Abstract
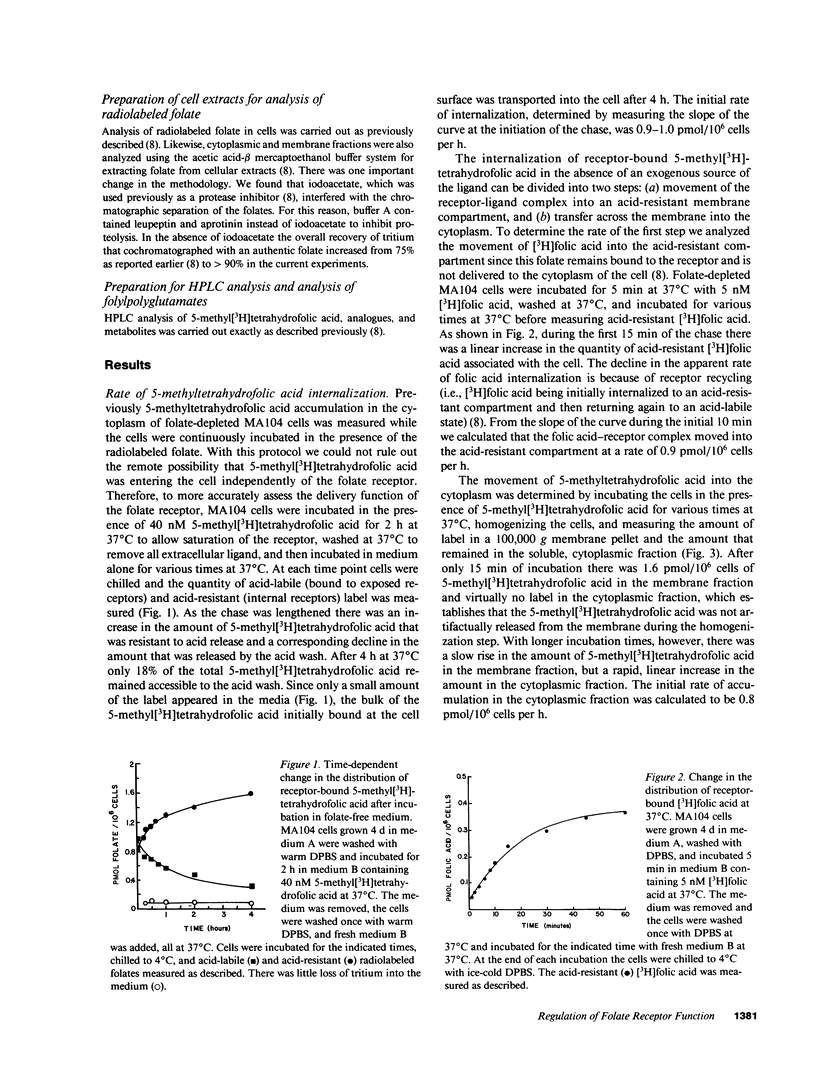
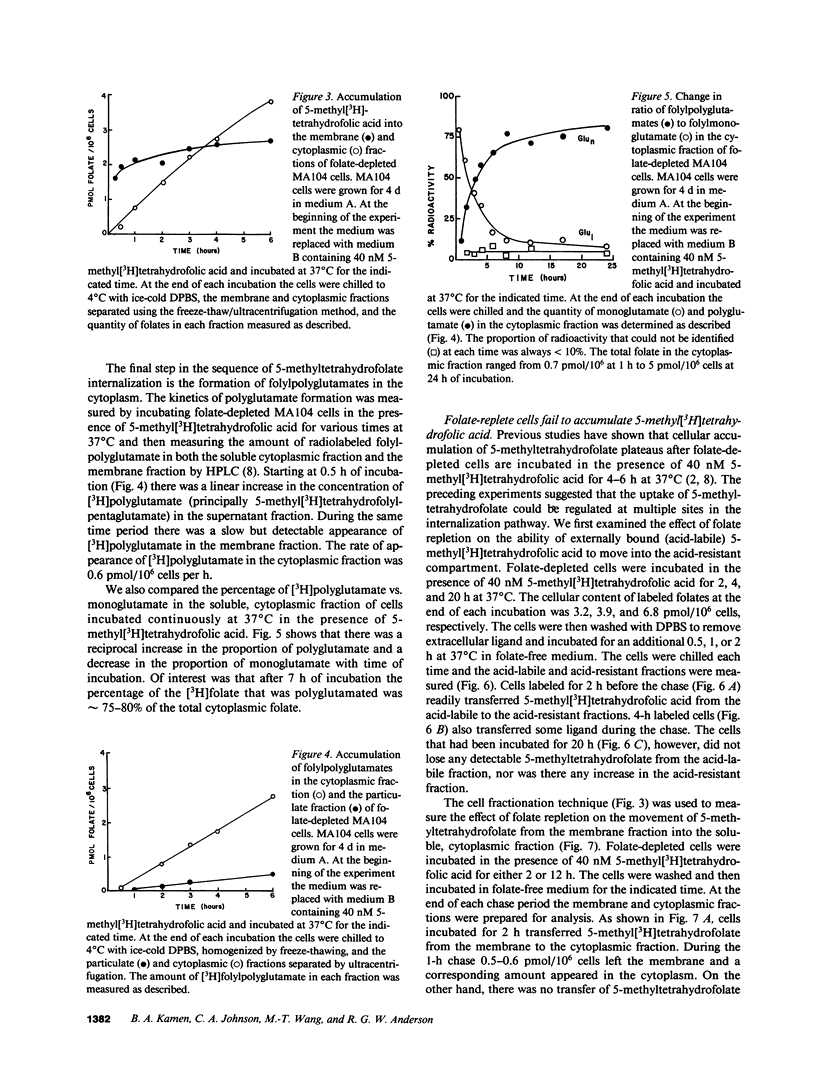
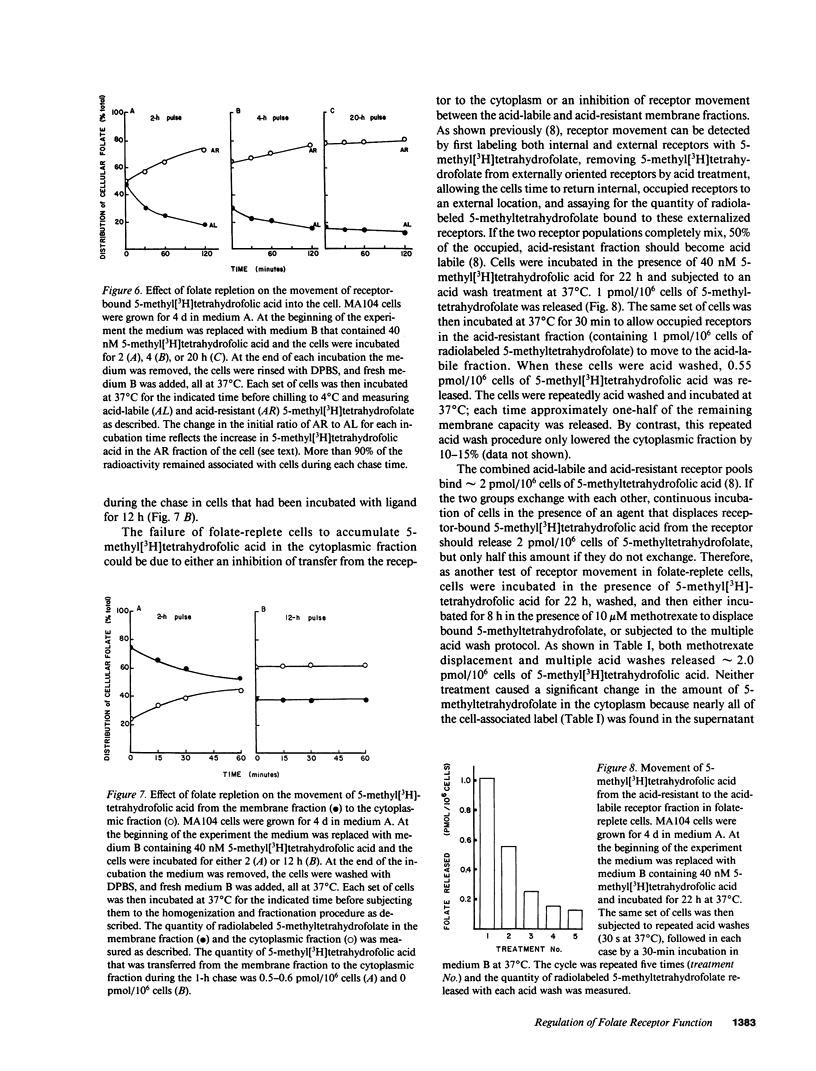
To better understand how the folate receptor (also known as the membrane folate binder) is able to deliver 5-methyltetrahydrofolic acid to the cytoplasm of folate-depleted MA104 cells, we have examined the kinetics of movement from the cell surface into the cytoplasm. Bound 5-methyltetrahydrofolic acid was transferred into an acid-resistant membrane compartment at the rate of 0.9-1.0 pmol/10(6) cells per h. This folate appeared in the cytoplasm at the same rate. Furthermore, cytoplasmic 5-methyltetrahydrofolic acid became polyglutamated at the rate of 0.6-0.7 pmol/10(6) cells per h. As soon as intracellular 5-methyltetrahydrofolate reached 5-7 pmol/10(6) cells, however, cytoplasmic accumulation was markedly inhibited even though the folate receptor remained functional. Therefore, the acute regulation of 5-methyltetrahydrofolic acid accumulation appears to be achieved by controlling the movement of the vitamin from the receptor into the cytoplasm of the cell.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amemura M., Makino K., Shinagawa H., Kobayashi A., Nakata A. Nucleotide sequence of the genes involved in phosphate transport and regulation of the phosphate regulon in Escherichia coli. J Mol Biol. 1985 Jul 20;184(2):241–250. doi: 10.1016/0022-2836(85)90377-8. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Portillo R. M., Elwood P. C., Kolhouse J. F. Studies of the role of a particulate folate-binding protein in the uptake of 5-methyltetrahydrofolate by cultured human KB cells. J Biol Chem. 1985 Dec 5;260(28):14911–14917. [PubMed] [Google Scholar]
- Antony A. C., Utley C., Van Horne K. C., Kolhouse J. F. Isolation and characterization of a folate receptor from human placenta. J Biol Chem. 1981 Sep 25;256(18):9684–9692. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Elwood P. C., Kane M. A., Portillo R. M., Kolhouse J. F. The isolation, characterization, and comparison of the membrane-associated and soluble folate-binding proteins from human KB cells. J Biol Chem. 1986 Nov 25;261(33):15416–15423. [PubMed] [Google Scholar]
- Harayama S., Bollinger J., Iino T., Hazelbauer G. L. Characterization of the mgl operon of Escherichia coli by transposon mutagenesis and molecular cloning. J Bacteriol. 1983 Jan;153(1):408–415. doi: 10.1128/jb.153.1.408-415.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. B., Grzelakowska-Sztabert B., Zevely E. M., Huennekens F. M. Binding properties of the 5-methyltetrahydrofolate/methotrexate transport system in L1210 cells. Arch Biochem Biophys. 1980 Jun;202(1):144–149. doi: 10.1016/0003-9861(80)90416-6. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Suresh M. R., Vitols K. S., Huennekens F. M. Transport of folate compounds in L1210 cells: kinetic evidence that folate influx proceeds via the high-affinity transport system for 5-methyltetrahydrofolate and methotrexate. Cancer Res. 1986 Apr;46(4 Pt 1):1639–1643. [PubMed] [Google Scholar]
- Henderson G. B., Tsuji J. M., Kumar H. P. Mediated uptake of folate by a high-affinity binding protein in sublines of L1210 cells adapted to nanomolar concentrations of folate. J Membr Biol. 1988 Mar;101(3):247–258. doi: 10.1007/BF01872839. [DOI] [PubMed] [Google Scholar]
- Henderson G. B., Zevely E. M. Affinity labeling of the 5-methyltetrahydrofolate/methotrexate transport protein of L1210 cells by treatment with an N-hydroxysuccinimide ester of [3H]methotrexate. J Biol Chem. 1984 Apr 10;259(7):4558–4562. [PubMed] [Google Scholar]
- Hengge R., Boos W. Maltose and lactose transport in Escherichia coli. Examples of two different types of concentrative transport systems. Biochim Biophys Acta. 1983 Aug 11;737(3-4):443–478. doi: 10.1016/0304-4157(83)90009-6. [DOI] [PubMed] [Google Scholar]
- Higgins C. F., Haag P. D., Nikaido K., Ardeshir F., Garcia G., Ames G. F. Complete nucleotide sequence and identification of membrane components of the histidine transport operon of S. typhimurium. Nature. 1982 Aug 19;298(5876):723–727. doi: 10.1038/298723a0. [DOI] [PubMed] [Google Scholar]
- Jansen G., Kathmann I., Rademaker B. C., Braakhuis B. J., Westerhof G. R., Rijksen G., Schornagel J. H. Expression of a folate binding protein in L1210 cells grown in low folate medium. Cancer Res. 1989 Apr 15;49(8):1959–1963. [PubMed] [Google Scholar]
- Jansen G., Westerhof G. R., Kathmann I., Rademaker B. C., Rijksen G., Schornagel J. H. Identification of a membrane-associated folate-binding protein in human leukemic CCRF-CEM cells with transport-related methotrexate resistance. Cancer Res. 1989 May 1;49(9):2455–2459. [PubMed] [Google Scholar]
- Kamen B. A., Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5983–5987. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Caston J. D. Purification of folate binding factor in normal umbilical cord serum. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4261–4264. doi: 10.1073/pnas.72.11.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamen B. A., Wang M. T., Streckfuss A. J., Peryea X., Anderson R. G. Delivery of folates to the cytoplasm of MA104 cells is mediated by a surface membrane receptor that recycles. J Biol Chem. 1988 Sep 25;263(27):13602–13609. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Kolhouse J. F. The interrelationship of the soluble and membrane-associated folate-binding proteins in human KB cells. J Biol Chem. 1986 Nov 25;261(33):15625–15631. [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Najfeld V., Finley A., Waxman S., Kolhouse J. F. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988 May;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey S. W., Sanders J. M., Rothberg K. G., Anderson R. G., Kamen B. A. Complementary DNA for the folate binding protein correctly predicts anchoring to the membrane by glycosyl-phosphatidylinositol. J Clin Invest. 1989 Aug;84(2):715–720. doi: 10.1172/JCI114220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G. Biochemistry of the glycosyl-phosphatidylinositol membrane protein anchors. Biochem J. 1987 May 15;244(1):1–13. doi: 10.1042/bj2440001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhrs C. A., Pitiranggon P., da Costa M., Rothenberg S. P., Slomiany B. L., Brink L., Tous G. I., Stein S. Purified membrane and soluble folate binding proteins from cultured KB cells have similar amino acid compositions and molecular weights but differ in fatty acid acylation. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6546–6549. doi: 10.1073/pnas.84.18.6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantzos J. D., Alevizou-Terzaki V., Gyftaki E. Folate binding in animal plasma. Acta Haematol. 1974;51(4):204–210. doi: 10.1159/000208295. [DOI] [PubMed] [Google Scholar]
- Price E. M., Freisheim J. H. Photoaffinity analogues of methotrexate as folate antagonist binding probes. 2. Transport studies, photoaffinity labeling, and identification of the membrane carrier protein for methotrexate from murine L1210 cells. Biochemistry. 1987 Jul 28;26(15):4757–4763. doi: 10.1021/bi00389a024. [DOI] [PubMed] [Google Scholar]
- Robbins A. R., Guzman R., Rotman B. Roles of individual mgl gene products in the beta-methylgalactoside transport system of Escherichia coli K12. J Biol Chem. 1976 May 25;251(10):3112–3116. [PubMed] [Google Scholar]
- Sadasivan E., Rothenberg S. P. Molecular cloning of the complementary DNA for a human folate binding protein. Proc Soc Exp Biol Med. 1988 Nov;189(2):240–244. doi: 10.3181/00379727-189-42804. [DOI] [PubMed] [Google Scholar]
- Sadasivan E., Rothenberg S. P. The complete amino acid sequence of a human folate binding protein from KB cells determined from the cDNA. J Biol Chem. 1989 Apr 5;264(10):5806–5811. [PubMed] [Google Scholar]
- Salter D. N., Ford J. E., Scott K. J., Andrews P. Isolation of the folate-binding protein from cow's milk by the use of affinity chromatography. FEBS Lett. 1972 Feb 15;20(3):302–306. doi: 10.1016/0014-5793(72)80092-9. [DOI] [PubMed] [Google Scholar]
- Sennett C., Rosenberg L. E., Mellman I. S. Transmembrane transport of cobalamin in prokaryotic and eukaryotic cells. Annu Rev Biochem. 1981;50:1053–1086. doi: 10.1146/annurev.bi.50.070181.005201. [DOI] [PubMed] [Google Scholar]
- Sirotnak F. M. Obligate genetic expression in tumor cells of a fetal membrane property mediating "folate" transport: biological significance and implications for improved therapy of human cancer. Cancer Res. 1985 Sep;45(9):3992–4000. [PubMed] [Google Scholar]
- Yang C. H., Sirotnak F. M., Mines L. S. Further studies on a novel class of genetic variants of the L1210 cell with increased folate analogue transport inward. Transport properties of a new variant, evidence for increased levels of a specific transport protein, and its partial characterization following affinity labeling. J Biol Chem. 1988 Jul 15;263(20):9703–9709. [PubMed] [Google Scholar]


