120–130. doi: 10.1016/j.pharmthera.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Etminan M, Gill SS, Samii A. Intake of vitamin E, vitamin C, and carotenoids and the risk of Parkinson’s disease: a meta-analysis. Lancet Neurol. 2005;4:362–365. doi: 10.1016/S1474-4422(05)70097-1. [DOI] [PubMed] [Google Scholar]
- 53.Andres RH, Huber AW, Schlattner U, Pérez-Bouza A, Krebs SH, Seiler RW, Wallimann T, Widmer HR. Effects of creatine treatment on the survival of dopaminergic neurons in cultured fetal ventral mesencephalic tissue. Neuroscience. 2005;133:701–713. doi: 10.1016/j.neuroscience.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 54.Klivenyi P, Gardian G, Calingasan NY, Yang L, Beal MF. Additive neuroprotective effects of creatine and a cyclooxygenase 2 inhibitor against dopamine depletion in the 1-methyl- 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. J. Mol. Neurosci. 2003;21:191–198. doi: 10.1385/jmn:21:3:191. [DOI] [PubMed] [Google Scholar]
- 55.Borah A, Mohanakumar KP. Melatonin inhibits 6-hydroxydopamine production in the brain to protect against experimental Parkinsonism in rodents. J. Pineal Res. 2009;47:293–300. doi: 10.1111/j.1600-079X.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 56.Bi J, Wang XB, Chen L, Hao S, An LJ, Jiang B, Guo L. Catalpol protects mesencephalic neurons against MPTP induced neurotoxicity via attenuation of mitochondrial dysfunction and MAO-B activity. Toxicol In Vitro. 2008;22:1883–1889. doi: 10.1016/j.tiv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 57.Bahat-Stroomza M, Gilgun-Sherki Y, Offen D, Panet H, Saada A, Krool-Galron N, Barzilai A, Atlas D, Melamed E. A novel thiol antioxidant that crosses the blood brain barrier protects dopaminergic neurons in experimental models of Parkinson’s disease. Eur. J. Neurosci. 2005;21:637–646. doi: 10.1111/j.1460-9568.2005.03889.x. [DOI] [PubMed] [Google Scholar]
- 58.Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. Therapeutic potential of neurotrophic factors in neurodegenerative diseases. BioDrugs. 2005;19:97–127. doi: 10.2165/00063030-200519020-00003. [DOI] [PubMed] [Google Scholar]
- 59.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005;102:216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 60.D’Astous M, Morissette M, Tanguay B, Callier S, Di Paolo T. Dehydroepiandrosterone (DHEA) such as 17beta-estradiol prevents MPTP-induced dopamine depletion in mice. Synapse. 2003;47:10–14. doi: 10.1002/syn.10145. [DOI] [PubMed] [Google Scholar]
- 61.D’Astous M, Morissette M, Di Paolo T. Effect of estrogen receptor agonists treatment in MPTP mice: evidence of neuroprotection by an ER alpha agonist. Neuropharmacology. 2004;47:1180–1188. doi: 10.1016/j.neuropharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 62.Morelli M, Carta AR, Kachroo A, Schwarzschild MA. Pathophysiological roles for purines: adenosine, caffeine and urate. Prog. Brain Res. 2010;183:183–208. doi: 10.1016/S0079-6123(10)83010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikeda K, Kurokawa M, Aoyama S, Kuwana Y. Neuroprotection by adenosine A2A receptor blockade in experimental models of Parkinson’s disease. J. Neurochem. 2002;80:262–270. doi: 10.1046/j.0022-3042.2001.00694.x. [DOI] [PubMed] [Google Scholar]
- 64.Xu K, Bastia E, Schwarzschild M. Therapeutic potential of adenosine A2A receptor antagonists in Parkinson’s disease. Pharmacol. Ther. 2005;105:267–310. doi: 10.1016/j.pharmthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Azam F, Ibn-Rajab IA, Alruiad AA. Adenosine A2A receptor antagonists as novel anti- Parkinsonian agents: a review of structure-activity relationships. Pharmazie. 2009;64:771–795. [PubMed] [Google Scholar]
- 66.García E, Villeda-Hernández J, Pedraza-Chaverrí J, Maldonado PD, Santamaría A. S-allylcysteine reduces the MPTP-induced striatal cell damage via inhibition of pro-inflammatory cytokine tumor necrosis factor-α and inducible nitric oxide synthase expressions in mice. Phytomedicine. 2010;18:65–73. doi: 10.1016/j.phymed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 67.Battaglia G, Busceti CL, Pontarelli F, Biagioni F, Fornai F, Paparelli A, Bruno V, Ruggieri S, Nicoletti F. Protective role of group-II metabotropic glutamate receptors against nigro-striatal degeneration induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Neuropharmacology. 2003;45:155–166. doi: 10.1016/s0028-3908(03)00146-1. [DOI] [PubMed] [Google Scholar]
- 68.Kim YK, Lim HH, Song YK, Lee HH, Lim S, Han SM, Kim CJ. Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci. Lett. 2005;384:133–138. doi: 10.1016/j.neulet.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 69.Li XM, Ma HB, Ma ZQ, Li LF, Xu CL, Qu R, Ma SP. Ameliorative and neuroprotective effect in MPTP model of Parkinson’s disease by Zhen-Wu-Tang (ZWT), a traditional Chinese medicine. J. Ethnopharmacol. 2010;130:19–27. doi: 10.1016/j.jep.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 70.Kurosaki R, Muramatsu Y, Kato H, Watanabe Y, Imai Y, Itoyama Y, Araki T. Effect of angiotensin-converting enzyme inhibitor perindopril on interneurons in MPTP-treated mice. Eur. Neuropsychopharmacol. 2005;15:57–67. doi: 10.1016/j.euroneuro.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 71.Singh N, Pillay V, Choonara YE. Advances in the treatment of Parkinson’s disease. Prog. Neurobiol. 2007;81:29–44. doi: 10.1016/j.pneurobio.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 72.Kotake Y, Ohta S. MPP+ analogs acting on mitochondria and inducing neuro-degeneration. Curr. Med. Chem. 2003;10:2507–2516. doi: 10.2174/0929867033456558. [DOI] [PubMed] [Google Scholar]
- 73.Nagatsu T. Isoquinoline neurotoxins in the brain and Parkinson’s disease. Neurosci. Res. 1997;29:99–111. doi: 10.1016/s0168-0102(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 74.Mazzio EA, Soliman KF. Effects of enhancing mitochondrial oxidative phosphorylation with reducing equivalents and ubiquinone on 1-methyl-4-phenylpyridinium toxicity and complex I-IV damage in neuroblastoma cells. Biochem. Pharmacol. 2004;67:1167–1184. doi: 10.1016/j.bcp.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 75.Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol. Sci. 1999;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- 76.Del Zompo M, Piccardi MP, Ruiu S, Quartu M, Gessa GL, Vaccari A. Selective MPP+ uptake into synaptic dopamine vesicles: possible involvement in MPTP neurotoxicity. Br. J. Pharmacol. 1993;109:411–414. doi: 10.1111/j.1476-5381.1993.tb13584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazzio E, Soliman KF. d-(+)-glucose rescue against 1-methyl-4-phenylpyridinium toxicity through anaerobic glycolysis in neuroblastoma cells. Brain Res. 2003;962:48–60. doi: 10.1016/s0006-8993(02)03695-8. [DOI] [PubMed] [Google Scholar]
- 78.Palacios JM, Wiederhold KH. Acute administration of 1-N-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP), a compound producing parkinsonism in humans, stimulates [2–14C]deoxyglucose uptake in the regions of the catecholaminergic cell bodies in the rat and guinea pig brains. Brain Res. 1984;301:187–191. doi: 10.1016/0006-8993(84)90422-0. [DOI] [PubMed] [Google Scholar]
- 79.Palombo E, Porrino LJ, Bankiewicz KS, Crane AM, Kopin IJ, Sokoloff L. Administration of MPTP acutely increases glucose utilization in the substantia nigra of primates. Brain Res. 1988;453:227–234. doi: 10.1016/0006-8993(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 80.Schwartzman RJ, Alexander GM. Changes in the local cerebral metabolic rate for glucose in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) primate model of Parkinson’s disease. Brain Res. 1985;358:137–143. doi: 10.1016/0006-8993(85)90957-6. [DOI] [PubMed] [Google Scholar]
- 81.Schwartzman RJ, Alexander GM, Ferraro TN, Grothusen JR, Stahl SM. Cerebral metabolism of parkinsonian primates 21 days after MPTP. Exp Neurol. 1988;102:307–313. doi: 10.1016/0014-4886(88)90224-5. [DOI] [PubMed] [Google Scholar]
- 82.Lagrue E, Abert B, Nadal L, Tabone L, Bodard S, Medja F, Lombes A, Chalon S, Castelnau P. MPTP intoxication in mice: a useful model of Leigh syndrome to study mitochondrial diseases in childhood. Metab. Brain Dis. 2009;24:321–335. doi: 10.1007/s11011-009-9132-y. [DOI] [PubMed] [Google Scholar]
- 83.Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Chan P, DeLanney LE, Irwin I, Langston JW, Di Monte D. MPTP-induced ATP loss in mouse brain. Ann. N. Y. Acad. Sci. 1992;648:306–308. doi: 10.1111/j.1749-6632.1992.tb24564.x. [DOI] [PubMed] [Google Scholar]
- 85.Koga K, Mori A, Ohashi S, Kurihara N, Kitagawa H, Ishikawa M, Mitsumoto Y, Nakai M. 1H MRS identifies lactate rise in the striatum of MPTP-treated C57BL/6 mice. Eur. J. Neurosci. 2006;23:1077–1081. doi: 10.1111/j.1460-9568.2006.04610.x. [DOI] [PubMed] [Google Scholar]
- 86.Brownell AL, Jenkins BG, Elmaleh DR, Deacon TW, Spealman RD, Isacson O. Combined PET/MRS brain studies show dynamic and long-term physiological changes in a primate model of Parkinson disease. Nat. Med. 1998;4:1308–1312. doi: 10.1038/3300. [DOI] [PubMed] [Google Scholar]
- 87.Pastoris O, Dossena M, Foppa P, Catapano M, Ferrari R, Dagani F. Biochemical evaluations in skeletal muscles of primates with MPTP Parkinson-like syndrome. Pharmacol. Res. 1995;31:361–369. doi: 10.1016/1043-6618(95)80090-5. [DOI] [PubMed] [Google Scholar]
- 88.Singh Y, Swanson E, Sokoloski E, Kutty RK, Krishna G. MPTP and MPTP analogs induced cell death in cultured rat hepatocytes involving the formation of pyridinium metabolites. Toxicol. Appl. Pharmacol. 1988;96:347–359. doi: 10.1016/0041-008x(88)90093-2. [DOI] [PubMed] [Google Scholar]
- 89.Singer TP, Ramsay RR, McKeown K, Trevor A, Castagnoli NE., Jr Mechanism of the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+) the toxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicology. 1988;49:17–23. doi: 10.1016/0300-483x(88)90169-2. [DOI] [PubMed] [Google Scholar]
- 90.Scotcher KP, Irwin I, DeLanney LE, Langston JW, Di Monte D. Effects of 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on ATP levels of mouse brain synaptosomes. J. Neurochem. 1990;54:1295–1301. doi: 10.1111/j.1471-4159.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 91.Di Monte DA, Wu EY, Delanney LE, Irwin I, Langston JW. Toxicity of 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine in primary cultures of mouse astrocytes. J. Pharmacol. Exp. Ther. 1992;261:44–49. [PubMed] [Google Scholar]
- 92.Mazzio EA, Soliman YI, Soliman KF. Variable toxicological response to the loss of OXPHOS through 1-methyl-4-phenylpyridinium-induced mitochondrial damage and anoxia in diverse neural immortal cell lines. Cell Biol. Toxicol. 2010;26:527–539. doi: 10.1007/s10565-010-9161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maruoka N, Murata T, Omata N, Takashima Y, Fujibayashi Y, Wada Y. Topological and chronological features of the impairment of glucose metabolism induced by 1-methyl-4-phenylpyridinium ion (MPP+) in rat brain slices. J. Neural. Transm. 2007;114:1155–1159. doi: 10.1007/s00702-007-0720-x. [DOI] [PubMed] [Google Scholar]
- 94.Clausen T, Khaldi A, Zauner A, Reinert M, Doppenberg E, Menzel M, Soukup J, Alves OL, Bullock MR. Cerebral acid-base homeostasis after severe traumatic brain injury. J. Neurosurg. 2005;103:597–607. doi: 10.3171/jns.2005.103.4.0597. [DOI] [PubMed] [Google Scholar]
- 95.Woo CW, Lee BS, Kim ST, Kim KS. Correlation between lactate and neuronal cell damage in the rat brain after focal ischemia: An in vivo 1H magnetic resonance spectroscopic (1H-MRS) study. Acta Radiol. 2010;51:344–350. doi: 10.3109/02841850903515395. [DOI] [PubMed] [Google Scholar]
- 96.Chow SL, Rooney ZJ, Cleary MA, Clayton PT, Leonard JV. The significance of elevated CSF lactate. Arch. Dis. Child. 2005;90:1188–1189. doi: 10.1136/adc.2005.075317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Makoroff KL, Cecil KM, Care M, Ball WS., Jr Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr. Radiol. 2005;35:668–676. doi: 10.1007/s00247-005-1441-7. [DOI] [PubMed] [Google Scholar]
- 98.Cavus I, Kasoff WS, Cassaday MP, Jacob R, Gueorguieva R, Sherwin RS, Krystal JH, Spencer DD, Abi-Saab WM. Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann. Neurol. 2005;57:226–235. doi: 10.1002/ana.20380. [DOI] [PubMed] [Google Scholar]
- 99.Brooks DJ. Imaging approaches to Parkinson disease. J. Nucl. Med. 2010;51:596–609. doi: 10.2967/jnumed.108.059998. [DOI] [PubMed] [Google Scholar]
- 100.Noda A, Ohba H, Kakiuchi T, Futatsubashi M, Tsukada H, Nishimura S. Age-related changes in cerebral blood flow and glucose metabolism in conscious rhesus monkeys. Brain Res. 2002;936:76–81. doi: 10.1016/s0006-8993(02)02558-1. [DOI] [PubMed] [Google Scholar]
- 101.Cunnane S, Nugent S, Roy M, Courchesne-Loyer A, Croteau E, Tremblay S, Castellano A, Pifferi F, Bocti C, Paquet N, Begdouri H, Bentourkia M, Turcotte E, Allard M, Barberger-Gateau P, Fulop T, Rapoport SI. Brain fuel metabolism, aging, and Alzheimer’s disease. Nutrition. 2011;27:3–20. doi: 10.1016/j.nut.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2009;219:334–340. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Halestrap AP. A pore way to die: the role of mitochondria in reperfusion injury and cardioprotection. Biochem. Soc. Trans. 2010;38:841–860. doi: 10.1042/BST0380841. [DOI] [PubMed] [Google Scholar]
- 104.Bisaglia M, Soriano ME, Arduini I, Mammi S, Bubacco L. Molecular characterization of dopamine-derived quinones reactivity toward NADH and glutathione: implications for mitochondrial dysfunction in Parkinson disease. Biochim. Biophys Acta. 2010;1802:699–706. doi: 10.1016/j.bbadis.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 105.Liou AK, Zhou Z, Pei W, Lim TM, Yin XM, Chen J. BimEL up-regulation potentiates AIF translocation and cell death in response to MPTP. FASEB J. 2005;19:1350–1352. doi: 10.1096/fj.04-3258fje. [DOI] [PubMed] [Google Scholar]
- 106.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 107.Bo J, Ming BY, Gang LZ, Lei C, Jia AL. Protection by puerarin against MPP+-induced neurotoxicity in PC12 cells mediated by inhibiting mitochondrial dysfunction and caspase-3-like activation. Neurosci. Res. 2005;53:183–188. doi: 10.1016/j.neures.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 108.Cappelletti G, Surrey T, Maci R. The Parkinsonism producing neurotoxin MPP+ affects microtubule dynamics by acting as a destabilising factor. FEBS Lett. 2005;579:4781–4786. doi: 10.1016/j.febslet.2005.07.058. [DOI] [PubMed] [Google Scholar]
- 109.Thomas B, Beal MF. Parkinson’s disease. Hum. Mol. Genet. 2007;16:R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- 110.Rollema H, de Vries JB, Damsma G, Westerink BH, Kranenborg GL, Kuhr WG, Horn AS. The use of in vivo brain dialysis of dopamine, acetylcholine, amino acids and lactic acid in studies on the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicology. 1988;49:503–511. doi: 10.1016/0300-483x(88)90036-4. [DOI] [PubMed] [Google Scholar]
- 111.Ofori S, Heikkila RE, Nicklas WJ. Attenuation by dopamine uptake blockers of the inhibitory effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and some of its analogs on NADH-linked metabolism in mouse neostriatal slices. J. Pharmacol. Exp. Ther. 1989;251:258–266. [PubMed] [Google Scholar]
- 112.Guillot TS, Miller GW. Protective actions of the vesicular monoamine transporter 2 (VMAT2) in monoaminergic neurons. Mol. Neurobiol. 2009;39:149–170. doi: 10.1007/s12035-009-8059-y. [DOI] [PubMed] [Google Scholar]
- 113.Tanaka R, Asaga H, Takeda M. Nucleoside triphosphate and cation requirement for dopamine uptake by plain synaptic vesicles isolated from rat cerebrums. Brain Res. 1976;115:273–283. doi: 10.1016/0006-8993(76)90512-6. [DOI] [PubMed] [Google Scholar]
- 114.Hossain MM, Filipov NM. Alteration of dopamine uptake into rat striatal vesicles and synaptosomes caused by an in vitro exposure to atrazine and some of its metabolites. Toxicology. 2008;248:52–58. doi: 10.1016/j.tox.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Choi HJ, Lee SY, Cho Y, Hwang O. Inhibition of vesicular monoamine transporter enhances vulnerability of dopaminergic cells: relevance to Parkinson’s disease. Neurochem. Int. 2005;46:329–335. doi: 10.1016/j.neuint.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 116.Ren Y, Liu W, Jiang H, Jiang Q, Feng J. Selective vulnerability of dopaminergic neurons to microtubule depolymerization. J. Biol. Chem. 2005;280:34105–34112. doi: 10.1074/jbc.M503483200. [DOI] [PubMed] [Google Scholar]
- 117.Chang GD, Ramirez VD. The mechanism of action of MPTP and MPP+ on endogenous dopamine release from the rat corpus striatum superfused in vitro. Brain Res. 1986;368:134–140. doi: 10.1016/0006-8993(86)91050-4. [DOI] [PubMed] [Google Scholar]
- 118.Kurosaki R, Muramatsu Y, Watanabe H, Michimata M, Matsubara M, Imai Y, Araki T. Role of dopamine transporter against MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in mice. Metab. Brain Dis. 2003;18:139–146. doi: 10.1023/a:1023863003093. [DOI] [PubMed] [Google Scholar]
- 119.Jourdain S, Morissette M, Morin N, Di Paolo T. Oestrogens prevent loss of dopamine transporter (DAT) and vesicular monoamine transporter (VMAT2) in substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice. J. Neuroendocrinol. 2005;17:509–517. doi: 10.1111/j.1365-2826.2005.01337.x. [DOI] [PubMed] [Google Scholar]
- 120.Hogan KA, Staal RG, Sonsalla PK. Analysis of VMAT2 binding after methamphetamine or MPTP treatment: disparity between homogenates and vesicle preparations. J. Neurochem. 2000;74:2217–2220. doi: 10.1046/j.1471-4159.2000.0742217.x. [DOI] [PubMed] [Google Scholar]
- 121.Harrington KA, Augood SJ, Kingsbury AE, Foster OJ, Emson PC. Dopamine transporter (Dat) and synaptic vesicle amine transporter (VMAT2) gene expression in the substantia nigra of control and Parkinson’s disease. Brain Res. Mol. Brain Res. 1996;36:157–162. doi: 10.1016/0169-328x(95)00278-z. [DOI] [PubMed] [Google Scholar]
- 122.Frey KA, Koeppe RA, Kilbourn MR, van der Borght TM, Albin RL, Gilman S, Kuhl DE. Presynaptic monoaminergic vesicles in Parkinson’s disease and normal aging. Ann. Neurol. 1996;40:873–884. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- 123.Reinhard JF, Jr, Carmichael SW, Daniels AJ. Mechanisms of toxicity and cellular resistance to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium in adrenomedullary chromaffin cell cultures. J. Neurochem. 1990;55:311–320. doi: 10.1111/j.1471-4159.1990.tb08853.x. [DOI] [PubMed] [Google Scholar]
- 124.Wimalasena DS, Perera RP, Heyen BJ, Balasooriya IS, Wimalasena K. Vesicular monoamine transporter substrate/inhibitor activity of MPTP/MPP+ derivatives: A structure-activity study. J. Med. Chem. 2008;51:760–768. doi: 10.1021/jm070875p. [DOI] [PubMed] [Google Scholar]
- 125.Przedborski S. Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat. Disord. 2005;11:S3–S7. doi: 10.1016/j.parkreldis.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 126.Sala G, Brighina L, Saracchi E, Fermi S, Riva C, Carrozza V, Pirovano M, Ferrarese C. Vesicular monoamine transporter 2 mRNA levels are reduced in platelets from patients with Parkinson’s disease. J. Neural. Transm. 2010;117:1093–1098. doi: 10.1007/s00702-010-0446-z. [DOI] [PubMed] [Google Scholar]
- 127.Serra PA, Sciola L, Delogu MR, Spano A, Monaco G, Miele E, Rocchitta G, Miele M, Migheli R, Desole MS. The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine induces apoptosis in mouse nigrostriatal glia. Relevance to nigral neuronal death and striatal neurochemical changes. J. Biol. Chem. 2002;277:34451–34461. doi: 10.1074/jbc.M202099200. [DOI] [PubMed] [Google Scholar]
- 128.Asanuma M, Miyazaki I, Diaz-Corrales FJ, Ogawa N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic Parkinson’s disease and neurotoxin-induced parkinsonism. Acta Med Okayama. 2004;58:221–233. doi: 10.18926/AMO/32105. [DOI] [PubMed] [Google Scholar]
- 129.Antunes F, Nunes C, Laranjinha J, Cadenas E. Redox interactions of nitric oxide with dopamine and its derivatives. Toxicology. 2005;208:207–212. doi: 10.1016/j.tox.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 130.Li HT, Lin DH, Luo XY, Zhang F, Ji LN, Du HN, Song GQ, Hu J, Zhou JW, Hu HY. Inhibition of alpha-synuclein fibrillization by dopamine analogs via reaction with the amino groups of alpha-synuclein. Implication for dopaminergic neurodegeneration. FEBS J. 2005;272:3661–3672. doi: 10.1111/j.1742-4658.2005.04792.x. [DOI] [PubMed] [Google Scholar]
- 131.Akagawa M, Ishii Y, Ishii T, Shibata T, Yotsu-Yamashita M, Suyama K, Uchida K. Metal-catalyzed oxidation of protein-bound dopamine. Biochemistry. 2006;45:15120–15128. doi: 10.1021/bi0614434. [DOI] [PubMed] [Google Scholar]
- 132.Smythies J, Galzigna L. The oxidative metabolism of catecholamines in the brain: a review. Biochim. Biophys Acta. 1998;1380:159–162. doi: 10.1016/s0304-4165(97)00131-1. [DOI] [PubMed] [Google Scholar]
- 133.Zecca L, Fariello R, Riederer P, Sulzer D, Gatti A, Tampellini D. The absolute concentration of nigral neuromelanin, assayed by a new sensitive method, increases throughout the life and is dramatically decreased in Parkinson’s disease. FEBS Lett. 2002;510:216–220. doi: 10.1016/s0014-5793(01)03269-0. [DOI] [PubMed] [Google Scholar]
- 134.Khan FH, Sen T, Maiti AK, Jana S, Chatterjee U, Chakrabarti S. Inhibition of rat brain mitochondrial electron transport chain activity by dopamine oxidation products during extended in vitro incubation: implications for Parkinson’s disease. Biochim. Biophys Acta. 2005;1741:65–74. doi: 10.1016/j.bbadis.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 135.Halliday GM, Ophof A, Broe M, Jensen PH, Kettle E, Fedorow H, Cartwright MI, Griffiths FM, Shepherd CE, Double KL. Alpha-synuclein redistributes to neuromelanin lipid in the substantia nigra early in Parkinson’s disease. Brain. 2005;28:2654–2664. doi: 10.1093/brain/awh584. [DOI] [PubMed] [Google Scholar]
- 136.García-Molina F, Fenoll LG, Morote JC, García-Ruiz PA, Rodríguez-López JN, García-Cánovas F, Tudela J. Opposite effects of peroxidase in the initial stages of tyrosinase-catalysed melanin biosynthesis. Int. J. Biochem. Cell Biol. 2005;37:1179–1196. doi: 10.1016/j.biocel.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 137.Alexi T, Borlongan CV, Faull RL, Williams CE, Clark RG, Gluckman PD, Hughes PE. Neuroprotective strategies for basal ganglia degeneration: Parkinson’s and Huntington’s diseases. Prog. Neurobiol. 2000;60:409–740. doi: 10.1016/s0301-0082(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 138.Double KL, Ben-Shachar D, Youdim MB, Zecca L, Riederer P, Gerlach M. Influence of neuromelanin on oxidative pathways within the human substantia nigra. Neurotoxicol. Teratol. 2002;24:621–628. doi: 10.1016/s0892-0362(02)00218-0. [DOI] [PubMed] [Google Scholar]
- 139.Gentile V, Cooper AJ. Transglutaminases—possible drug targets in human diseases. Curr. Drug Targets CNS Neurol. Disord. 2004;3:99–104. doi: 10.2174/1568007043482552. [DOI] [PubMed] [Google Scholar]
- 140.Caccamo D, Currò M, Condello S, Ferlazzo N, Ientile R. Critical role of transglutaminase and other stress proteins during neurodegenerative processes. Amino Acids. 2010;38:653–658. doi: 10.1007/s00726-009-0428-3. [DOI] [PubMed] [Google Scholar]
- 141.Klivenyi P, Beal MF, Ferrante RJ, Andreassen OA, Wermer M, Chin MR, Bonventre JV. Mice deficient in group IV cytosolic phospholipase A2 are resistant to MPTP neurotoxicity. J. Neurochem. 1998;71:2634–2637. doi: 10.1046/j.1471-4159.1998.71062634.x. [DOI] [PubMed] [Google Scholar]
- 142.Feng ZH, Wang TG, Li DD, Fung P, Wilson BC, Liu B, Ali SF, Langenbach R, Hong JS. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1,2,3,6- tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci. Lett. 2002;329:354–358. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- 143.Zhang J, Graham DG, Montine TJ, Ho YS. Enhanced N-methyl-4-phenyl-1,2,3,6- tetrahydropyridine toxicity in mice deficient in CuZn-superoxide dismutase or glutathione peroxidase. J. Neuropathol. Exp. Neurol. 2000;59:53–61. doi: 10.1093/jnen/59.1.53. [DOI] [PubMed] [Google Scholar]
- 144.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 145.Mohanakumar KP, Muralikrishnan D, Thomas B. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Brain Res. 2000;864:281–290. doi: 10.1016/s0006-8993(00)02189-2. [DOI] [PubMed] [Google Scholar]
- 146.Gupta A, Dhir A, Kumar A, Kulkarni SK. Effect of preferential cyclooxygenase-2 (COX-2) inhibitor against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced striatal lesions in rats: behavioral, biochemical and histological evidences. Indian J. Exp. Biol. 2010;48:577–585. [PubMed] [Google Scholar]
- 147.Teismann P, Ferger B. Inhibition of the cyclooxygenase isoenzymes COX-1 and COX-2 provide neuroprotection in the MPTP-mouse model of Parkinson’s disease. Synapse. 2001;39:167–174. doi: 10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 148.Tariq M, Khan HA, Al Moutaery K, Al Deeb S. Protective effect of quinacrine on striatal dopamine levels in 6-OHDA and MPTP models of Parkinsonism in rodents. Brain Res Bullutin. 2001;54:77–82. doi: 10.1016/s0361-9230(00)00427-5. [DOI] [PubMed] [Google Scholar]
- 149.Nishino Y, Ando M, Makino R, Ueda K, Okamoto Y, Kojima N. Different mechanisms between copper and iron in catecholamines-mediated oxidative DNA damage and disruption of gene expression in vitro. Neurotox Res. 2010 doi: 10.1007/s12640-010-9226-7. in press. [DOI] [PubMed] [Google Scholar]
- 150.Sayre LM, Perry G, Smith MA. Redox metals and neurodegenerative disease. Curr. Opin. Chem. Biol. 1999;3:220–225. doi: 10.1016/S1367-5931(99)80035-0. [DOI] [PubMed] [Google Scholar]
- 151.Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA., dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog. Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 152.Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog. Neurobiol. 1996;48:1–19. doi: 10.1016/0301-0082(95)00029-1. [DOI] [PubMed] [Google Scholar]
- 153.Annepu J, Ravindranath V. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced complex I inhibition is reversed by disulfide reductant, dithiothreitol in mouse brain. Neurosci. Lett. 2000;289:209–212. doi: 10.1016/s0304-3940(00)01300-8. [DOI] [PubMed] [Google Scholar]
- 154.Asanuma M, Miyazaki I, Diaz-Corrales FJ, Ogawa N. Quinone formation as dopaminergic neuron-specific oxidative stress in the pathogenesis of sporadic Parkinson’s disease and neurotoxin-induced parkinsonism. Acta Med Okayama. 2004;58:221–233. doi: 10.18926/AMO/32105. [DOI] [PubMed] [Google Scholar]
- 155.Hare DJ, George JL, Grimm R, Wilkins S, Adlard PA, Cherny RA, Bush AI, Finkelstein DI, Doble P. Three-dimensional elemental bio-imaging of Fe, Zn, Cu, Mn and P in a 6-hydroxydopamine lesioned mouse brain. Metallomics. 2010;2:745–753. doi: 10.1039/c0mt00039f. [DOI] [PubMed] [Google Scholar]
- 156.Barnham KJ, Bush AI. Metals in Alzheimer’s and Parkinson’s diseases. Curr. Opin. Chem. Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 157.Hirsch EC. Iron transport in Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:S209–S211. doi: 10.1016/S1353-8020(09)70816-8. [DOI] [PubMed] [Google Scholar]
- 158.Jameson GN, Jameson RF, Linert W. New insights into iron release from ferritin: direct observation of the neurotoxin 6-hydroxydopamine entering ferritin and reaching redox equilibrium with the iron core. Org. Biomol. Chem. 2004;2:2346–2351. doi: 10.1039/B408044K. [DOI] [PubMed] [Google Scholar]
- 159.Kobayashi H, Oikawa S, Umemura S, Hirosawa I, Kawanishi S. Mechanism of metalmediated DNA damage and apoptosis induced by 6-hydroxydopamine in neuroblastoma SH-SY5Y cells. Free Radic. Res. 2008;42:651–660. doi: 10.1080/10715760802270334. [DOI] [PubMed] [Google Scholar]
- 160.Gauthier MA, Eibl JK, Crispo JA, Ross GM. Covalent arylation of metallothionein by oxidized dopamine products: a possible mechanism for zinc-mediated enhancement of dopaminergic neuron survival. Neurotox. Res. 2008;14:317–328. doi: 10.1007/BF03033856. [DOI] [PubMed] [Google Scholar]
- 161.Jiang H, Song N, Xu H, Zhang S, Wang J, Xie J. Up-regulation of divalent metal transporter 1 in 6-hydroxydopamine intoxication is IRE/IRP dependent. Cell Res. 2010;20:345–356. doi: 10.1038/cr.2010.20. [DOI] [PubMed] [Google Scholar]
- 162.Nicolaus BJ. A critical review of the function of neuromelanin and an attempt to provide a unified theory. Med Hypotheses. 2005;65:791–796. doi: 10.1016/j.mehy.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 163.Koeppen AH. The history of iron in the brain. J. Neurol. Sci. 1995;134:1–9. doi: 10.1016/0022-510x(95)00202-d. [DOI] [PubMed] [Google Scholar]
- 164.Kaur D, Andersen J. Does cellular iron dysregulation play a causative role in Parkinson’s disease? Ageing Res. Rev. 2004;3:327–343. doi: 10.1016/j.arr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 165.Takanashi M, Mochizuki H, Yokomizo K, Hattori N, Mori H, Yamamura Y, Mizuno Y. Iron accumulation in the substantia nigra of autosomal recessive juvenile parkinsonism (ARJP) Parkinsonism Relat. Disord. 2001;7:311–314. doi: 10.1016/s1353-8020(00)00050-x. [DOI] [PubMed] [Google Scholar]
- 166.Faucheux BA, Nillesse N, Damier P, Spik G, Mouatt-Prigent A, Pierce A, Leveugle B, Kubis N, Hauw JJ, Agid Y, et al. Expression of lactoferrin receptors is increased in the mesencephalon of patients with Parkinson disease. Proc. Natl. Acad. Sci USA. 1995;92:9603–9607. doi: 10.1073/pnas.92.21.9603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jiang H, Qian ZM, Xie JX. Increased DMT1 expression and iron content in MPTP-treated C57BL/6 mice. Sheng Li Xue Bao. 2003;55:571–576. [PubMed] [Google Scholar]
- 168.Zucca FA, Giaveri G, Gallorini M, Albertini A, Toscani M, Pezzoli G, Lucius R, Wilms H, Sulzer D, Ito S, Wakamatsu K, Zecca L. The neuromelanin of human substantia nigra: physiological and pathogenic aspects. Pigment Cell Res. 2004;17:610–617. doi: 10.1111/j.1600-0749.2004.00201.x. [DOI] [PubMed] [Google Scholar]
- 169.Zhang J, Zhang Y, Wang J, Cai P, Luo C, Qian Z, Dai Y, Feng H. Characterizing iron deposition in Parkinson’s disease using susceptibility-weighted imaging: an in vivo MR study. Brain Res. 2010;1330:124–130. doi: 10.1016/j.brainres.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 170.Andersen JK. Iron dysregulation and Parkinson’s disease. J. Alzheimers Dis. 2004;6:S47–S52. doi: 10.3233/jad-2004-6s602. [DOI] [PubMed] [Google Scholar]
- 171.Bou-Abdallah F, McNally J, Liu XX, Melman A. Oxygen catalyzed mobilization of iron from ferritin by iron(iii) chelate ligands. Chem. Commun. 2011;47:731–733. doi: 10.1039/c0cc03454a. [DOI] [PubMed] [Google Scholar]
- 172.Qian ZM, Wang Q. Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Res. Rev. 1998;27:257–267. doi: 10.1016/s0165-0173(98)00012-5. [DOI] [PubMed] [Google Scholar]
- 173.Ke Y, Ming Qian Z. Iron misregulation in the brain: a primary cause of neurodegenerative disorders. Lancet Neurol. 2003;2:246–253. doi: 10.1016/s1474-4422(03)00353-3. [DOI] [PubMed] [Google Scholar]
- 174.Schipper HM, Liberman A, Stopa EG. Neural heme oxygenase-1 expression in idiopathic Parkinson’s disease. Exp. Neurol. 1998;150:60–68. doi: 10.1006/exnr.1997.6752. [DOI] [PubMed] [Google Scholar]
- 175.Shamoto-Nagai M, Maruyama W, Yi H, Akao Y, Tribl F, Gerlach M, Osawa T, Riederer P, Naoi M. Neuromelanin induces oxidative stress in mitochondria through release of iron: mechanism behind the inhibition of 26S proteasome. J. Neural. Transm. 2006;113:633–644. doi: 10.1007/s00702-005-0410-5. [DOI] [PubMed] [Google Scholar]
- 176.Hirsch EC. Iron transport in Parkinson’s disease. Parkinsonism Relat. Disord. 2009;15:S209–S211. doi: 10.1016/S1353-8020(09)70816-8. [DOI] [PubMed] [Google Scholar]
- 177.Levenson CW, Cutler RG, Ladenheim B, Cadet JL, Hare J, Mattson MP. Role of dietary iron restriction in a mouse model of Parkinson’s disease. Exp. Neurol. 2004;190:506–514. doi: 10.1016/j.expneurol.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 178.Gal S, Fridkin M, Amit T, Zheng H, Youdim MB. M30, a novel multifunctional neuroprotective drug with potent iron chelating and brain selective monoamine oxidase-ab inhibitory activity for Parkinson’s disease. J. Neural. Transm. Suppl. 2006;70:447–456. doi: 10.1007/978-3-211-45295-0_68. [DOI] [PubMed] [Google Scholar]
- 179.Kaur D, Yantiri F, Rajagopalan S, Kumar J, Mo JQ, Boonplueang R, Viswanath V, Jacobs R, Yang L, Beal MF, DiMonte D, Volitaskis I, Ellerby L, Cherny RA, Bush AI, Andersen JK. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson’s disease. Neuron. 2003;37:899–909. doi: 10.1016/s0896-6273(03)00126-0. [DOI] [PubMed] [Google Scholar]
- 180.Zecca L, Berg D, Arzberger T, Ruprecht P, Rausch WD, Musicco M, Tampellini D, Riederer P, Gerlach M, Becker G. In vivo detection of iron and neuromelanin by transcranial sonography: a new approach for early detection of substantia nigra damage. Mov. Disord. 2005;20:1278–1285. doi: 10.1002/mds.20550. [DOI] [PubMed] [Google Scholar]
- 181.Naoi M, Maruyama W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharm. Des. 2010;16:2799–2817. doi: 10.2174/138161210793176527. [DOI] [PubMed] [Google Scholar]
- 182.Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv. Drug Deliv. Rev. 2008;60:1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li SW, Lin TS, Minteer S, Burke WJ. 3,4-Dihydroxyphenylacetaldehyde and hydrogen peroxide generate a hydroxyl radical: possible role in Parkinson’s disease pathogenesis. Brain Res. Mol. Brain Res. 2001;93:1–7. doi: 10.1016/s0169-328x(01)00120-6. [DOI] [PubMed] [Google Scholar]
- 184.Tabner BJ, Turnbull S, El-Agnaf OM, Allsop D. Formation of hydrogen peroxide and hydroxyl radicals from A(beta) and alpha-synuclein as a possible mechanism of cell death in Alzheimer’s disease and Parkinson’s disease. Free Radic. Biol. Med. 2002;32:1076–1083. doi: 10.1016/s0891-5849(02)00801-8. [DOI] [PubMed] [Google Scholar]
- 185.Matsubara K, Aoyama K, Suno M, Awaya T. N-methylation underlying Parkinson’s disease. Neurotoxicol. Teratol. 2002;24:593–598. doi: 10.1016/s0892-0362(02)00212-x. [DOI] [PubMed] [Google Scholar]
- 186.Parsons RB, Smith SW, Waring RH, Williams AC, Ramsden DB. High expression of nicotinamide N-methyltransferase in patients with idiopathic Parkinson’s disease. Neurosci. Lett. 2003;342:13–16. doi: 10.1016/s0304-3940(03)00218-0. [DOI] [PubMed] [Google Scholar]
- 187.Naoi M, Maruyama W, Nagy GM. Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology. 2004;25:193–204. doi: 10.1016/S0161-813X(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 188.DeCuypere M, Lu Y, Miller DD, LeDoux MS. Regional distribution of tetrahydroisoquinoline derivatives in rodent, human, and Parkinson’s disease brain. J. Neurochem. 2008;107:1398–1413. doi: 10.1111/j.1471-4159.2008.05709.x. [DOI] [PubMed] [Google Scholar]
- 189.Antkiewicz-Michaluk L. Endogenous risk factors in Parkinson’s disease: dopamine and tetrahydroisoquinolines. Pol. J. Pharmacol. 2002;54:567–572. [PubMed] [Google Scholar]
- 190.Soto-Otero R, Méndez-Alvarez E, Sánchez-Sellero I, Cruz-Landeira A, López-Rivadulla Lamas M. Reduction of rat brain levels of the endogenous dopaminergic proneurotoxins 1,2,3,4- tetrahydroisoquinoline and 1,2,3,4-tetrahydro-beta-carboline by cigarette smoke. Neurosci. Lett. 2001;298:187–190. doi: 10.1016/s0304-3940(00)01746-8. [DOI] [PubMed] [Google Scholar]
- 191.Gearhart DA, Neafsey EJ, Collins MA. Phenylethanolamine N-methyltransferase has beta-carboline 2N-methyltransferase activity: hypothetical relevance to Parkinson’s disease. Neurochem. Int. 2002;40:611–620. doi: 10.1016/s0197-0186(01)00115-2. [DOI] [PubMed] [Google Scholar]
- 192.Wu YN, Johnson SW. Rotenone potentiates NMDA currents in substantia nigra dopamine neurons. Neurosci. Lett. 2007;421:96–100. doi: 10.1016/j.neulet.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 193.Gao WJ, Goldman-Rakic PS. NMDA receptor-mediated epileptiform persistent activity requires calcium release from intracellular stores in prefrontal neurons. Exp. Neurol. 2006;197:495–504. doi: 10.1016/j.expneurol.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 194.Nizami S, Lee VW, Davies J, Long P, Jovanovic JN, Sihra TS. Presynaptic roles of intracellular Ca(2+) stores in signalling and exocytosis. Biochem. Soc. Trans. 2010;38:529–535. doi: 10.1042/BST0380529. [DOI] [PubMed] [Google Scholar]
- 195.Maurois P, Pages N, Bac P, German-Fattal M, Agnani G, Delplanque B, Durlach J. Threshold to N-methyl-d-aspartate-induced seizures in mice undergoing chronic nutritional magnesium deprivation is lowered in a way partly responsive to acute magnesium and antioxidant administrations. Br. J. Nutr. 2009;101:317–321. doi: 10.1017/S0007114508006752. [DOI] [PubMed] [Google Scholar]
- 196.Gu Z, Nakamura T, Lipton SA. Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases. Mol. Neurobiol. 2010;41:55–72. doi: 10.1007/s12035-010-8113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Martínez A, Portero-Otin M, Pamplona R, Ferrer I. Protein targets of oxidative damage in human neurodegenerative diseases with abnormal protein aggregates. Brain Pathol. 2010;20:281–297. doi: 10.1111/j.1750-3639.2009.00326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adamczyk A, KaŸmierczak A, Czapski GA, Strosznajder JB. Alpha-synuclein induced cell death in mouse hippocampal (HT22) cells is mediated by nitric oxide-dependent activation of caspase-3. FEBS Lett. 2010;584:3504–3508. doi: 10.1016/j.febslet.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 199.Adamczyk A, Czapski GA, KaŸmierczak A, Strosznajder JB. Effect of N-methyl-d-aspartate (NMDA) receptor antagonists on alpha-synuclein-evoked neuronal nitric oxide synthase activation in the rat brain. Pharmacol. Rep. 2009;61:1078–1085. doi: 10.1016/s1734-1140(09)70170-7. [DOI] [PubMed] [Google Scholar]
- 200.Meredith GE, Totterdell S, Beales M, Meshul CK. Impaired glutamate homeostasis and programmed cell death in a chronic MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2009;219:334–340. doi: 10.1016/j.expneurol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nimmrich V, Reymann KG, Strassburger M, Schöder UH, Gross G, Hahn A, Schoemaker H, Wicke K, Möller A. Inhibition of calpain prevents NMDA-induced cell death and beta-amyloid-induced synaptic dysfunction in hippocampal slice cultures. Br J Pharmacol. 2010;159 :1523–1531. doi: 10.1111/j.1476-5381.2010.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Ma T, Zhao Y, Kwak YD, Yang Z, Thompson R, Luo Z, Xu H, Liao FF. Statin’s excitoprotection is mediated by sAPP and the subsequent attenuation of calpain-induced truncation events, likely via rho-ROCK signaling. J. Neurosci. 2009;29:11226–11236. doi: 10.1523/JNEUROSCI.6150-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pan J, Xiao Q, Sheng CY, Hong Z, Yang HQ, Wang G, Ding JQ, Chen SD. Blockade of the translocation and activation of c-Jun N-terminal kinase 3 (JNK3) attenuates dopaminergic neuronal damage in mouse model of Parkinson’s disease. Neurochem. Int. 2009;54:418–425. doi: 10.1016/j.neuint.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 204.Wang AL, Liou YM, Pawlak CR, Ho YJ. Involvement of NMDA receptors in both MPTP-induced neuroinflammation and deficits in episodic-like memory in Wistar rats. Behav. Brain Res. 2010;208:38–46. doi: 10.1016/j.bbr.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 205.Hald A, van Beek J, Lotharius J. Inflammation in Parkinson’s disease: causative or epiphenomenal? Subcell. Biochem. 2007;42:249–279. [PubMed] [Google Scholar]
- 206.Kurkowska-Jastrzebska I, Wrońska A, Kohutnicka M, Członkowski A, Członkowska A. MHC class II positive microglia and lymphocytic infiltration are present in the substantia nigra and striatum in mouse model of Parkinson’s disease. Acta Neurobiol. Exp. 1999;59:1–8. doi: 10.55782/ane-1999-1289. [DOI] [PubMed] [Google Scholar]
- 207.Ouchi Y, Yoshikawa E, Sekine Y, Futatsubashi M, Kanno T, Ogusu T, Torizuka T. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann. Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 208.Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr. Pharm. Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- 209.McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann. N. Y. Acad. Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- 210.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J. Neural. Transm. Suppl. 2000;60:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 211.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. FASEB J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 212.Onyango IG, Tuttle JB, Bennett JP., Jr Activation of p38 and N-acetylcysteine-sensitive c-Jun NH2-terminal kinase signaling cascades is required for induction of apoptosis in Parkinson’s disease cybrids. Mol. Cell Neurosci. 2005;28:452–461. doi: 10.1016/j.mcn.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 213.Hirsch EC, Hunot S, Hartmann A. Neuroinflammatory processes in Parkinson’s disease. Parkinsonism Relat. Disord. 2005;11:S9–S15. doi: 10.1016/j.parkreldis.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 214.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 216.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 217.Ji H, Wang H, Zhang F, Li X, Xiang L, Aiguo S. PPARγ agonist pioglitazone inhibits microglia inflammation by blocking p38 mitogen-activated protein kinase signaling pathways. Inflamm. Res. 2010;59:921–929. doi: 10.1007/s00011-010-0203-7. [DOI] [PubMed] [Google Scholar]
- 218.Kim SH, Kim J, Sharma RP. Inhibition of p38 and ERK MAP kinases blocks endotoxininduced nitric oxide production and differentially modulates cytokine expression. Pharmacol. Res. 2004;49:433–439. doi: 10.1016/j.phrs.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 219.Thomas T, Timmer M, Cesnulevicius K, Hitti E, Kotlyarov A, Gaestel M. MAPKAP kinase 2-deficiency prevents neurons from cell death by reducing neuroinflammation—relevance in a mouse model of Parkinson’s disease. J. Neurochem. 2008;105:2039–2052. doi: 10.1111/j.1471-4159.2008.05310.x. [DOI] [PubMed] [Google Scholar]
- 220.Willesen MG, Gammeltoft S, Vaudano E. Activation of the c-Jun N terminal kinase pathway in an animal model of Parkinson’s disease. Ann. N. Y. Acad. Sci. 2002;973:237–240. doi: 10.1111/j.1749-6632.2002.tb04640.x. [DOI] [PubMed] [Google Scholar]
- 221.Lee DY, Oh YJ, Jin BK. Thrombin-activated microglia contribute to death of dopaminergic neurons in rat mesencephalic cultures: dual roles of mitogen-activated protein kinase signaling pathways. Glia. 2005;51:98–110. doi: 10.1002/glia.20190. [DOI] [PubMed] [Google Scholar]
- 222.Kao SJ, Lei HC, Kuo CT, Chang MS, Chen BC, Chang YC, Chiu WT, Lin CH. Lipoteichoic acid induces nuclear factor-kappaB activation and nitric oxide synthase expression via phosphatidylinositol 3-kinase, Akt, and p38 MAPK in RAW 264.7 macrophages. Imunology. 2005;115:366–374. doi: 10.1111/j.1365-2567.2005.02160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chio CC, Chang YH, Hsu YW, Chi KH, Lin WW. PKA-dependent activation of PKC.; p38 MAPK and IKK in macrophage: implication in the induction of inducible nitric oxide synthase and interleukin-6 by dibutyryl cAMP. Cell Signal. 2004;16:565–575. doi: 10.1016/j.cellsig.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 224.Lee JK, Choi SS, Won JS, Suh HW. The regulation of inducible nitric oxide synthase gene expression induced by lipopolysaccharide and tumor necrosis factor-alpha in C6 cells: involvement of AP-1 and NFkappaB. Life Sci. 2003;73:595–609. doi: 10.1016/s0024-3205(03)00317-5. [DOI] [PubMed] [Google Scholar]
- 225.Wang MJ, Lin WW, Chen HL, Chang YH, Ou HC, Kuo JS, Hong JS, Jeng KC. Silymarin protects dopaminergic neurons against lipopolysaccharide-induced neurotoxicity by inhibiting microglia activation. Eur. J. Neurosci. 2002;16:2103–2112. doi: 10.1046/j.1460-9568.2002.02290.x. [DOI] [PubMed] [Google Scholar]
- 226.Hua LL, Zhao ML, Cosenza M, Kim MO, Huang H, Tanowitz HB, Brosnan CF, Lee SC. Role of mitogen-activated protein kinases in inducible nitric oxide synthase and TNFalpha expression in human fetal astrocytes. J. Neuroimmunol. 2002;126:180–189. doi: 10.1016/s0165-5728(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 227.Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 228.Ban HS, Suzuki K, Lim SS, Jung SH, Lee S, Ji J, Lee HS, Lee YS, Shin KH, Ohuchi K. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase and tumor necrosis factor-alpha by 2′-hydroxychalcone derivatives in RAW 264.7 cells. Biochem. Pharmacol. 2004;67:1549–1557. doi: 10.1016/j.bcp.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 229.Wang W, Ma C, Mao Z, Li M. JNK inhibition as a potential strategy in treating Parkinson’s disease. Drug News Perspect. 2004;17:646–654. doi: 10.1358/dnp.2004.17.10.873916. [DOI] [PubMed] [Google Scholar]
- 230.Saporito MS, Thomas BA, Scott RW. MPTP activates c-Jun NH(2)-terminal kinase (JNK) and its upstream regulatory kinase MKK4 in nigrostriatal neurons in vivo. J. Neurochem. 2000;75:1200–1208. doi: 10.1046/j.1471-4159.2000.0751200.x. [DOI] [PubMed] [Google Scholar]
- 231.Saporito MS, Brown EM, Miller MS, Carswell S. CEP-1347/KT-7515, an inhibitor of c-jun N-terminal kinase activation, attenuates the 1-methyl-4-phenyl tetrahydropyridine-mediated loss of nigrostriatal dopaminergic neurons in vivo. J. Pharmacol. Exp. Ther. 1999;288:421–427. [PubMed] [Google Scholar]
- 232.Kurkowska-Jastrzebska I, Babiuch M, Joniec I, Przybyłkowski A, Członkowski A, Członkowska A. Indomethacin protects against neurodegeneration caused by MPTP intoxication in mice. Int. Immunopharmacol. 2002;2:1213–1218. doi: 10.1016/s1567-5769(02)00078-4. [DOI] [PubMed] [Google Scholar]
- 233.Fahrig T, Gerlach I, Horváth E. A synthetic derivative of the natural product rocaglaol is a potent inhibitor of cytokine-mediated signaling and shows neuroprotective activity in vitro and in animal models of Parkinson’s disease and traumatic brain injury. Mol. Pharmacol. 2005;67:1544–1555. doi: 10.1124/mol.104.008177. [DOI] [PubMed] [Google Scholar]
- 234.Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi DK, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc. Natl. Acad. Sci USA. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee HG, Kim H, Oh WK, Yu KA, Choe YK, Ahn JS, Kim DS, Kim SH, Dinarello CA, Kim K, Yoon DY. Tetramethoxy hydroxyflavone p7F downregulates inflammatory mediators via the inhibition of nuclear factor kappaB. Ann. N. Y. Acad. Sci. 2004;1030:555–568. doi: 10.1196/annals.1329.065. [DOI] [PubMed] [Google Scholar]
- 237.Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 238.Tieu K, Ischiropoulos H, Przedborski S. Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life. 2003;55:329–335. doi: 10.1080/1521654032000114320. [DOI] [PubMed] [Google Scholar]
- 239.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci USA. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB. Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J. Neurochem. 2000;74:2213–2216. doi: 10.1046/j.1471-4159.2000.0742213.x. [DOI] [PubMed] [Google Scholar]
- 241.Klivenyi P, St Clair D, Wermer M, Yen HC, Oberley T, Yang L, Beal MF. Manganese superoxide dismutase overexpression attenuates MPTP toxicity. Neurobiol. Dis. 1998;5:253–258. doi: 10.1006/nbdi.1998.0191. [DOI] [PubMed] [Google Scholar]
- 242.Andreassen OA, Ferrante RJ, Dedeoglu A, Albers DW, Klivenyi P, Carlson EJ, Epstein CJ, Beal MF. Mice with a partial deficiency of manganese superoxide dismutase show increased vulnerability to the mitochondrial toxins malonate, 3-nitropropionic acid, and MPTP. Exp. Neurol. 2001;167:189–195. doi: 10.1006/exnr.2000.7525. [DOI] [PubMed] [Google Scholar]
- 243.Callio J, Oury TD, Chu CT. Manganese superoxide dismutase protects against 6-hydroxydopamine injury in mouse brains. J. Biol. Chem. 2005;280:18536–18542. doi: 10.1074/jbc.M413224200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (−)-epigallocatechin-3-gallate prevents N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced dopaminergic neurodegeneration. J. Neurochem. 2001;78:1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 245.Kurosaki R, Muramatsu Y, Michimata M, Matsubara M, Kato H, Imai Y, Itoyama Y, Araki T. Role of nitric oxide synthase against MPTP neurotoxicity in mice. Neurol. Res. 2002;24:655–662. doi: 10.1179/016164102101200717. [DOI] [PubMed] [Google Scholar]
- 246.Choi JY, Park CS, Kim DJ, Cho MH, Jin BK, Pie JE, Chung WG. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology. 2002;23:367–374. doi: 10.1016/s0161-813x(02)00079-7. [DOI] [PubMed] [Google Scholar]
- 247.Mazzio EA, Soliman KF. Cytoprotection of pyruvic acid and reduced beta-nicotinamide adenine dinucleotide against hydrogen peroxide toxicity in neuroblastoma cells. Neurochem. Res. 2003;28:733–741. doi: 10.1023/a:1022813817743. [DOI] [PubMed] [Google Scholar]
- 248.Mazzio EA, Reams RR, Soliman KF. The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res. 2004;1004:29–44. doi: 10.1016/j.brainres.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 249.Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004;19:169–175. doi: 10.1023/b:mebr.0000043967.78763.5b. [DOI] [PubMed] [Google Scholar]
- 250.Mazzio E, Soliman KF. Pyruvic acid cytoprotection against 1-methyl-4-phenylpyridinium, 6-hydroxydopamine and hydrogen peroxide toxicities in vitro. Neurosci. Lett. 2003;337:77–80. doi: 10.1016/s0304-3940(02)01327-7. [DOI] [PubMed] [Google Scholar]
- 251.Gonzalez SV, Nguyen NH, Rise F, Hassel B. Brain metabolism of exogenous pyruvate. J. Neurochem. 2005;95:284–293. doi: 10.1111/j.1471-4159.2005.03365.x. [DOI] [PubMed] [Google Scholar]
- 252.Lee JY, Kim YH, Koh JY. Protection by pyruvate against transient forebrain ischemia in rats. J Neurosci. 2001;21:RC171. doi: 10.1523/JNEUROSCI.21-20-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Izumi Y, Zorumski CF. Neuroprotective effects of pyruvate following NMDA-mediated excitotoxic insults in hippocampal slices. Neurosci. Lett. 2010;478:131–135. doi: 10.1016/j.neulet.2010.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cosi C, Marien M. Decreases in mouse brain NAD+ and ATP induced by 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP): prevention by the poly(ADP-ribose) polymerase inhibitor, benzamide. Brain Res. 1998;809:58–67. doi: 10.1016/s0006-8993(98)00829-4. [DOI] [PubMed] [Google Scholar]
- 255.Cosi C, Marien M. Implication of poly (ADP-ribose) polymerase (PARP) in neurodegeneration and brain energy metabolism. Decreases in mouse brain NAD+ and ATP caused by MPTP are prevented by the PARP inhibitor benzamide. Ann. N. Y. Acad. Sci. 1999;890:227–239. doi: 10.1111/j.1749-6632.1999.tb07998.x. [DOI] [PubMed] [Google Scholar]
- 256.Iwashita A, Yamazaki S, Mihara K, Hattori K, Yamamoto H, Ishida J, Matsuoka N, Mutoh S. Neuroprotective effects of a novel poly(ADP-ribose) polymerase-1 inhibitor, 2-[3-[4-(4-chlorophenyl)-1-piperazinyl] propyl]-4(3H)-quinazolinone (FR255595), in an in vitro model of cell death and in mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2004;309:1067–1078. doi: 10.1124/jpet.103.064642. [DOI] [PubMed] [Google Scholar]
- 257.Yokoyama H, Kuroiwa H, Tsukada T, Uchida H, Kato H, Araki T. Poly(ADPribose) polymerase inhibitor can attenuate the neuronal death after 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-induced neurotoxicity in mice. J. Neurosci. Res. 2010;88:1522–1536. doi: 10.1002/jnr.22310. [DOI] [PubMed] [Google Scholar]
- 258.Anderson DW, Bradbury KA, Schneider JS. Broad neuroprotective profile of nicotinamide in different mouse models of MPTP-induced Parkinsonism. Eur. J. Neurosci. 2008;28:610–617. doi: 10.1111/j.1460-9568.2008.06356.x. [DOI] [PubMed] [Google Scholar]
- 259.Mukherjee SK, Klaidman LK, Yasharel R, Adams JD., Jr Increased brain NAD prevents neuronal apoptosis in vivo. Eur. J. Pharmacol. 1997;330:27–34. doi: 10.1016/s0014-2999(97)00171-4. [DOI] [PubMed] [Google Scholar]
- 260.Yokoyama H, Kuroiwa H, Tsukada T, Uchida H, Kato H, Araki T. Poly(ADPribose) polymerase inhibitor can attenuate the neuronal death after 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-induced neurotoxicity in mice. J. Neurosci. Res. 2010;88:1522–1536. doi: 10.1002/jnr.22310. [DOI] [PubMed] [Google Scholar]
- 261.Mandir AS, Simbulan-Rosenthal CM, Poitras MF, Lumpkin JR, Dawson VL, Smulson ME, Dawson TM. A novel in vivo post-translational modification of p53 by PARP-1 in MPTP-induced parkinsonism. J. Neurochem. 2002;83:186–192. doi: 10.1046/j.1471-4159.2002.01144.x. [DOI] [PubMed] [Google Scholar]
- 262.Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 263.Fukushima T, Ohta M, Tanaka K, Kaneko S-Y, Maeda T, Sasaki A. Niacin metabolism and Parkinson’s disease. Asia Pac. J. Clin. Nutr. 2004;13:S176. [Google Scholar]
- 264.Alston TA, Abeles RH. Substrate specificity of nicotinamide methyltransferase isolated from porcine liver. Arch. Biochem. Biophys. 1988;260:601–618. doi: 10.1016/0003-9861(88)90487-0. [DOI] [PubMed] [Google Scholar]
- 265.Ashihara H, Crozier A. Caffeine: a well known but little mentioned compound in plant science. Trends Plant Sci. 2001;6:407–413. doi: 10.1016/s1360-1385(01)02055-6. [DOI] [PubMed] [Google Scholar]
- 266.Koshiishi C, Kato A, Yama S, Crozier A, Ashihara H. A new caffeine biosynthetic pathway in tea leaves: utilisation of adenosine released from the S-adenosyl-l-methionine cycle. FEBS Lett. 2001;499:50–54. doi: 10.1016/s0014-5793(01)02512-1. [DOI] [PubMed] [Google Scholar]
- 267.Góngora-Alfaro JL. Caffeine as a preventive drug for Parkinson’s disease: epidemiologic evidence and experimental support. Rev. Neurol. 2010;50:221–229. [PubMed] [Google Scholar]
- 268.Singh K, Singh S, Singhal NK, Sharma A, Parmar D, Singh MP. Nicotine- and caffeinemediated changes in gene expression patterns of MPTP-lesioned mouse striatum: Implications in neuroprotection mechanism. Chem. Biol. Interact. 2010;185:81–93. doi: 10.1016/j.cbi.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 269.Upmeier B, Gross W, Köster S, Barz W. Purification and properties of S-adenosyl-l-methionine: nicotinic acid-N-methyltransferase from cell suspension cultures of Glycine max L. Arch. Biochem. Biophys. 1988;262:445–454. doi: 10.1016/0003-9861(88)90396-7. [DOI] [PubMed] [Google Scholar]
- 270.Oyanagi K. The nature of the parkinsonism-dementia complex and amyotrophic lateral sclerosis of Guam and magnesium deficiency. Parkinsonism Relat. Disord. 2005;11:S17–S23. doi: 10.1016/j.parkreldis.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 271.Barbiroli B, Martinelli P, Patuelli A, Lodi R, Iotti S, Cortelli P, Montagna P. Phosphorus magnetic resonance spectroscopy in multiple system atrophy and Parkinson’s disease. Mov. Disord. 1999;14:430–435. doi: 10.1002/1531-8257(199905)14:3<430::aid-mds1007>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 272.Philippu A, Matthaei H, Lentzen H. Uptake of dopamine into fractions of pig caudate nucleus homogenates. Naunyn Schmiedebergs Arch. Pharmacol. 1975;287:181–190. doi: 10.1007/BF00510449. [DOI] [PubMed] [Google Scholar]
- 273.Schümann HJ, Althoff B. Effects of calcium and phosphate on catecholamines, ATP and dopamine beta-hydroxylase of chromaffin medullary granules. Naunyn Schmiedebergs Arch. Pharmacol. 1976;293:67–74. doi: 10.1007/BF00498872. [DOI] [PubMed] [Google Scholar]
- 274.Baker PF, Knight DE. Gaining access to the site of exocytosis in bovine adrenal medullary cells. J. Physiol. 1980;76:497–504. [PubMed] [Google Scholar]
- 275.Yang YC, Lee CH, Kuo CC. Ionic flow enhances low-affinity binding: a revised mechanistic view into Mg2+ block of NMDA receptors. J. Physiol. 2010;588:633–650. doi: 10.1113/jphysiol.2009.178913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Safar MM, Abdallah DM, Arafa NM, Abdel-Aziz MT. Magnesium supplementation enhances the anticonvulsant potential of valproate in pentylenetetrazol-treated rats. Brain Res. 2010;1334:58–64. doi: 10.1016/j.brainres.2010.03.076. [DOI] [PubMed] [Google Scholar]
- 277.Lin JY, Chung SY, Lin M, Cheng FC. Effects of magnesium sulfate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002;71:803–811. doi: 10.1016/s0024-3205(02)01738-1. [DOI] [PubMed] [Google Scholar]
- 278.Johnson S. Micronutrient accumulation and depletion in schizophrenia, epilepsy, autism and Parkinson’s disease? Med Hypotheses. 2001;56:641–645. doi: 10.1054/mehy.2000.1302. [DOI] [PubMed] [Google Scholar]
- 279.Brosnan JT, Jacobs RL, Stead LM, Brosnan ME. Methylation demand: a key determinant of homocysteine metabolism. Acta Biochim. Pol. 2004;51:405–413. [PubMed] [Google Scholar]
- 280.Zoccolella S, Lamberti P, Armenise E, de Mari M, Lamberti SV, Mastronardi R, Fraddosio A, Iliceto G, Livrea P. Plasma homocysteine levels in Parkinson’s disease: role of antiparkinsonian medications. Parkinsonism Relat. Disord. 2005;11:131–133. doi: 10.1016/j.parkreldis.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 281.Lamberti P, Zoccolella S, Armenise E, Lamberti SV, Fraddosio A, de Mari M, Iliceto G, Livrea P. Hyperhomocysteinemia in L-dopa treated Parkinson’s disease patients: effect of cobalamin and folate administration. Eur. J. Neurol. 2005;12:365–368. doi: 10.1111/j.1468-1331.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 282.McCully KS. Chemical pathology of homocysteine. IV. Excitotoxicity, oxidative stress, endothelial dysfunction, and inflammation. Ann. Clin. Lab. Sci. 2009;39:219–232. [PubMed] [Google Scholar]
- 283.dos Santos EF, Busanello EN, Miglioranza A, Zanatta A, Barchak AG, Vargas CR, Saute J, Rosa C, Carrion MJ, Camargo D, Dalbem A, da Costa JC, de Sousa Miguel SR, de Mello Rieder CR, Wajner M. Evidence that folic acid deficiency is a major determinant of hyperhomocysteinemia in Parkinson’s disease. Metab. Brain Dis. 2009;24:257–269. doi: 10.1007/s11011-009-9139-4. [DOI] [PubMed] [Google Scholar]
- 284.Miller JW. Homocysteine, folate deficiency, and Parkinson’s disease. Nutr. Rev. 2002;60:410–413. doi: 10.1301/002966402320964089. [DOI] [PubMed] [Google Scholar]
- 285.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J. Neurochem. 2002;80:101–110. doi: 10.1046/j.0022-3042.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 286.Yagisawa M, Okawa N, Shigematsu N, Nakata R. Effects of intravenous betaine on methionine-loading-induced plasma homocysteine elevation in rats. J. Nutr. Biochem. 2004;15:666–671. doi: 10.1016/j.jnutbio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 287.Kharbanda KK, Rogers DD, II, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: protection by betaine. Biochem. Pharmacol. 2005;70:1883–1890. doi: 10.1016/j.bcp.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 288.Yeh Y-Y, Lim H-S, Yeh S-M, Picciano MF. Garlic extract attenuates hyperhomocysteinemia caused by folic acid deficiency in the rat. Nutr. Res. 2005;25:93–102. [Google Scholar]
- 289.Sled VD, Rudnitzky NI, Hatefi Y, Ohnishi T. Thermodynamic analysis of flavin in mitochondrial NADH:ubiquinone oxidoreductase (complex I) Biochemistry. 1994;33:10069–10075. doi: 10.1021/bi00199a034. [DOI] [PubMed] [Google Scholar]
- 290.Gerards M, van den Bosch BJ, Danhauser K, Serre V, van Weeghel M, Wanders RJ, Nicolaes GA, Sluiter W, Schoonderwoerd K, Scholte HR, Prokisch H, Rötig A, de Coo IF, Smeets HJ. Riboflavin-responsive oxidative phosphorylation complex I deficiency caused by defective ACAD9: new function for an old gene. Brain. 2011;134:210–219. doi: 10.1093/brain/awq273. [DOI] [PubMed] [Google Scholar]
- 291.Bar-Meir M, Elpeleg ON, Saada A. Effect of various agents on adenosine triphosphate synthesis in mitochondrial complex I deficiency. J. Pediatr. 2001;139:868–870. doi: 10.1067/mpd.2001.118885. [DOI] [PubMed] [Google Scholar]
- 292.Griebel V, Krägeloh-Mann I, Ruitenbeek W, Trijbels JM, Paulus W. A mitochondrial myopathy in an infant with lactic acidosis. Dev. Med. Child Neurol. 1990;32:528–531. doi: 10.1111/j.1469-8749.1990.tb16979.x. [DOI] [PubMed] [Google Scholar]
- 293.Antozzi C, Garavaglia B, Mora M, Rimoldi M, Morandi L, Ursino E, DiDonato S. Late-onset riboflavin-responsive myopathy with combined multiple acyl coenzyme A dehydrogenase and respiratory chain deficiency. Neurology. 1994;44:2153–2158. doi: 10.1212/wnl.44.11.2153. [DOI] [PubMed] [Google Scholar]
- 294.Ogle RF, Christodoulou J, Fagan E, Blok RB, Kirby DM, Seller KL, Dahl HH, Thorburn DR. Mitochondrial myopathy with tRNA(Leu(UUR)) mutation and complex I deficiency responsive to riboflavin. J. Pediatr. 1997;130:138–145. doi: 10.1016/s0022-3476(97)70323-8. [DOI] [PubMed] [Google Scholar]
- 295.Kerr DS. Treatment of mitochondrial electron transport chain disorders: a review of clinical trials over the past decade. Mol. Genet. Metab. 2010;99:246–255. doi: 10.1016/j.ymgme.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 296.Jia H, Liu Z, Li X, Feng Z, Hao J, Li X, Shen W, Zhang H, Liu J. Synergistic anti-Parkinsonism activity of high doses of B vitamins in a chronic cellular model. Neurobiol Aging. 2010;31:636–646. doi: 10.1016/j.neurobiolaging.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 297.Brownell AL, Jenkins BG, Isacson O. Dopamine imaging markers and predictive mathematical models for progressive degeneration in Parkinson’s disease. Biomed. Pharmacother. 1999;53:131–140. doi: 10.1016/S0753-3322(99)80078-X. [DOI] [PubMed] [Google Scholar]
- 298.Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah- Daouk R, Beal MF. Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp. Neurol. 1999;157:142–149. doi: 10.1006/exnr.1999.7049. [DOI] [PubMed] [Google Scholar]
- 299.McCarty MF. The therapeutic potential of glucose tolerance factor. Med Hypotheses. 1980;6:1177–1189. doi: 10.1016/0306-9877(80)90140-1. [DOI] [PubMed] [Google Scholar]
- 300.McCarty MF. High-dose biotin, an inducer of glucokinase expression, may synergize with chromium picolinate to enable a definitive nutritional therapy for type II diabetes. Med Hypotheses. 1999;52:401–406. doi: 10.1054/mehy.1997.0682. [DOI] [PubMed] [Google Scholar]
- 301.Aguilar MV, Jiménez-Jiménez FJ, Molina JA, Meseguer I, Mateos-Vega CJ, González- Muñoz MJ, de Bustos F, Gómez-Escalonilla C, Ort-Pareja M, Zurdo M, Martínez-Para MC. Cerebrospinal fluid selenium and chromium levels in patients with Parkinson’s disease. J. Neural Transm. 1998;105:1245–1251. doi: 10.1007/s007020050127. [DOI] [PubMed] [Google Scholar]
- 302.Greggio E, Bergantino E, Carter D, Ahmad R, Costin GE, Hearing VJ, Clarimon J, Singleton A, Eerola J, Hellström O, Tienari PJ, Miller DW, Beilina A, Bubacco L, Cookson MR. Tyrosinase exacerbates dopamine toxicity but is not genetically associated with Parkinson’s disease. J. Neurochem. 2005;93:246–256. doi: 10.1111/j.1471-4159.2005.03019.x. [DOI] [PubMed] [Google Scholar]
- 303.Boissy RE, Visscher M, DeLong MA. DeoxyArbutin: a novel reversible tyrosinase inhibitor with effective in vivo skin lightening potency. Exp. Dermatol. 2005;14:601–608. doi: 10.1111/j.0906-6705.2005.00337.x. [DOI] [PubMed] [Google Scholar]
- 304.Galeazzi MA. Behavior of polyphenoloxidases in food. Arch. Lat. Nutr. 1984;34:269–289. [PubMed] [Google Scholar]
- 305.Matheis G, Belitz HD. Studies on enzymic browning of potatoes (Solanum tuberosum). III. Kinetics of potato phenoloxidase (EC 1.14.18.1 monophenol, dihydroxyphenylalanine: oxygenoxidoreductase) Z. Lebensm. Unters. Forsch. 1977;163:191–195. doi: 10.1007/BF01459856. [DOI] [PubMed] [Google Scholar]
- 306.Henderson HM, Eskin NA, Pinsky C, Bose R, Ashique AM. Pyridine and other coal tar constituents as inhibitors of potato polyphenol oxidase: a non-animal model for neurochemical studies. Life Sci. 1992;51:PL207–210. doi: 10.1016/0024-3205(92)90317-i. [DOI] [PubMed] [Google Scholar]
- 307.Khatib S, Nerya O, Musa R, Shmuel M, Tamir S, Vaya J. Chalcones as potent tyrosinase inhibitors: the importance of a 2,4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005;13:433–441. doi: 10.1016/j.bmc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 308.Nerya O, Musa R, Khatib S, Tamir S, Vaya J. Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry. 2004;65:1389–1395. doi: 10.1016/j.phytochem.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 309.Lee NK, Son KH, Chang HW, Kang SS, Park H, Heo MY, Kim HP. Prenylated flavonoids as tyrosinase inhibitors. Arch. Pharm. Res. 2004;27:1132–1135. doi: 10.1007/BF02975118. [DOI] [PubMed] [Google Scholar]
- 310.Kim SJ, Son KH, Chang HW, Kang SS, Kim HP. Tyrosinase inhibitory prenylated flavonoids from Sophora flavescens. Biol. Pharm Bullutin. 2003;26:1348–1350. doi: 10.1248/bpb.26.1348. [DOI] [PubMed] [Google Scholar]
- 311.Son JK, Park JS, Kim JA, Kim Y, Chung SR, Lee SH. Prenylated flavonoids from the roots of Sophora flavescens with tyrosinase inhibitory activity. Planta Med. 2003;69:559–561. doi: 10.1055/s-2003-40643. [DOI] [PubMed] [Google Scholar]
- 312.Tan C, Zhu W, Lu Y. Aloin, cinnamic acid and sophorcarpidine are potent inhibitors of tyrosinase. Chin. Med. J. (Engl ) 2002;115:1859–1862. [PubMed] [Google Scholar]
- 313.Shi Y, Chen Q-X, Wang Q, Song KK, Qiu L. Inhibitory effects of cinnamic acid and its derivatives on the diphenolase activity of mushroom (Agaricus bisporus) tyrosinase. Food Chem. 2005;92:707–712. [Google Scholar]
- 314.Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S. Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots. J. Agric. Food Chem. 2003;51:1201–1207. doi: 10.1021/jf020935u. [DOI] [PubMed] [Google Scholar]
- 315.Fu B, Li H, Wang X, Lee FS, Cui S. Isolation and identification of flavonoids in licorice and a study of their inhibitory effects on tyrosinase. J. Agric. Food Chem. 2005;53:7408–7414. doi: 10.1021/jf051258h. [DOI] [PubMed] [Google Scholar]
- 316.Xie LP, Chen QX, Huang H, Wang HZ, Zhang RQ. Inhibitory effects of some flavonoids on the activity of mushroom tyrosinase. Biochemistry. 2003;68:487–491. doi: 10.1023/a:1023620501702. [DOI] [PubMed] [Google Scholar]
- 317.Masamoto Y, Murata Y, Baba K, Shimoishi Y, Tada M, Takahata K. Inhibitory effects of esculetin on melanin biosynthesis. Biol. Pharm Bullutin. 2004;27:422–425. doi: 10.1248/bpb.27.422. [DOI] [PubMed] [Google Scholar]
- 318.Chen QX, Ke LN, Song KK, Huang H, Liu XD. Inhibitory effects of hexylresorcinol and dodecylresorcinol on mushroom (Agaricus bisporus) tyrosinase. Protein J. 2004;23:135–141. doi: 10.1023/b:jopc.0000020080.21417.ff. [DOI] [PubMed] [Google Scholar]
- 319.Shin NH, Ryu SY, Choi EJ, Kang SH, Chang IM, Min KR, Kim Y. Oxyresveratrol as the potent inhibitor on dopa oxidase activity of mushroom tyrosinase. Biochem. Biophys. Res. Commun. 1998;243:801–803. doi: 10.1006/bbrc.1998.8169. [DOI] [PubMed] [Google Scholar]
- 320.Ohguchi K, Tanaka T, Iliya I, Ito T, Iinuma M, Matsumoto K, Akao Y, Nozawa Y. Gnetol as a potent tyrosinase inhibitor from genus Gnetum. Biosci. Biotechnol. Biochem. 2003;67:663–665. doi: 10.1271/bbb.67.663. [DOI] [PubMed] [Google Scholar]
- 321.Kim DS, Park SH, Kwon SB, Li K, Youn SW, Park KC. (−)-Epigallocatechin-3- gallate and hinokitiol reduce melanin synthesis via decreased MITF production. Arch. Pharm. Res. 2004;27:334–339. doi: 10.1007/BF02980069. [DOI] [PubMed] [Google Scholar]
- 322.No JK, Soung DY, Kim YJ, Shim KH, Jun YS, Rhee SH, Yokozawa T, Chung HY. Inhibition of tyrosinase by green tea components. Life Sci. 1999;65:PL241–246. doi: 10.1016/s0024-3205(99)00492-0. [DOI] [PubMed] [Google Scholar]
- 323.Zocca F, Lomolino G, Lante A. Antibrowning potential of Brassicacaea processing water. Bioresour. Technol. 2010;101:3791–3795. doi: 10.1016/j.biortech.2009.12.126. [DOI] [PubMed] [Google Scholar]
- 324.Negishi O, Ozawa T. Inhibition of enzymatic browning and protection of sulfhydryl enzymes by thiol compounds. Phytochemistry. 2000;54:481–487. doi: 10.1016/s0031-9422(00)00125-4. [DOI] [PubMed] [Google Scholar]
- 325.Nagai T, Suzuki N. Partial purification of polyphenol oxidase from Chinese cabbage Brassica rapa L. J. Agric. Food Chem. 2001;49:3922–3926. doi: 10.1021/jf000694v. [DOI] [PubMed] [Google Scholar]
- 326.Yang CP, Fujita S, Kohno K, Kusubayashi A, Ashrafuzzaman M, Hayashi N. Partial purification and characterization of polyphenol oxidase from banana (Musa sapientum L.) peel. J. Agric. Food Chem. 2001;49:1446–1449. doi: 10.1021/jf001051i. [DOI] [PubMed] [Google Scholar]
- 327.Pérez-Gilabert M, García-Carmona F. Dimethyl sulfide, a volatile flavor constituent, is a slow-binding inhibitor of tyrosinase. Biochem. Biophys. Res. Commun. 2001;285:257–261. doi: 10.1006/bbrc.2001.5189. [DOI] [PubMed] [Google Scholar]
- 328.Graf E, Empson KL, Eaton JW. Phytic acid. A natural antioxidant. J. Biol. Chem. 1987;262:11647–11650. [PubMed] [Google Scholar]
- 329.Kubo I, Kinst-Hori I, Nihei K, Soria F, Takasaki M, Calderón JS, Céspedes CL. Tyrosinase inhibitors from galls of Rhus javanica leaves and their effects on insects. Z. Naturforsch C. 2003;58:719–725. doi: 10.1515/znc-2003-9-1022. [DOI] [PubMed] [Google Scholar]
- 330.Sasaki K, Yoshizaki F. Nobiletin as a tyrosinase inhibitor from the peel of Citrus fruit. Biol. Pharm Bullutin. 2002;25:806–808. doi: 10.1248/bpb.25.806. [DOI] [PubMed] [Google Scholar]
- 331.Kubo I, Kinst-Hori I. Flavonols from saffron flower: tyrosinase inhibitory activity and inhibition mechanism. J. Agric. Food Chem. 1999;47:4121–4125. doi: 10.1021/jf990201q. [DOI] [PubMed] [Google Scholar]
- 332.Kubo I, Kinst-Hori I, Chaudhuri SK, Kubo Y, Sánchez Y, Ogura T. Flavonols from Heterotheca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg. Med. Chem. 2000;8:1749–1755. doi: 10.1016/s0968-0896(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 333.Masuda T, Yamashita D, Takeda Y, Yonemori S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005;69:197–201. doi: 10.1271/bbb.69.197. [DOI] [PubMed] [Google Scholar]
- 334.Gómez-Cordovés C, Bartolomé B, Vieira W, Virador VM. Effects of wine phenolics and sorghum tannins on tyrosinase activity and growth of melanoma cells. J. Agric. Food Chem. 2001;49:1620–1624. doi: 10.1021/jf001116h. [DOI] [PubMed] [Google Scholar]
- 335.An BJ, Kwak JH, Son JH, Park JM, Lee JY, Park TS, Kim SY, Kim YS, Jo C, Byun MW. Physiological activity of irradiated green tea polyphenol on the human skin. Am. J. Chin. Med. 2005;33:535–546. doi: 10.1142/S0192415X05003144. [DOI] [PubMed] [Google Scholar]
- 336.Shoji T, Masumoto S, Moriichi N, Kobori M, Kanda T, Shinmoto H, Tsushida T. Procyanidin trimers to pentamers fractionated from apple inhibit melanogenesis in B16 mouse melanoma cells. J. Agric. Food Chem. 2005;53:6105–6111. doi: 10.1021/jf050418m. [DOI] [PubMed] [Google Scholar]
- 337.Yamakoshi J, Otsuka F, Sano A, Tokutake S, Saito M, Kikuchi M, Kubota Y. Lightening effect on ultraviolet-induced pigmentation of guinea pig skin by oral administration of a proanthocyanidin-rich extract from grape seeds. Pigment Cell Res. 2003;16:629–638. doi: 10.1046/j.1600-0749.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 338.Kubo I, Kinst-Hori I, Kubo Y, Yamagiwa Y, Kamikawa T, Haraguchi H. Molecular design of antibrowning agents. J. Agric. Food Chem. 2000;48:1393–1399. doi: 10.1021/jf990926u. [DOI] [PubMed] [Google Scholar]
- 339.Roh JS, Han JY, Kim JH, Hwang JK. Inhibitory effects of active compounds isolated from safflower (Carthamus tinctorius L.) seeds for melanogenesis. Biol. Pharm Bullutin. 2004;27:1976–1978. doi: 10.1248/bpb.27.1976. [DOI] [PubMed] [Google Scholar]
- 340.Kubo I, Chen QX, Nihei K, Calderón JS, Céspedes CL. Tyrosinase inhibition kinetics of anisic acid. Z. Naturforsch C. 2003;58:713–718. doi: 10.1515/znc-2003-9-1021. [DOI] [PubMed] [Google Scholar]
- 341.Kubo I, Kinst-Hori I. Tyrosinase inhibitory activity of the olive oil flavor compounds. J. Agric. Food Chem. 1999;47:4574–4578. doi: 10.1021/jf990165v. [DOI] [PubMed] [Google Scholar]
- 342.Kim HP, Son KH, Chang HW, Kang SS. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004;96:229–245. doi: 10.1254/jphs.crj04003x. [DOI] [PubMed] [Google Scholar]
- 343.Francis JA, Rumbeiha W, Nair MG. Constituents in Easter lily flowers with medicinal activity. Life Sci. 2004;76:671–683. doi: 10.1016/j.lfs.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 344.Hou DX, Fujii M, Terahara N, Yoshimoto M. Molecular Mechanisms Behind the Chemopreventive Effects of Anthocyanidins. J. Biomed. Biotechnol. 2004;5:321–325. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Seeram NP, Zhang Y, Nair MG. Inhibition of proliferation of human cancer cells and cyclooxygenase enzymes by anthocyanidins and catechins. Nutr Cancer. 2003;46:101–106. doi: 10.1207/S15327914NC4601_13. [DOI] [PubMed] [Google Scholar]
- 346.Woo KJ, Jeong YJ, Inoue H, Park JW, Kwon TK. Chrysin suppresses lipopolysaccharide-induced cyclooxygenase-2 expression through the inhibition of nuclear factor for IL-6 (NF-IL6) DNA-binding activity. FEBS Lett. 2005;579:705–711. doi: 10.1016/j.febslet.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 347.Liang YC, Huang YT, Tsai SH, Lin-Shiau SY, Chen CF, Lin JK. Suppression of inducible cyclooxygenase and inducible nitric oxide synthase by apigenin and related flavonoids in mouse macrophages. Carcinogenesis. 1999;20:1945–1952. doi: 10.1093/carcin/20.10.1945. [DOI] [PubMed] [Google Scholar]
- 348.Raso GM, Meli R, Di Carlo G, Pacilio M, Di Carlo R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001;68:921–931. doi: 10.1016/s0024-3205(00)00999-1. [DOI] [PubMed] [Google Scholar]
- 349.Chi YS, Cheon BS, Kim HP. Effect of wogonin, a plant flavone from Scutellaria radix, on the suppression of cyclooxygenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW 264.7 cells. Biochem. Pharmacol. 2001;61:1195–1203. doi: 10.1016/s0006-2952(01)00597-4. [DOI] [PubMed] [Google Scholar]
- 350.O’Leary KA, de Pascual-Tereasa S, Needs PW, Bao YP, O’Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 351.Mutoh M, Takahashi M, Fukuda K, Komatsu H, Enya T, Matsushima-Hibiya Y, Mutoh H, Sugimura T, Wakabayashi K. Suppression by flavonoids of cyclooxygenase-2 promoterdependent transcriptional activity in colon cancer cells: structure-activity relationship. Jpn. J. Cancer Res. 2000;91:686–691. doi: 10.1111/j.1349-7006.2000.tb01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Huss U, Ringbom T, Perera P, Bohlin L, Vasänge M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J. Nat. Prod. 2002;65:1517–1521. doi: 10.1021/np020023m. [DOI] [PubMed] [Google Scholar]
- 353.Seaver B, Smith JR. Inhibition of COX isoforms by nutraceuticals. J. Herb. Pharmacother. 2004;4:11–18. [PubMed] [Google Scholar]
- 354.Murias M, Handler N, Erker T, Pleban K, Ecker G, Saiko P, Szekeres T, Jäger W. Resveratrol analogues as selective cyclooxygenase-2 inhibitors: synthesis and structure-activity relationship. Bioorg. Med. Chem. 2004;12:5571–5578. doi: 10.1016/j.bmc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 355.Chi YS, Jong HG, Son KH, Chang HW, Kang SS, Kim HP. Effects of naturally occurring prenylated flavonoids on enzymes metabolizing arachidonic acid: cyclooxygenases and lipoxygenases. Biochem. Pharmacol. 2001;62:1185–1191. doi: 10.1016/s0006-2952(01)00773-0. [DOI] [PubMed] [Google Scholar]
- 356.Selvam C, Jachak SM, Bhutani KK. Cyclooxygenase inhibitory flavonoids from the stem bark of Semecarpus anacardium Linn. Phytother. Res. 2004;18:582–584. doi: 10.1002/ptr.1492. [DOI] [PubMed] [Google Scholar]
- 357.Yoo SW, Kim JS, Kang SS, Son KH, Chang HW, Kim HP, Bae K, Lee CO. Constituents of the fruits and leaves of Euodia daniellii. Arch. Pharm. Res. 2002;25:824–830. doi: 10.1007/BF02976999. [DOI] [PubMed] [Google Scholar]
- 358.Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent Fatty Acids. 1998;58:17–24. doi: 10.1016/s0952-3278(98)90125-9. [DOI] [PubMed] [Google Scholar]
- 359.Chen Y, Yang L, Lee TJ. Oroxylin A inhibition of lipopolysaccharide-induced iNOS and COX-2 gene expression via suppression of nuclear factor-kappaB activation. Biochem. Pharmacol. 2000;59:1445–1457. doi: 10.1016/s0006-2952(00)00255-0. [DOI] [PubMed] [Google Scholar]
- 360.Rossi A, Ligresti A, Longo R, Russo A, Borrelli F, Sautebin L. The inhibitory effect of propolis and caffeic acid phenethyl ester on cyclooxygenase activity in J774 macrophages. Phytomedicine. 2002;9:530–535. doi: 10.1078/09447110260573164. [DOI] [PubMed] [Google Scholar]
- 361.You KM, Jong HG, Kim HP. Inhibition of cyclooxygenase/lipoxygenase from human platelets by polyhydroxylated/methoxylated flavonoids isolated from medicinal plants. Arch. Pharm. Res. 1999;22:18–24. doi: 10.1007/BF02976430. [DOI] [PubMed] [Google Scholar]
- 362.Prasad NS, Raghavendra R, Lokesh BR, Naidu KA. Spice phenolics inhibit human PMNL 5-lipoxygenase. Prostaglandins Leukot. Essent Fatty Acids. 2004;70:521–528. doi: 10.1016/j.plefa.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 363.O’Prey J, Brown J, Fleming J, Harrison PR. Effects of dietary flavonoids on major signal transduction pathways in human epithelial cells. Biochem. Pharmacol. 2003;66:2075–2088. doi: 10.1016/j.bcp.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 364.Hsieh RJ, German JB, Kinsella JE. Relative inhibitory potencies of flavonoids on 12-lipoxygenase of fish gill. Lipids. 1988;23:322–326. doi: 10.1007/BF02537342. [DOI] [PubMed] [Google Scholar]
- 365.Sekiya K, Okuda H, Arichi S. Selective inhibition of platelet lipoxygenase by esculetin. Biochim. Biophys Acta. 1982;713:68–72. [PubMed] [Google Scholar]
- 366.Sadik CD, Sies H, Schewe T. Inhibition of 15-lipoxygenases by flavonoids: structure-activity relations and mode of action. Biochem. Pharmacol. 2003;65:773–781. doi: 10.1016/s0006-2952(02)01621-0. [DOI] [PubMed] [Google Scholar]
- 367.Nakadate T, Yamamoto S, Aizu E, Kato R. Effects of flavonoids and antioxidants on 12-Otetradecanoyl- phorbol-13-acetate-caused epidermal ornithine decarboxylase induction and tumor promotion in relation to lipoxygenase inhibition by these compounds. Gann. 1984;75:214–222. [PubMed] [Google Scholar]
- 368.Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Nicolaides DN. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 369.Malterud KE, Rydland KM. Inhibitors of 15-lipoxygenase from orange peel. J. Agric. Food Chem. 2000;48:5576–5580. doi: 10.1021/jf000613v. [DOI] [PubMed] [Google Scholar]
- 370.Rui YC. Advances in pharmacological studies of silymarin. Mem. Inst Oswaldo Cruz. 1991;86:79–85. doi: 10.1590/s0074-02761991000600020. [DOI] [PubMed] [Google Scholar]
- 371.Robak J, Duniec Z, Rzadkowska-Bodalska H, Olechnowicz-Stepień W, Cisowski W. The effect of some flavonoids on non-enzymatic lipid oxidation and enzymatic oxidation of arachidonic acid. Pol. J. Pharmacol. Pharm. 1986;38:483–491. [PubMed] [Google Scholar]
- 372.Ferrándiz ML, Nair AG, Alcaraz MJ. Inhibition of sheep platelet arachidonate metabolism by flavonoids from Spanish and Indian medicinal herbs. Pharmazie. 1990;45:206–208. [PubMed] [Google Scholar]
- 373.Yoshimoto T, Furukawa M, Yamamoto S, Horie T, Watanabe-Kohno S. Flavonoids: potent inhibitors of arachidonate 5-lipoxygenase. Biochem. Biophys. Res. Commun. 1983;116:612–618. doi: 10.1016/0006-291x(83)90568-5. [DOI] [PubMed] [Google Scholar]
- 374.Schewe T, Kühn H, Sies H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002;132:1825–1829. doi: 10.1093/jn/132.7.1825. [DOI] [PubMed] [Google Scholar]
- 375.Oomah BD, Corbé A, Balasubramanian P. Antioxidant and anti-inflammatory activities of bean (Phaseolus vulgaris L.) hulls. J. Agric. Food. Chem. 2010;58:8225–8230. doi: 10.1021/jf1011193. [DOI] [PubMed] [Google Scholar]
- 376.Abad MJ, Bermejo P, Villar A. The activity of flavonoids extracted from Tanacetum microphyllum DC. (Compositae) on soybean lipoxygenase and prostaglandin synthetase. Gen. Pharmacol. 1995;26:815–819. doi: 10.1016/0306-3623(94)00242-f. [DOI] [PubMed] [Google Scholar]
- 377.Mattammal MB, Strong R, Lakshmi VM, Chung HD, Stephenson AH. Prostaglandin H synthetase-mediated metabolism of dopamine: implication for Parkinson’s disease. J. Neurochem. 1995;64:1645–1654. doi: 10.1046/j.1471-4159.1995.64041645.x. [DOI] [PubMed] [Google Scholar]
- 378.Zhou LE, Wang WJ, Bai JY, Cheng GF. Effects of ginkgolide B on arachidonic acid metabolizing enzymes and level of intracellular calcium in rat polymorphonuclear leukocytes. Yao Xue Xue Bao. 2001;36:92–95. [PubMed] [Google Scholar]
- 379.Kim HR, Pham HT, Ziboh VA. Flavonoids differentially inhibit guinea pig epidermal cytosolic phospholipase A2. Prostaglandins Leukot. Essent Fatty Acids. 2001;65:281–286. doi: 10.1054/plef.2001.0326. [DOI] [PubMed] [Google Scholar]
- 380.Grataroli R, Léonardi J, Charbonnier M, Lafont R, Lafont H, Nalbone G. Effects of dietary corn oil and salmon oil on lipids and prostaglandin E2 in rat gastric mucosa. Lipids. 1988;23:666–670. doi: 10.1007/BF02535665. [DOI] [PubMed] [Google Scholar]
- 381.Grataroli R, Vamecq J, Poupaert JH, Léonardi J, Termine E, Lafont H, Nalbone G. Effects of dietary n−6/n−3 ratios on lipid and prostaglandin E2 metabolism in rat gastric mucosa. J. Lipid Mediat. 1992;5:227–236. [PubMed] [Google Scholar]
- 382.Han CK, Son MJ, Chang HW, Chi YS, Park H, Kim HP. Inhibition of prostaglandin production by a structurally-optimized flavonoid derivative, 2′,4′,7-trimethoxyflavone and cellular action mechanism. Biol. Pharm Bullutin. 2005;28:1366–1370. doi: 10.1248/bpb.28.1366. [DOI] [PubMed] [Google Scholar]
- 383.Tanaka S, Sato T, Akimoto N, Yano M, Ito A. Prevention of UVB-induced photoinflammation and photoaging by a polymethoxy flavonoid, nobiletin, in human keratinocytes in vivo and in vitro. Biochem. Pharmacol. 2004;68:433–439. doi: 10.1016/j.bcp.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 384.Ticli FK, Hage LI, Cambraia RS, Pereira PS, Magro AJ, Fontes MR, Stábeli RG, Giglio JR, França SC, Soares AM, Sampaio SV. Rosmarinic acid, a new snake venom phospholipase A2 inhibitor from Cordia verbenacea (Boraginaceae): antiserum action potentiation and molecular interaction. Toxicon. 2005;46:318–327. doi: 10.1016/j.toxicon.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 385.Adam O. Dietary fatty acids and immune reactions in synovial tissue. Eur. J. Med. Res. 2003;8:381–387. [PubMed] [Google Scholar]
- 386.Shao ZH, Vanden Hoek TL, Li CQ, Schumacker PT, Becker LB, Chan KC, Qin Y, Yin JJ, Yuan CS. Synergistic effect of Scutellaria baicalensis and grape seed proanthocyanidins on scavenging reactive oxygen species in vitro. Am. J. Chin. Med. 2004;32:89–95. doi: 10.1142/S0192415X04001722. [DOI] [PubMed] [Google Scholar]
- 387.Dew TP, Day AJ, Morgan MR. Xanthine oxidase activity in vitro: effects of food extracts and components. J. Agric. Food Chem. 2005;53:6510–6515. doi: 10.1021/jf050716j. [DOI] [PubMed] [Google Scholar]
- 388.Lin JK, Chen PC, Ho CT, Lin-Shiau SY. Inhibition of xanthine oxidase and suppression of intracellular reactive oxygen species in HL-60 cells by theaflavin-3,3′-digallate, (−)-epigallocatechin-3-gallate, and propyl gallate. J. Agric. Food Chem. 2000;48:2736–2743. doi: 10.1021/jf000066d. [DOI] [PubMed] [Google Scholar]
- 389.Kurisawa M, Chung JE, Uyama H, Kobayashi S. Oxidative coupling of epigallocatechin gallate amplifies antioxidant activity and inhibits xanthine oxidase activity. Chem. Commun. 2004;7:294–295. doi: 10.1039/b312311a. [DOI] [PubMed] [Google Scholar]
- 390.van Hoorn DE, Nijveldt RJ, van Leeuwen PA, Hofman Z, M’Rabet L, de Bont DB, van Norren K. Accurate prediction of xanthine oxidase inhibition based on the structure of flavonoids. Eur. J. Pharmacol. 2002;451:111–118. doi: 10.1016/s0014-2999(02)02192-1. [DOI] [PubMed] [Google Scholar]
- 391.Selloum L, Reichl S, Müller M, Sebihi L, Arnhold J. Effects of flavonols on the generation of superoxide anion radicals by xanthine oxidase and stimulated neutrophils. Arch. Biochem. Biophys. 2001;395:49–56. doi: 10.1006/abbi.2001.2562. [DOI] [PubMed] [Google Scholar]
- 392.Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999;63:1787–1790. doi: 10.1271/bbb.63.1787. [DOI] [PubMed] [Google Scholar]
- 393.Iio M, Ono Y, Kai S, Fukumoto M. Effects of flavonoids on xanthine oxidation as well as on cytochrome c reduction by milk xanthine oxidase. J. Nutr. Sci. Vitaminol. 1986;32:635–642. doi: 10.3177/jnsv.32.635. [DOI] [PubMed] [Google Scholar]
- 394.Zhu JX, Wang Y, Kong LD, Yang C, Zhang X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2004;93:133–140. doi: 10.1016/j.jep.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 395.Moridani MY, Pourahmad J, Bui H, Siraki A, O’Brien PJ. Dietary flavonoid iron complexes as cytoprotective superoxide radical scavengers. Free Radic. Biol. Med. 2003;34:243–253. doi: 10.1016/s0891-5849(02)01241-8. [DOI] [PubMed] [Google Scholar]
- 396.Foppoli C, Coccia R, Cini C, Rosei MA. Catecholamines oxidation by xanthine oxidase. Biochim. Biophys Acta. 1997;1334:200–206. doi: 10.1016/s0304-4165(96)00093-1. [DOI] [PubMed] [Google Scholar]
- 397.Day AJ, Bao Y, Morgan MR, Williamson G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000;29:1234–1243. doi: 10.1016/s0891-5849(00)00416-0. [DOI] [PubMed] [Google Scholar]
- 398.Lin CM, Chen CS, Chen CT, Liang YC, Lin JK. Molecular modeling of flavonoids that inhibits xanthine oxidase. Biochem. Biophys. Res. Commun. 2002;294:167–172. doi: 10.1016/S0006-291X(02)00442-4. [DOI] [PubMed] [Google Scholar]
- 399.Beiler JM, Martin GJ. The inhibition of xanthine oxidase by flavonoids and related compounds. J. Biol. Chem. 1951;192:831–834. [PubMed] [Google Scholar]
- 400.Yoshizumi K, Nishioka N, Tsuji T. Xanthine oxidase inhibitory activity and hypouricemia effect of propolis in rats. Yakugaku Zasshi. 2005;125:315–321. doi: 10.1248/yakushi.125.315. [DOI] [PubMed] [Google Scholar]
- 401.Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73:S21–S29. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 402.Tapia A, Rodriguez J, Theoduloz C, Lopez S, Feresin GE, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Baccharis grisebachii. J. Ethnopharmacol. 2004;95:155–161. doi: 10.1016/j.jep.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 403.Huang Y, Tsang SY, Yao X, Chen ZY. Biological properties of baicalein in cardiovascular system. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005;5:177–184. doi: 10.2174/1568006043586206. [DOI] [PubMed] [Google Scholar]
- 404.Shieh DE, Liu LT, Lin CC. Antioxidant and free radical scavenging effects of baicalein, baicalin and wogonin. Anticancer Res. 2000;20:2861–2865. [PubMed] [Google Scholar]
- 405.Chang WS, Lee YJ, Lu FJ, Chiang HC. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13:2165–2170. [PubMed] [Google Scholar]
- 406.Varga Z, Ujhelyi L, Kiss A, Balla J, Czompa A, Antus S. Effect of silybin on phorbol myristate actetate-induced protein kinase C translocation NADPH oxidase activity and apoptosis in human neutrophils. Phytomedicine. 2004;11:206–212. doi: 10.1078/0944-7113-00358. [DOI] [PubMed] [Google Scholar]
- 407.Sheu SY, Lai CH, Chiang HC. Inhibition of xanthine oxidase by purpurogallin and silymarin group. Anticancer Res. 1998;18:263–267. [PubMed] [Google Scholar]
- 408.Łuczaj W, Skrzydlewska E. Antioxidative properties of black tea. Prev. Med. 2005;40:910–918. doi: 10.1016/j.ypmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 409.Wang Y, Zhu JX, Kong LD, Yang C, Cheng CH, Zhang X. Administration of procyanidins from grape seeds reduces serum uric acid levels and decreases hepatic xanthine dehydrogenase/oxidase activities in oxonate-treated mice. Basic Clin. Pharmacol. Toxicol. 2004;94:232–237. doi: 10.1111/j.1742-7843.2004.pto940506.x. [DOI] [PubMed] [Google Scholar]
- 410.Packer L, Rimbach G, Virgili F. Antioxidant activity and biologic properties of a procyanidin-rich extract from pine (Pinus maritima) bark, pycnogenol. Free Radic. Biol. Med. 1999;27:704–724. doi: 10.1016/s0891-5849(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 411.Moini H, Guo Q, Packer L. Enzyme inhibition and protein-binding action of the procyanidinrich french maritime pine bark extract, pycnogenol: effect on xanthine oxidase. J. Agric. Food Chem. 2000;48:5630–5639. doi: 10.1021/jf000618s. [DOI] [PubMed] [Google Scholar]
- 412.Moini H, Guo Q, Packe L. Xanthine oxidase and xanthine dehydrogenase inhibition by the procyanidin-rich French maritime pine bark extract, pycnogenol: a protein binding effect. Adv. Exp. Med. Biol. 2002;505:141–149. doi: 10.1007/978-1-4757-5235-9_13. [DOI] [PubMed] [Google Scholar]
- 413.Acquaviva R, Russo A, Galvano F, Galvano G, Barcellona ML, Li Volti G, Vanella A. Cyanidin and cyanidin 3-O-beta-d-glucoside as DNA cleavage protectors and antioxidants. Cell Biol. Toxicol. 2003;19:243–252. doi: 10.1023/b:cbto.0000003974.27349.4e. [DOI] [PubMed] [Google Scholar]
- 414.Cioffi G, D’Auria M, Braca A, Mendez J, Castillo A, Morelli I, de Simone F, de Tommasi N. Antioxidant and free-radical scavenging activity of constituents of the leaves of Tachigalia paniculata. J. Nat. Prod. 2002;65:1526–1529. doi: 10.1021/np0200764. [DOI] [PubMed] [Google Scholar]
- 415.Robak J, Gryglewski RJ. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988;37:837–841. doi: 10.1016/0006-2952(88)90169-4. [DOI] [PubMed] [Google Scholar]
- 416.Ignatov S, Shishniashvili D, Ge B, Scheller FW, Lisdat F. Amperometric biosensor based on a functionalized gold electrode for the detection of antioxidants. Biosens. Bioelectron. 2002;17:191–199. doi: 10.1016/s0956-5663(01)00283-4. [DOI] [PubMed] [Google Scholar]
- 417.Marfak A, Trouillas P, Allais DP, Champavier Y, Calliste CA, Duroux JL. Radiolysis of kaempferol in water/methanol mixtures. Evaluation of antioxidant activity of kaempferol and products formed. J. Agric. Food Chem. 2003;51:1270–1277. doi: 10.1021/jf020836g. [DOI] [PubMed] [Google Scholar]
- 418.Lu Y, Foo LY. Antioxidant activities of polyphenols from sage (Salvia officinalis) Food Chem. 2001;75:197–202. [Google Scholar]
- 419.Beyer G, Melzig MF. Effects of selected flavonoids and caffeic acid derivatives on hypoxanthine-xanthine oxidase-induced toxicity in cultivated human cells. Planta Med. 2003;69:1125–1129. doi: 10.1055/s-2003-45194. [DOI] [PubMed] [Google Scholar]
- 420.Park YH, Han DW, Suh H, Ryu GH, Hyon SH, Cho BK, Park JC. Protective effects of green tea polyphenol against reactive oxygen species-induced oxidative stress in cultured rat calvarial osteoblast. Cell Biol. Toxicol. 2003;19:325–337. doi: 10.1023/b:cbto.0000004986.51081.c5. [DOI] [PubMed] [Google Scholar]
- 421.Rah DK, Han DW, Baek HS, Hyon SH, Park JC. Prevention of reactive oxygen species-induced oxidative stress in human microvascular endothelial cells by green tea polyphenol. Toxicol. Lett. 2005;155:269–275. doi: 10.1016/j.toxlet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 422.Liu H, Yang XL, Wang Y, Tang XQ, Jiang DY, Xu HB. Protective effects of scutellarin on superoxide-induced oxidative stress in rat cortical synaptosomes. Acta Pharmacol. Sin. 2003;24:1113–1117. [PubMed] [Google Scholar]
- 423.Taubert D, Breitenbach T, Lazar A, Censarek P, Harlfinger S, Berkels R, Klaus W, Roesen R. Reaction rate constants of superoxide scavenging by plant antioxidants. Free Radic. Biol. Med. 2003;35:1599–1607. doi: 10.1016/j.freeradbiomed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 424.Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.) J. Agric Food Chem. 2005;53:2511–2517. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 425.Moridani MY, O’Brien PJ. Iron complexes of deferiprone and dietary plant catechols as cytoprotective superoxide radical scavengers. Biochem. Pharmacol. 2001;62:1579–1585. doi: 10.1016/s0006-2952(01)00821-8. [DOI] [PubMed] [Google Scholar]
- 426.Shi H, Zhao B, Xin W. Scavenging effects of baicalin on free radicals and its protection on erythrocyte membrane from free radical injury. Biochem. Mol. Biol. Int. 1995;35:981–994. [PubMed] [Google Scholar]
- 427.Toyo’oka T, Kashiwazaki T, Kato M. On-line screening methods for antioxidants scavenging superoxide anion radical and hydrogen peroxide by liquid chromatography with indirect chemiluminescence detection. Talanta. 2003;60:467–475. doi: 10.1016/S0039-9140(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 428.Stinefelt B, Leonard SS, Blemings KP, Shi X, Klandorf H. Free radical scavenging, DNA protection, and inhibition of lipid peroxidation mediated by uric acid. Ann. Clin. Lab. Sci. 2005;35:37–45. [PubMed] [Google Scholar]
- 429.Mishra B, Priyadarsini KI, Kumar MS, Unnikrishnan MK, Mohan H. Effect of O-glycosilation on the antioxidant activity and free radical reactions of a plant flavonoid, chrysoeriol. Bioorg. Med. Chem. 2003;11:2677–2685. doi: 10.1016/s0968-0896(03)00232-3. [DOI] [PubMed] [Google Scholar]
- 430.Candan F. Effect of Rhus coriaria L. (Anacardiaceae) on superoxide radical scavenging and xanthine oxidase activity. J. Enzyme Inhib. Med. Chem. 2003;18:59–62. doi: 10.1080/1475636031000069273. [DOI] [PubMed] [Google Scholar]
- 431.Ozgová S, Hermánek J, Gut I. Different antioxidant effects of polyphenols on lipid peroxidation and hydroxyl radicals in the NADPH-, Fe-ascorbate- and Fe-microsomal systems. Biochem. Pharmacol. 2003;66:1127–1137. doi: 10.1016/s0006-2952(03)00425-8. [DOI] [PubMed] [Google Scholar]
- 432.Wei IH, Wu YC, Wen CY, Shieh JY. Green tea polyphenol (−)-epigallocatechin gallate attenuates the neuronal NADPH-d/nNOS expression in the nodose ganglion of acute hypoxic rats. Brain Res. 2004;999:73–80. doi: 10.1016/j.brainres.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 433.Chen S, Deng PS, Swiderek K, Li M, Chan SI. Interaction of flavones and their bromoacetyl derivatives with NAD(P)H:quinone acceptor oxidoreductase. Mol. Pharmacol. 1995;47:419–424. [PubMed] [Google Scholar]
- 434.Liu XF, Liu ML, Iyanagi T, Legesse K, Lee TD, Chen SA. Inhibition of rat liver NAD(P)H:quinone acceptor oxidoreductase (DT-diaphorase) by flavonoids isolated from the Chinese herb scutellariae radix (Huang Qin) Mol. Pharmacol. 1990;37:911–915. [PubMed] [Google Scholar]
- 435.Tamura M, Kagawa S, Tsuruo Y, Ishimura K, Morita K. Effects of flavonoid compounds on the activity of NADPH diaphorase prepared from the mouse brain. Jpn. J. Pharmacol. 1994;65:371–373. doi: 10.1254/jjp.65.371. [DOI] [PubMed] [Google Scholar]
- 436.Terland O, Flatmark T, Tangerås A, Grønberg M. Dopamine oxidation generates an oxidative stress mediated by dopamine semiquinone and unrelated to reactive oxygen species. J. Mol. Cell Cardiol. 1997;29:1731–1738. doi: 10.1006/jmcc.1997.0412. [DOI] [PubMed] [Google Scholar]
- 437.Mocchegiani E, Bertoni-Freddari C, Marcellini F, Malavolta M. Brain, aging and neurodegeneration: role of zinc ion availability. Prog. Neurobiol. 2005;75:367–390. doi: 10.1016/j.pneurobio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 438.Kukreja RC, Loesser KE, Kearns AA, Naseem SA, Hess ML. Protective effects of histidine during ischemia-reperfusion in isolated perfused rat hearts. Am. J. Physiol. 1993;264:H1370–H1381. doi: 10.1152/ajpheart.1993.264.5.H1370. [DOI] [PubMed] [Google Scholar]
- 439.Obata T, Aomine M, Yamanaka Y. Protective effect of histidine on iron (II)-induced hydroxyl radical generation in rat hearts. J. Physiol Paris. 1999;93:213–218. doi: 10.1016/s0928-4257(99)80153-3. [DOI] [PubMed] [Google Scholar]
- 440.Obata T, Inada T. Protective effect of histidine on MPP+-induced hydroxyl radical generation in rat striatum. Brain Res. 1999;817:206–208. doi: 10.1016/s0006-8993(98)01225-6. [DOI] [PubMed] [Google Scholar]
- 441.Lundvig D, Lindersson E, Jensen PH. Pathogenic effects of alpha-synuclein aggregation. Brain Res. Mol. Brain Res. 2005;134:3–17. doi: 10.1016/j.molbrainres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 442.O’Dowd Y, Driss F, Dang PM, Elbim C, Gougerot-Pocidalo MA, Pasquier C, El-Benna J. Antioxidant effect of hydroxytyrosol, a polyphenol from olive oil: scavenging of hydrogen peroxide but not superoxide anion produced by human neutrophils. Biochem. Pharmacol. 2004;68:2003–2008. doi: 10.1016/j.bcp.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 443.Everse J, Coates PW. Role of peroxidases in Parkinson disease: a hypothesis. Free Radic. Biol. Med. 2005;38:1296–1310. doi: 10.1016/j.freeradbiomed.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 444.Thiruchelvam M, Prokopenko O, Cory-Slechta DA, Buckley B, Mirochnitchenko O. Overexpression of superoxide dismutase or glutathione peroxidase protects against the paraquat + maneb-induced Parkinson disease phenotype. J. Biol. Chem. 2005;280:22530–22539. doi: 10.1074/jbc.M500417200. [DOI] [PubMed] [Google Scholar]
- 445.Fornai F, Carrì MT, Ferri A, Paolucci E, Prisco S, Bernardi G, Rotilio G, Mercuri NB. Resistance to striatal dopamine depletion induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice expressing human mutant Cu,Zn superoxide dismutase. Neurosci. Lett. 2002;325:124–128. doi: 10.1016/s0304-3940(02)00252-5. [DOI] [PubMed] [Google Scholar]
- 446.Zhao Y, Gao Z, Li H, Xu H. Hemin/nitrite/H2O2 induces brain homogenate oxidation and nitration: effects of some flavonoids. Biochim. Biophys Acta. 2004;1675:105–112. doi: 10.1016/j.bbagen.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 447.Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4- phenyl-1,2,5,6-tetrahydropyridine. J. Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Biswas SK, McClure D, Jimenez LA, Megson IL, Rahman I. Curcumin induces glutathione biosynthesis and inhibits NF-kappaB activation and interleukin-8 release in alveolar epithelial cells: mechanism of free radical scavenging activity. Antioxid. Redox Signal. 2005;7:32–41. doi: 10.1089/ars.2005.7.32. [DOI] [PubMed] [Google Scholar]
- 449.Ferrari CK. Functional foods, herbs and nutraceuticals: towards biochemical mechanisms of healthy aging. Biogerontology. 2004;5:275–289. doi: 10.1007/s10522-004-2566-z. [DOI] [PubMed] [Google Scholar]
- 450.Mansouri A, Makris DP, Kefalas P. Determination of hydrogen peroxide scavenging activity of cinnamic and benzoic acids employing a highly sensitive peroxyoxalate chemiluminescencebased assay: structure-activity relationships. J. Pharm. Biomed. Anal. 2005;39:22–26. doi: 10.1016/j.jpba.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 451.Yilmaz Y, Toledo RT. Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food Chem. 2004;52:255–260. doi: 10.1021/jf030117h. [DOI] [PubMed] [Google Scholar]
- 452.Sroka Z, Cisowski W. Hydrogen peroxide scavenging, antioxidant and anti-radical activity of some phenolic acids. Food Chem. Toxicol. 2003;41:753–758. doi: 10.1016/s0278-6915(02)00329-0. [DOI] [PubMed] [Google Scholar]
- 453.Vanzani P, Rossetto M, Rigo A, Vrhovsek U, Mattivi F, D’Amato E, Scarpa M. Major phytochemicals in apple cultivars: contribution to peroxyl radical trapping efficiency. J. Agric. Food Chem. 2005;53:3377–3382. doi: 10.1021/jf040482o. [DOI] [PubMed] [Google Scholar]
- 454.López-Alarcón C, Lissi E. Interaction of pyrogallol red with peroxyl radicals. A basis for a simple methodology for the evaluation of antioxidant capabilities. Free Radic. Res. 2005;39:729–736. doi: 10.1080/10715760500143452. [DOI] [PubMed] [Google Scholar]
- 455.Mazzio EA, Reams RR, Soliman KF. The role of oxidative stress, impaired glycolysis and mitochondrial respiratory redox failure in the cytotoxic effects of 6-hydroxydopamine in vitro. Brain Res. 2004;1004:29–44. doi: 10.1016/j.brainres.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 456.Kabuto H, Nishizawa M, Tada M, Higashio C, Shishibori T, Kohno M. Zingerone [4-(4-hydroxy-3-methoxyphenyl)-2-butanone] prevents 6-hydroxydopamine-induced dopamine depression in mouse striatum and increases superoxide scavenging activity in serum. Neurochem. Res. 2005;30:325–332. doi: 10.1007/s11064-005-2606-3. [DOI] [PubMed] [Google Scholar]
- 457.Caillet S, Yu H, Lessard S, Lamoureux G, Ajdukovic D, Lacroix M. Fenton reaction applied for screening natural antioxidants. Food Chem. 2007;100:542–552. [Google Scholar]
- 458.van Acker SA, van den Berg DJ, Tromp MN, Griffioen DH, van Bennekom WP, van der Vijgh WJ, Bast A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996;20:331–342. doi: 10.1016/0891-5849(95)02047-0. [DOI] [PubMed] [Google Scholar]
- 459.Brown JE, Khodr H, Hider RC, Rice-Evans CA. Structural dependence of flavonoid interactions with Cu2+ ions: implications for their antioxidant properties. Biochem. J. 1998;330:1173–1178. doi: 10.1042/bj3301173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Arora A, Nair MG, Strasburg GM. Structure-activity relationships for antioxidant activities of a series of flavonoids in a liposomal system. Free Radic. Biol. Med. 1998;24:1355–1363. doi: 10.1016/s0891-5849(97)00458-9. [DOI] [PubMed] [Google Scholar]
- 461.Fernandez MT, Mira ML, Florêncio MH, Jennings KR. Iron and copper chelation by flavonoids: an electrospray mass spectrometry study. J. Inorg. Biochem. 2002;92:105–111. doi: 10.1016/s0162-0134(02)00511-1. [DOI] [PubMed] [Google Scholar]
- 462.Cheng IF, Breen K. On the ability of four flavonoids, baicilein, luteolin, naringenin, and quercetin, to suppress the Fenton reaction of the iron-ATP complex. Biometals. 2000;13:77–83. doi: 10.1023/a:1009229429250. [DOI] [PubMed] [Google Scholar]
- 463.Aherne SA, O’Brien NM. Mechanism of protection by the flavonoids, quercetin and rutin, against tert-butylhydroperoxide- and menadione-induced DNA single strand breaks in Caco-2 cells. Free Radic. Biol. Med. 2000;29:507–514. doi: 10.1016/s0891-5849(00)00360-9. [DOI] [PubMed] [Google Scholar]
- 464.Mahakunakorn P, Tohda M, Murakami Y, Matsumoto K, Watanabe H. Antioxidant and free radical-scavenging activity of Choto-san and its related constituents. Biol. Pharm Bullutin. 2004;27:38–46. doi: 10.1248/bpb.27.38. [DOI] [PubMed] [Google Scholar]
- 465.Yoshida H, Ishikawa T, Hosoai H, Suzukawa M, Ayaori M, Hisada T, Sawada S, Yonemura A, Higashi K, Ito T, Nakajima K, Yamashita T, Tomiyasu K, Nishiwaki M, Ohsuzu F, Nakamura H. Inhibitory effect of tea flavonoids on the ability of cells to oxidize low density lipoprotein. Biochem. Pharmacol. 1999;58:1695–1703. doi: 10.1016/s0006-2952(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 466.O’Coinceanainn M, Bonnely S, Baderschneider B, Hynes MJ. Reaction of iron(III) with theaflavin: complexation and oxidative products. J. Inorg. Biochem. 2004;98:657–663. doi: 10.1016/j.jinorgbio.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 467.Fraga CG, Oteiza PI. Iron toxicity and antioxidant nutrients. Toxicology. 2002;180:23–32. doi: 10.1016/s0300-483x(02)00379-7. [DOI] [PubMed] [Google Scholar]
- 468.Hynes MJ, Coinceanainn MO. The kinetics and mechanisms of the reaction of iron(III) with gallic acid, gallic acid methyl ester and catechin. J. Inorg. Biochem. 2001;85:131–142. doi: 10.1016/s0162-0134(01)00205-7. [DOI] [PubMed] [Google Scholar]
- 469.Borsari M, Gabbi C, Ghelfi F, Grandi R, Saladini M, Severi S, Borella F. Silybin, a new iron-chelating agent. J. Inorg. Biochem. 2001;85:123–129. doi: 10.1016/s0162-0134(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 470.Kostyuk VA, Potapovich AI. Antiradical and chelating effects in flavonoid protection against silica-induced cell injury. Arch. Biochem. Biophys. 1998;355:43–48. doi: 10.1006/abbi.1998.0708. [DOI] [PubMed] [Google Scholar]
- 471.Schipper HM. Heme oxygenase expression in human central nervous system disorders. Free Radic. Biol. Med. 2004;37:1995–2011. doi: 10.1016/j.freeradbiomed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 472.Abate A, Yang G, Wong RJ, Schroder H, Stevenson DK, Dennery PA. Apigenin decreases hemin-mediated heme oxygenase-1 induction. Free Radic. Biol. Med. 2005;39:711–718. doi: 10.1016/j.freeradbiomed.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 473.Kantengwa S, Polla BS. Flavonoids, but not protein kinase C inhibitors, prevent stress protein synthesis during erythrophagocytosis. Biochem. Biophys. Res. Commun. 1991;180:308–314. doi: 10.1016/s0006-291x(05)81293-8. [DOI] [PubMed] [Google Scholar]
- 474.Zatta P, Lucchini R, van Rensburg SJ, Taylor A. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res Bullutin. 2003;62:15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 475.Schweizer U, Bräuer AU, Köhrle J, Nitsch R, Savaskan NE. Selenium and brain function: a poorly recognized liaison. Brain Res. Brain Res. Rev. 2004;45:164–178. doi: 10.1016/j.brainresrev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 476.Johnson S. Is Parkinson’s disease the heterozygote form of Wilson’s disease: PD = 1/2 WD? Med Hypotheses. 2001;56:171–173. doi: 10.1054/mehy.2000.1134. [DOI] [PubMed] [Google Scholar]
- 477.Sziráki I, Mohanakumar KP, Rauhala P, Kim HG, Yeh KJ, Chiueh CC. Manganese: a transition metal protects nigrostriatal neurons from oxidative stress in the iron-induced animal model of Parkinsonism. Neuroscience. 1998;85:1101–1111. doi: 10.1016/s0306-4522(97)00660-x. [DOI] [PubMed] [Google Scholar]
- 478.Cuajungco MP, Lees GJ. Zinc metabolism in the brain: relevance to human neurodegenerative disorders. Neurobiol. Dis. 1997;4:137–169. doi: 10.1006/nbdi.1997.0163. [DOI] [PubMed] [Google Scholar]
- 479.Bush AI. Metals and neuroscience. Curr. Opin. Chem. Biol. 2000;4:184–191. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 480.Zago MP, Mackenzie GG, Adamo AM, Keen CL, Oteiza PI. Differential modulation of MAP kinases by zinc deficiency in IMR-32 cells: role of H2O2. Antioxid. Redox Signal. 2005;7:1773–1782. doi: 10.1089/ars.2005.7.1773. [DOI] [PubMed] [Google Scholar]
- 481.Blonska M, Bronikowska J, Pietsz G, Czuba ZP, Scheller S, Krol W. Effects of ethanol extract of propolis (EEP) and its flavones on inducible gene expression in J774A.1 macrophages. J. Ethnopharmacol. 2004;91:25–30. doi: 10.1016/j.jep.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 482.Cho H, Yun CW, Park WK, Kong JY, Kim KS, Park Y, Lee S, Kim BK. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS.; by chrysin derivatives. Pharmacol. Res. 2004;49:37–43. doi: 10.1016/s1043-6618(03)00248-2. [DOI] [PubMed] [Google Scholar]
- 483.Chen JC, Ho FM, Pei-Dawn LC, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen TW, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur. J. Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 484.Martínez-Flórez S, Gutiérrez-Fernández B, Sánchez-Campos S, González-Gallego J, Tuñón MJ. Quercetin attenuates nuclear factor-kappaB activation and nitric oxide production in interleukin-1beta-activated rat hepatocytes. J. Nutr. 2005;135:1359–1365. doi: 10.1093/jn/135.6.1359. [DOI] [PubMed] [Google Scholar]
- 485.Jung WJ, Sung MK. Effects of major dietary antioxidants on inflammatory markers of RAW 264.7 macrophages. Biofactors. 2004;21:113–117. doi: 10.1002/biof.552210122. [DOI] [PubMed] [Google Scholar]
- 486.Cho SY, Park SJ, Kwon MJ, Jeong TS, Bok SH, Choi WY, Jeong WI, Ryu SY, Do SH, Lee CS, Song JC, Jeong KS. Quercetin suppresses proinflammatory cytokines production through MAP kinases andNF-kappaB pathway in lipopolysaccharide-stimulated macrophage. Mol. Cell Biochem. 2003;243:153–160. doi: 10.1023/a:1021624520740. [DOI] [PubMed] [Google Scholar]
- 487.van Meeteren ME, Hendriks JJ, Dijkstra CD, van Tol EA. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem. Pharmacol. 2004;67:967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 488.Scuro LS, Simioni PU, Grabriel DL, Saviani EE, Modolo LV, Tamashiro WM, Salgado I. Suppression of nitric oxide production in mouse macrophages by soybean flavonoids accumulated in response to nitroprusside and fungal elicitation. BMC Biochem. 2004;21:5. doi: 10.1186/1471-2091-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Hu C, Kitts DD. Luteolin and luteolin-7-O-glucoside from dandelion flower suppress iNOS and COX-2 in RAW264.7 cells. Mol. Cell Biochem. 2004;265:107–113. doi: 10.1023/b:mcbi.0000044364.73144.fe. [DOI] [PubMed] [Google Scholar]
- 490.Kim SJ, Park H, Kim HP. Inhibition of nitric oxide production from lipopolysaccharidetreated RAW 264.7 cells by synthetic flavones: structure-activity relationship and action mechanism. Arch. Pharm. Res. 2004;27:937–943. doi: 10.1007/BF02975847. [DOI] [PubMed] [Google Scholar]
- 491.Matsuda H, Morikawa T, Ando S, Toguchida I, Yoshikawa M. Structural requirements of flavonoids for nitric oxide production inhibitory activity and mechanism of action. Bioorg. Med. Chem. 2003;11:1995–2000. doi: 10.1016/s0968-0896(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 492.Rojas J, Payá M, Devesa I, Dominguez JN, Ferrándiz ML. Therapeutic administration of 3,4,5-trimethoxy-4′-fluorochalcone, a selective inhibitor of iNOS expression, attenuates the development of adjuvant-induced arthritis in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2003;368:225–233. doi: 10.1007/s00210-003-0780-x. [DOI] [PubMed] [Google Scholar]
- 493.Ko HH, Tsao LT, Yu KL, Liu CT, Wang JP, Lin CN. Structure-activity relationship studies on chalcone derivatives. The potent inhibition of chemical mediators release. Bioorg. Med. Chem. 2003;11:105–111. doi: 10.1016/s0968-0896(02)00312-7. [DOI] [PubMed] [Google Scholar]
- 494.Chiu FL, Lin JK. HPLC analysis of naturally occurring methylated catechins, 3″- and 4″-methyl-epigallocatechin gallate, in various fresh tea leaves and commercial teas and their potent inhibitory effects on inducible nitric oxide synthase in macrophages. J. Agric. Food Chem. 2005;53:7035–7042. doi: 10.1021/jf0507442. [DOI] [PubMed] [Google Scholar]
- 495.Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I. Neuroprotective effects of (−)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J. 2005;19:258–260. doi: 10.1096/fj.04-2806fje. [DOI] [PubMed] [Google Scholar]
- 496.Singh R, Ahmed S, Islam N, Goldberg VM, Haqqi TM. Epigallocatechin-3-gallate inhibits interleukin-1beta-induced expression of nitric oxide synthase and production of nitric oxide in human chondrocytes: suppression of nuclear factor kappaB activation by degradation of the inhibitor of nuclear factor kappaB. Arthritis Rheum. 2002;46:2079–2086. doi: 10.1002/art.10443. [DOI] [PubMed] [Google Scholar]
- 497.Lee SH, Seo GS, Sohn DH. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW 264.7 cells. Biochem. Biophys. Res. Commun. 2004;323:125–132. doi: 10.1016/j.bbrc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 498.Sautebin L, Rossi A, Serraino I, Dugo P, Di Paola R, Mondello L, Genovese T, Britti D, Peli A, Dugo G, Caputi AP, Cuzzocrea S. Effect of anthocyanins contained in a blackberry extract on the circulatory failure and multiple organ dysfunction caused by endotoxin in the rat. Planta Med. 2004;70:745–752. doi: 10.1055/s-2004-827206. [DOI] [PubMed] [Google Scholar]
- 499.Liu H, Yang X, Tang R, Liu J, Xu H. Effect of scutellarin on nitric oxide production in early stages of neuron damage induced by hydrogen peroxide. Pharmacol. Res. 2005;51:205–210. doi: 10.1016/j.phrs.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 500.Lin CM, Huang ST, Liang YC, Lin MS, Shih CM, Chang YC, Chen TY, Chen CT. Isovitexin suppresses lipopolysaccharide-mediated inducible nitric oxide synthase through inhibition of NF-kappa B in mouse macrophages. Planta Med. 2005;71:748–753. doi: 10.1055/s-2005-871287. [DOI] [PubMed] [Google Scholar]
- 501.Sakata K, Hirose Y, Qiao Z, Tanaka T, Mori H. Inhibition of inducible isoforms of cyclooxygenase and nitric oxide synthase by flavonoid hesperidin in mouse macrophage cell line. Cancer Lett. 2003;199:139–145. doi: 10.1016/s0304-3835(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 502.Kanno S, Shouji A, Tomizawa A, Hiura T, Osanai Y, Ujibe M, Obara Y, Nakahata N, Ishikawa M. Inhibitory effect of naringin on lipopolysaccharide (LPS)-induced endotoxin shock in mice and nitric oxide production in RAW 264.7 macrophages. Life Sci. 2006;78:673–681. doi: 10.1016/j.lfs.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 503.Lin HY, Shen SC, Chen YC. Anti-inflammatory effect of heme oxygenase 1: glycosylation and nitric oxide inhibition in macrophages. J. Cell Physiol. 2005;202:579–590. doi: 10.1002/jcp.20160. [DOI] [PubMed] [Google Scholar]
- 504.Chen CJ, Raung SL, Liao SL, Chen SY. Inhibition of inducible nitric oxide synthase expression by baicalein in endotoxin/cytokine-stimulated microglia. Biochem. Pharmacol. 2004;67:957–965. doi: 10.1016/j.bcp.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 505.Lin HY, Juan SH, Shen SC, Hsu FL, Chen YC. Inhibition of lipopolysaccharideinduced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003;66:1821–1832. doi: 10.1016/s0006-2952(03)00422-2. [DOI] [PubMed] [Google Scholar]
- 506.Schümann J, Prockl J, Kiemer AK, Vollmar AM, Bang R, Tiegs G. Silibinin protects mice from T cell-dependent liver injury. J. Hepatol. 2003;39:333–340. doi: 10.1016/s0168-8278(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 507.Kang JS, Jeon YJ, Kim HM, Han SH, Yang KH. Inhibition of inducible nitric-oxide synthase expression by silymarin in lipopolysaccharide-stimulated macrophages. J. Pharmacol. Exp. Ther. 2002;302:138–144. doi: 10.1124/jpet.302.1.138. [DOI] [PubMed] [Google Scholar]
- 508.Banerjee T, van der Vliet A, Ziboh VA. Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot. Essent Fatty Acids. 2002;66:485–492. doi: 10.1054/plef.2002.0387. [DOI] [PubMed] [Google Scholar]
- 509.Takahashi T, Takasuka N, Iigo M, Baba M, Nishino H, Tsuda H, Okuyama T. Isoliquiritigenin, a flavonoid from licorice, reduces prostaglandin E2 and nitric oxide, causes apoptosis, and suppresses aberrant crypt foci development. Cancer Sci. 2004;95:448–453. doi: 10.1111/j.1349-7006.2004.tb03230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Lee H, Kim YO, Kim H, Kim SY, Noh HS, Kang SS, Cho GJ, Choi WS, Suk K. lavonoid wogonin from medicinal herb is neuroprotective by inhibiting inflammatory activation of microglia. FASEB J. 2003;17:1943–1944. doi: 10.1096/fj.03-0057fje. [DOI] [PubMed] [Google Scholar]
- 511.Markovic M, Miljkovic Dj, Trajkovic V. Regulation of inducible nitric oxide synthase by cAMP-elevating phospho-diesterase inhibitors. Curr. Drug Targets Inflamm Allergy. 2003;2:63–79. doi: 10.2174/1568010033344471. [DOI] [PubMed] [Google Scholar]
- 512.Hulley P, Hartikka J, Abdel’Al S, Engels P, Buerki HR, Wiederhold KH, Müller T, Kelly P, Lowe D, Lübbert H. Inhibitors of type IV phosphodiesterases reduce the toxicity of MPTP in substantia nigra neurons in vivo. Eur. J. Neurosci. 1995;7:2431–2440. doi: 10.1111/j.1460-9568.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 513.Yu SM, Cheng ZJ, Kuo SC. Endothelium-dependent relaxation of rat aorta by butein, a novel cyclic AMP-specific phosphodiesterase inhibitor. Eur. J. Pharmacol. 1995;280:69–77. doi: 10.1016/0014-2999(95)00190-v. [DOI] [PubMed] [Google Scholar]
- 514.Girotti C, Ginet M, Demarne FC, Lagarde M, Géloën A. Lipolytic activity of cirsimarin extracted from Microtea debilis. Planta Med. 2005;71:1170–1172. doi: 10.1055/s-2005-873146. [DOI] [PubMed] [Google Scholar]
- 515.Dell’agli M, Bellosta S, Rizzi L, Galli GV, Canavesi M, Rota F, Parente R, Bosisio E, Romeo S. A structure-activity study for the inhibition of metalloproteinase-9 activity and gene expression by analogues of gallocatechin-3-gallate. Cell Mol. Life Sci. 2005;62:2896–2903. doi: 10.1007/s00018-005-5422-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 516.Ko WC, Shih CM, Lai YH, Chen JH, Huang HL. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem. Pharmacol. 2004;68:2087–2094. doi: 10.1016/j.bcp.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 517.Paliyath G, Poovaiah BW. Identification of naturally occurring calmodulin inhibitors in plants and their effects on calcium- and calmodulin-promoted protein phosphorylation. Plant Cell Physiol. 1985;26:201–209. [PubMed] [Google Scholar]
- 518.Campos-Toimil M, Lugnier C, Droy-Lefaix MT, Takeda K. Inhibition of type 4 phosphodiesterase by rolipram and Ginkgo biloba extract (EGb 761) decreases agonist-induced rises in internal calcium in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2000;20:E34–E40. doi: 10.1161/01.atv.20.9.e34. [DOI] [PubMed] [Google Scholar]
- 519.Nichols MR, Morimoto BH. Differential inhibition of multiple cAMP phosphodiesterase isozymes by isoflavones and tyrphostins. Mol. Pharmacol. 2000;57:738–745. doi: 10.1124/mol.57.4.738. [DOI] [PubMed] [Google Scholar]
- 520.Satake N, Imanishi M, Shibata S. Increased nitroglycerin-induced relaxation by genistein in rat aortic rings. Eur. J. Pharmacol. 1999;377:193–197. doi: 10.1016/s0014-2999(99)00412-4. [DOI] [PubMed] [Google Scholar]
- 521.Saponara R, Bosisio E. Inhibition of cAMP-phosphodiesterase by biflavones of Ginkgo biloba in rat adipose tissue. J. Nat. Prod. 1998;61:1386–1387. doi: 10.1021/np970569m. [DOI] [PubMed] [Google Scholar]
- 522.Kuppusamy UR, Das NP. Effects of flavonoids on cyclic AMP phosphodiesterase and lipid mobilization in rat adipocytes. Biochem. Pharmacol. 1992;44:1307–1315. doi: 10.1016/0006-2952(92)90531-m. [DOI] [PubMed] [Google Scholar]
- 523.Orallo F, Camiña M, Alvarez E, Basaran H, Lugnier C. Implication of cyclic nucleotide phosphodiesterase inhibition in the vasorelaxant activity of the citrus-fruits flavonoid (+/−)-naringenin. Planta Med. 2005;71:99–107. doi: 10.1055/s-2005-837774. [DOI] [PubMed] [Google Scholar]
- 524.Kumar S, Sarkar A, Sundar D. Controlling aggregation propensity in A53T mutant of alphasynuclein causing Parkinson’s disease. Biochem. Biophys. Res. Commun. 2009;387:305–309. doi: 10.1016/j.bbrc.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 525.McCormack AL, Mak SK, Shenasa M, Langston WJ, Forno LS, Di Monte DA. Pathologic modifications of alpha-synuclein in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated squirrel monkeys. J. Neuropathol. Exp. Neurol. 2008;67:793–802. doi: 10.1097/NEN.0b013e318180f0bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Ulusoy A, Febbraro F, Jensen PH, Kirik D, Romero-Ramos M. Co-expression of C-terminal truncated alpha-synuclein enhances full-length alpha-synuclein-induced pathology. Eur. J. Neurosci. 2010;32:409–422. doi: 10.1111/j.1460-9568.2010.07284.x. [DOI] [PubMed] [Google Scholar]
- 527.Paxinou E, Chen Q, Weisse M, Giasson BI, Norris EH, Rueter SM, Trojanowski JQ, Lee VM, Ischiropoulos H. Induction of alpha-synuclein aggregation by intracellular nitrative insult. J. Neurosci. 2001;21:8053–8061. doi: 10.1523/JNEUROSCI.21-20-08053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 528.Santner A, Uversky VN. Metalloproteomics and metal toxicology of α-synuclein. Metallomics. 2010;2:378–392. doi: 10.1039/b926659c. [DOI] [PubMed] [Google Scholar]
- 529.Perez RG, Hastings TG. Could a loss of alpha-synuclein function put dopaminergic neurons at risk? J. Neurochem. 2004;89:1318–1324. doi: 10.1111/j.1471-4159.2004.02423.x. [DOI] [PubMed] [Google Scholar]
- 530.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar alpha-synuclein is sensitive to Parkinson’s disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 531.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. Alpha-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int J. Biochem. Cell Biol. 2009;41:2015–2024. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 532.Reindl W, Yuan J, Krämer A, Strebhardt K, Berg T. Inhibition of polo-like kinase 1 by blocking polo-box domain-dependent protein-protein interactions. Chem Biol. 2008;15:459–466. doi: 10.1016/j.chembiol.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 533.Mbefo MK, Paleologou KE, Boucharaba A, Oueslati A, Schell H, Fournier M, Olschewski D, Yin G, Zweckstetter M, Masliah E, Kahle PJ, Hirling H, Lashuel HA. Phosphorylation of synucleins by members of the Polo-like kinase family. J. Biol. Chem. 2010;285:2807–2822. doi: 10.1074/jbc.M109.081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 534.Cozza G, Bonvini P, Zorzi E, Poletto G, Pagano MA, Sarno S, Donella-Deana A, Zagotto G, Rosolen A, Pinna LA, Meggio F, Moro S. Identification of ellagic acid as potent inhibitor of protein kinase CK2: a successful example of a virtual screening application. J. Med. Chem. 2006;49:2363–2366. doi: 10.1021/jm060112m. [DOI] [PubMed] [Google Scholar]
- 535.Chen L, Feany MB. Alpha-synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 2005;8:657–663. doi: 10.1038/nn1443. [DOI] [PubMed] [Google Scholar]
- 536.Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci. Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 537.Mandel SA, Fishman-Jacob T, Youdim MB. Modeling sporadic Parkinson’s disease by silencing the ubiquitin E3 ligase component, SKP1A. Parkinsonism Relat. Disord. 2009;15:S148–S151. doi: 10.1016/S1353-8020(09)70803-X. [DOI] [PubMed] [Google Scholar]
- 538.Yasuda T, Mochizuki H. The regulatory role of α-synuclein and parkin in neuronal cell apoptosis; possible implications for the pathogenesis of Parkinson’s disease. Apoptosis. 2010;15:1312–1321. doi: 10.1007/s10495-010-0486-8. [DOI] [PubMed] [Google Scholar]
- 539.Xie W, Li X, Li C, Zhu W, Jankovic J, Le W. Proteasome inhibition modeling nigral neuron degeneration in Parkinson’s disease. J. Neurochem. 2010;115:188–199. doi: 10.1111/j.1471-4159.2010.06914.x. [DOI] [PubMed] [Google Scholar]
- 540.Hyun DH, Lee M, Halliwell B, Jenner P. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J. Neurochem. 2003;86:363–373. doi: 10.1046/j.1471-4159.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 541.Zhu W, Xie W, Pan T, Xu P, Fridkin M, Zheng H, Jankovic J, Youdim MB, Le W. Prevention and restoration of lactacystin-induced nigrostriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J. 2007;21:3835–3844. doi: 10.1096/fj.07-8386com. [DOI] [PubMed] [Google Scholar]
- 542.Zhou ZD, Lim TM. Dopamine (DA) induced irreversible proteasome inhibition via DA derived quinones. Free Radic. Res. 2009;43:417–430. doi: 10.1080/10715760902801533. [DOI] [PubMed] [Google Scholar]
- 543.McNaught KS, Jnobaptiste R, Jackson T, Jengelley TA. The pattern of neuronal loss and survival may reflect differential expression of proteasome activators in Parkinson’s disease. Synapse. 2010;64:241–250. doi: 10.1002/syn.20719. [DOI] [PubMed] [Google Scholar]
- 544.Olanow CW. The pathogenesis of cell death in Parkinson’s disease—2007. Mov. Disord. 2007;22:S335–S342. doi: 10.1002/mds.21675. [DOI] [PubMed] [Google Scholar]
- 545.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 546.Lee JY, Nagano Y, Taylor JP, Lim KL, Yao TP. Disease-causing mutations in parkin impair mitochondrial ubiquitination, aggregation, and HDAC6-dependent mitophagy. J. Cell Biol. 2010;189:671–679. doi: 10.1083/jcb.201001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 547.Renna M, Jimenez-Sanchez M, Sarkar S, Rubinsztein DC. Chemical inducers of autophagy that enhance the clearance of mutant proteins in neurodegenerative diseases. J. Biol. Chem. 2010;285:11061–11067. doi: 10.1074/jbc.R109.072181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 548.Cherra SJ, III, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, Chu CT. Regulation of the autophagy protein LC3 by phosphorylation. J. Cell Biol. 2010;190:533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Sevlever D, Jiang P, Yen SH. Cathepsin D is the main lysosomal enzyme involved in the degradation of alpha-synuclein and generation of its carboxy-terminally truncated species. Biochemistry. 2008;47:9678–9687. doi: 10.1021/bi800699v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Murgas Torrazza R, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA. Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J. Nutr. 2010;140:2145–5212. doi: 10.3945/jn.110.128421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Bauchart-Thevret C, Cui L, Wu G, Burrin DG. Arginine-induced stimulation of protein synthesis and survival in IPEC-J2 cells is mediated by mTOR but not nitric oxide. Am. J. Physiol. Endocrinol. Metab. 2010;299:E899–E909. doi: 10.1152/ajpendo.00068.2010. [DOI] [PubMed] [Google Scholar]
- 552.Kim E. Mechanisms of amino acid sensing in mTOR signaling pathway. Nutr. Res. Pract. 2009;3:64–71. doi: 10.4162/nrp.2009.3.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 553.Machiya Y, Hara S, Arawaka S, Fukushima S, Sato H, Sakamoto M, Koyama S, Kato T. Phosphorylated {alpha}-Synuclein at Ser-129 Is Targeted to the Proteasome Pathway in a Ubiquitin-independent Manner. Biol. Chem. 2010;285:40732–40744. doi: 10.1074/jbc.M110.141952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 554.Crews L, Spencer B, Desplats P, Patrick C, Paulino A, Rockenstein E, Hansen L, Adame A, Galasko D, Masliah E. Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS One. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 555.Tang FY, Chiang EP, Pai MH. Consumption of S-Allylcysteine Inhibits the Growth of Human Non-Small-Cell Lung Carcinoma in a Mouse Xenograft Model. J Agric Food Chem. 2010 doi: 10.1021/jf102539k. in press. [DOI] [PubMed] [Google Scholar]
- 556.Petrovski G, Das DK. Does autophagy take a front seat in lifespan extension? J. Cell Mol. Med. 2010;14:2543–2551. doi: 10.1111/j.1582-4934.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Zhou H, Luo Y, Huang S. Updates of mTOR inhibitors. Anticancer Agents Med. Chem. 2010;10:571–581. doi: 10.2174/187152010793498663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 558.Lee YK, Lee WS, Kim GS, Park OJ. Anthocyanins are novel AMPKα1 stimulators that suppress tumor growth by inhibiting mTOR phosphorylation. Oncol. Rep. 2010;24:1471–1477. doi: 10.3892/or_00001007. [DOI] [PubMed] [Google Scholar]
- 559.Lee YK, Park SY, Kim YM, Kim DC, Lee WS, Surh YJ, Park OJ. Suppression of mTOR via Akt-dependent and -independent mechanisms in selenium-treated colon cancer cells: involvement of AMPKalpha1. Carcinogenesis. 2010;31:1092–1099. doi: 10.1093/carcin/bgq040. [DOI] [PubMed] [Google Scholar]
- 560.Tang FY, Cho HJ, Pai MH, Chen YH. Concomitant supplementation of lycopene and eicosapentaenoic acid inhibits the proliferation of human colon cancer cells. J. Nutr. Biochem. 2009;20:426–434. doi: 10.1016/j.jnutbio.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 561.Balgi AD, Fonseca BD, Donohue E, Tsang TC, Lajoie P, Proud CG, Nabi IR, Roberge M. Screen for chemical modulators of autophagy reveals novel therapeutic inhibitors of mTORC1 signaling. PLoS One. 2009;4:e7124. doi: 10.1371/journal.pone.0007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 562.Bruno P, Calastretti A, Priulla M, Asnaghi L, Scarlatti F, Nicolin A, Canti G. Cell survival under nutrient stress is dependent on metabolic conditions regulated by Akt and not by autophagic vacuoles. Cell Signal. 2007;19:2118–2126. doi: 10.1016/j.cellsig.2007.06.008. [DOI] [PubMed] [Google Scholar]