Abstract
Purpose
There is an unmet need for biomarkers for identifying patients likely to benefit from anticancer treatments, selecting dose, and understanding mechanisms of resistance. Plasma vascular endothelial growth factor (VEGF) and soluble VEGF receptor 2 (sVEGFR-2) are known to be modulated by VEGF pathway inhibitors. It is unknown whether chemotherapy or VEGFR inhibitor/chemotherapy combinations induce changes in these or other cytokines and angiogenic factors (CAFs) and whether such changes could be markers of benefit.
Methods
Thirty-five plasma CAFs were analyzed using multiplexed bead arrays and enzyme-linked immunosorbent assays from 123 patients with non–small-cell lung cancer in a randomized phase II study who received vandetanib, a VEGFR and epidermal growth factor receptor inhibitor, monotherapy carboplatin and paclitaxel (CP), or the combination (VCP). Changes in CAFs at days 8, 22, and 43 from baseline were correlated with progression risk.
Results
VEGF increased and sVEGFR-2 decreased by day 43 in the vandetanib arm, whereas a distinct pattern was observed in the CP and VCP arms, with significant decreases in interleukin (IL) -12, IL-1 receptor antagonist, and matrix metalloproteinase 9 (MMP-9) and increased macrophage chemoattractant protein 1. In each treatment arm, changes in different markers were associated with progression risk. For example, increases in IL-8 with VCP, MMP-9 with CP, and VEGF with vandetanib monotherapy were associated with increased progression risk, and increase in intercellular adhesion molecule 1 with vandetanib was associated with decreased risk.
Conclusion
Vandetanib and chemotherapy treatment led to distinct patterns of CAF changes; the combination resembled chemotherapy alone. Changes in specific CAFs correlated with clinical outcome, but markers differed for each treatment arm. CAF profiling may provide insights into the biologic effects of treatment and identify drug-specific markers of activity and clinical benefit.
INTRODUCTION
Angiogenesis is an essential process for tumor growth and metastatic spread.1,2 The balance of proangiogenic and antiangiogenic factors, including growth factors, cytokines, and chemokines, that regulate physiologic angiogenesis is disrupted during tumorigenesis.3–5 Vascular endothelial growth factor (VEGF) is a critical proangiogenic factor that is upregulated in tumors.4 Inhibitors of VEGF signaling, including bevacizumab, sorafenib, and sunitinib, have proven clinical benefit for the treatment of several solid tumors, and many similar agents are in development.6–13
However, clinical trials using such molecularly targeted therapies present some problems that do not typically occur in trials of cytotoxic agents. The optimal antitumor effect of these agents may occur at doses below the clinically defined maximum-tolerated dose. This has made determination of the recommended dose for phase II and III testing difficult, as demonstrated by the various doses of bevacizumab used in pivotal phase III trials.6–9,14 Furthermore, antiangiogenic agents may be cytostatic, rather than cytotoxic, which has made determination of their clinical efficacy and optimal dosing challenging.
Clinical evaluation and use of antiangiogenic agents would be greatly facilitated by the identification of biomarkers that are modulated by the therapies. Such modulated biomarkers could have the potential to be used as activity biomarkers to determine the optimal antitumor dose,15 to predict clinical benefit early in the course of therapy, to monitor responses to treatment, and to enhance our understanding of the mechanisms of action of and resistance to therapeutic agents.
Increases in VEGF and decreases in soluble VEGF receptor 2 (sVEGFR-2) have been commonly reported in phase I and II studies of VEGFR tyrosine kinase inhibitors (TKIs) and seem to be a class effect of these agents.16–19 However, only some studies have found associations between these factor changes and clinical benefit.16,18–22 Recently, Ebos et al16 showed that these VEGF and VEGFR-2 changes in tumor-bearing and non–tumor-bearing mice treated with sunitinib (VEGFR/platelet-derived growth factor receptor/c-kit inhibitor) occur as a result of a systemic, tumor-independent response that is dose dependent and coincides with the predetermined optimal antitumor dose of sunitinib. The impact of VEGFR TKIs and other therapeutic agents, such as chemotherapy, on the broader profile of cytokines and angiogenic factors (CAFs) in cancer patients is not well understood. Recent preclinical studies suggest that such changes may be biologically important.23
Vandetanib is an orally administered TKI of VEGFR-2, epidermal growth factor receptor (EGFR), and RET that, as monotherapy or in combination with chemotherapy, has improved progression-free survival (PFS) in patients with advanced non–small-cell lung cancer (NSCLC) in three phase II studies and is now being evaluated in phase III settings.24–26 We performed serial assessments of plasma levels of 35 CAFs, including VEGF and sVEGFR-2, among the patients in a randomized phase II trial who were randomly assigned to vandetanib monotherapy, carboplatin and paclitaxel (CP) chemotherapy, or vandetanib in combination with CP (VCP) for the first-line treatment of advanced NSCLC. In this trial, VCP demonstrated a PFS benefit compared with CP, but vandetanib was inferior to CP.24 The three-arm design of this study provided the unique opportunity to identify patterns of changes in CAF concentrations over time during therapy with vandetanib, CP chemotherapy, and the VCP combination and to correlate these changes with progression risk.
METHODS
Patients and Study Design
The multicenter, randomized, phase II clinical trial is described in detail elsewhere.24 Patients with chemotherapy-naïve stage IIIB or IV NSCLC (N = 181) were assigned (2:1:1) to vandetanib 300 mg by mouth once daily until disease progression or intolerance, CP (carboplatin area under the curve 6; paclitaxel 200 mg/m2) intravenously once every 3 weeks for six cycles, or same-dose CP for six cycles in combination with vandetanib 300 mg/d until progression or intolerance. The primary objectives were to determine whether vandetanib monotherapy was noninferior to CP and whether VCP prolonged PFS compared with CP. This clinical study was approved by all relevant institutional ethical committees or review bodies and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Each patient provided written informed consent. Consent for plasma sample collection for biomarker analyses was optional, and only patients who consented are included in this analysis.
Plasma Sample Collection and Analyses
Plasma samples were prepared from venous blood samples collected at baseline (day −7 to pretreatment on day 1), day 8 (D8; ± 1 day), day 22 (D22; ± 3 days), and day 43 (D43; ± 3 days), frozen, and stored at −70 to −80°C until analysis (further handling details are in the Appendix, online only). We analyzed the plasma samples blinded to clinical outcome. Plasma concentrations of 35 CAFs (Table 1) were measured at each of the four time points. Thirty-three factors were analyzed with commercially available multiplexed bead suspension arrays, and osteopontin and sVEGFR-2 were analyzed by enzyme-linked immunosorbent assays (product/manufacturer details can be found in the Appendix), all per the manufacturers' instructions. Each sample was analyzed in duplicate. CAF concentrations from all time points for each patient were analyzed in the same enzyme-linked immunosorbent assays and multiplexed bead suspension arrays to minimize interexperimental variability.
Table 1.
Cytokines and Angiogenic Factors Analyzed
| Factor Analyzed |
|---|
| Proangiogenic factors |
| VEGF |
| bFGF |
| EGF |
| TNF-α |
| IL-6 |
| IL-8 |
| IL-1b |
| MMP-9 |
| HGF |
| MCP-1 |
| Antiangiogenic factors |
| IL-12p40/70 |
| INF-α |
| INF-γ |
| MIG |
| IP-10 |
| Inflammatory markers |
| ICAM-1 |
| Markers of hypoxia |
| Osteopontin |
| Hematopoietic growth factors |
| GM-CSF |
| G-CSF |
| Markers of endothelial cell function or damage |
| E-selectin |
| sVEGFR-2 |
| Other interleukins |
| IL-1RA |
| IL-2 |
| sIL-2R |
| IL-4 |
| IL-5 |
| IL-7 |
| IL-10 |
| IL-13 |
| IL-15 |
| IL-17 |
| Other cytokines and chemokines |
| RANTES |
| MIP-1α |
| MIP-1β |
| Eotaxin |
Abbreviations: VEGF, vascular endothelial growth factor; bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; TNF, tumor necrosis factor; IL, interleukin; MMP, matrix metalloproteinase; HGF, hepatocyte growth factor; MCP-1, macrophage chemoattractant protein-1; INF, interferon; MIG, monokine induced by interferon gamma; IP-10, interferon gamma–induced protein 10; ICAM-1, intercellular adhesion molecule 1; GM-CSF, granulocyte-macrophage colony-stimulating factor; G-CSF, granulocyte colony-stimulating factor; sVEGFR-2, soluble vascular endothelial growth factor receptor 2; IL-1RA, interleukin-1 receptor antagonist; sIL-2R, soluble interleukin-2 receptor; MIP, macrophage inflammatory protein.
Statistical Methods
The patient demographics and disease characteristics of the clinical study patients with and without CAF data were compared using the χ2 test for categoric variables and the Wilcoxon rank sum test for continuous variables. Linear mixed models were used to study the marker changes over time.27 The transformation of logarithm to the base 2 of a marker was used in this analysis to satisfy the normality assumption. Treatment × time interaction on marker levels was assessed, and subgroup analyses by treatment arm were carried out. Regression analyses using the Cox proportional hazards model were conducted on PFS. The change over time of a CAF compared with baseline was calculated as the difference of CAF concentration at each time point from concentration at baseline on the log-transformed scale. We tested the interaction between the change over time of each CAF and treatment first. Subgroup analyses were performed to further test the effect of CAF change on PFS within each treatment group in the Cox model. Similar results were obtained with and without adjusting for sex and smoking status (smoker v nonsmoker), which were found to be significant baseline prognostic and/or predictive factors; adjusted results are presented here. All P values are two-sided. We considered P < .05 to be significant. We did not control for multiple analyses because these analyses are exploratory. SAS Version 9.1 (SAS Institute, Cary, NC) and S-Plus Version 7.0 (Statistical Sciences, Seattle, WA) were used to carry out the computations for all analyses.
RESULTS
Patient Population
Baseline plasma samples were available from 123 (68%) of 181 patients in the trial (55 from the vandetanib arm, 32 from the CP arm, and 36 from the VCP arm). The characteristics of patients with baseline plasma samples and the number of samples analyzed per treatment arm and time point are listed in Table 2. The characteristics of these 123 patients did not differ significantly from those of the 58 patients without plasma available for analysis (data not shown). D8, D22, and D43 plasma samples were available from 104, 94, and 80 patients, respectively.
Table 2.
Patient and Disease Characteristics
| Characteristic | No. of Patients (N = 123) | % |
|---|---|---|
| Median age, years | 61 | |
| Sex | ||
| Male | 86 | 70 |
| Female | 37 | 30 |
| Histology | ||
| Adenocarcinoma | 56 | 46 |
| Squamous | 28 | 23 |
| Large cell | 17 | 14 |
| Adenocarcinoma with BAC features/BAC | 5 | 4 |
| Other | 17 | 14 |
| Stage | ||
| IIIB | 18 | 15 |
| IV | 105 | 85 |
| Race | ||
| White | 112 | 91 |
| Black | 3 | 2 |
| Asian | 2 | 1.5 |
| Other | 6 | 5 |
| Smoking status | ||
| Current | 31 | 25 |
| Former | 66 | 54 |
| Never | 25 | 20 |
| Unknown | 1 | < 1 |
| V arm, No. of plasma samples | ||
| Baseline | 55 | 45 |
| Day 8 | 45 | |
| Day 22 | 35 | |
| Day 43 | 31 | |
| CP arm, No. of plasma samples | ||
| Baseline | 32 | 26 |
| Day 8 | 27 | |
| Day 22 | 30 | |
| Day 43 | 23 | |
| VCP arm, No. of plasma samples | ||
| Baseline | 36 | 29 |
| Day 8 | 32 | |
| Day 22 | 29 | |
| Day 43 | 26 |
Abbreviations: BAC, bronchioloalveolar carcinoma; V, vandetanib; CP, carboplatin and paclitaxel; VCP, vandetanib, carboplatin, and paclitaxel.
CAF Changes During Vandetanib, Chemotherapy, and Combination Treatment
When patients from all three treatment arms were considered together, significant changes in the plasma concentrations of interleukin (IL) -12, IL-1 receptor antagonist (IL-1RA), IL-8, macrophage chemoattractant protein 1 (MCP-1), matrix metalloproteinase 9 (MMP-9), and sVEGFR-2 over time were detected. IL-12, IL-1RA, MMP-9, and sVEGFR-2 concentrations were lower at D8 compared with baseline (P < .001, P = .003, P < .001, and P = .001, respectively). IL-8 and MCP-1 concentrations were higher at D8 than at baseline (P = .09 and P < .001, respectively). Changes in basic fibroblast growth factor (bFGF), IL-12, and interferon-inducible protein 10 (IP-10) over time differed by treatment arm, with P = .035, .0021, and .023, respectively, for the interactions between treatment and these factor changes. There were also interactions of borderline significance between treatment and changes in IL-1RA and VEGF (P < .10).
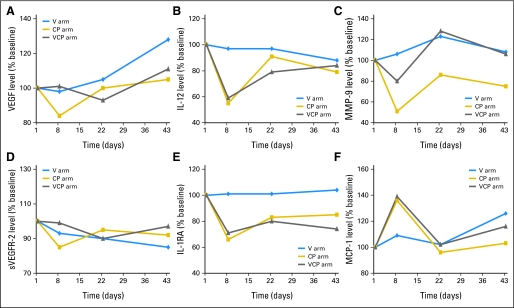
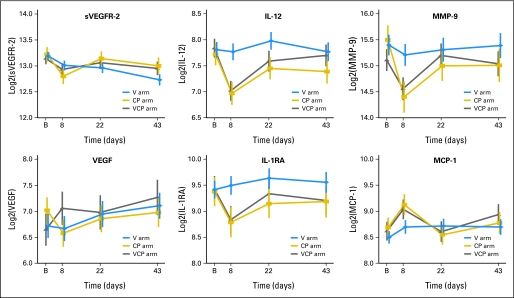
We then performed subgroup analyses to assess per treatment arm for significant changes in each marker from baseline to each of the time points (D8, D22, and D43). The changes in CAF concentrations over time are reported here and are illustrated in Table 3, Figure 1, and Appendix Figure 1 (online only).
Table 3.
Changes in Plasma Concentrations of Cytokines and Angiogenic Factors During Treatment
| CAF and Treatment Arm | Baseline |
Day 8 |
Day 22 |
Day 43 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | Median (pg/mL) | Range (pg/mL) | No. of Patients | Median (pg/mL) | Range (pg/mL) | % Baseline* | No. of Patients | Median (pg/mL) | Range (pg/mL) | % Baseline* | No. of Patients | Median (pg/mL) | Range (pg/mL) | % Baseline* | |
| VEGF | |||||||||||||||
| V | 55 | 102 | 0-1,246 | 45 | 105 | 0-1,160 | 98 | 35 | 97 | 0-880 | 105 | 31 | 128 | 0-289 | 138† |
| CP | 32 | 121 | 34-1,087 | 27 | 127 | 0-546 | 84 | 30 | 119 | 24-1,397 | 100 | 23 | 114 | 40-552 | 105 |
| VCP | 36 | 118 | 0-4,024 | 32 | 140 | 16-3,606 | 101 | 29 | 124 | 16-3,744 | 93 | 26 | 117 | 6-1,041 | 111 |
| sVEGFR-2 | |||||||||||||||
| V | 54 | 9,593 | 4,100-16,732 | 45 | 8,443 | 2,257-21,598 | 93 | 34 | 8,414 | 3,429-14,483 | 90 | 29 | 7,290 | 3,429-14,483 | 85† |
| CP | 29 | 8,611 | 5,543-17,764 | 27 | 9,153 | 249-13,431 | 86† | 28 | 8,877 | 3,110-15,457 | 95 | 22 | 9,097 | 939-16,897 | 92 |
| VCP | 34 | 9,201 | 2,953-17,594 | 30 | 8,529 | 1,294-17,673 | 99 | 29 | 8,994 | 3,895-15,995 | 90 | 25 | 8,638 | 1,111-20,221 | 97 |
| IL-12 | |||||||||||||||
| V | 55 | 219 | 38-1,230 | 45 | 211 | 54-1,292 | 97 | 35 | 276 | 44-1,178 | 97 | 31 | 258 | 54-1,406 | 88 |
| CP | 32 | 193 | 53-1,424 | 27 | 110 | 38-1,690 | 55† | 30 | 199 | 37-1,774 | 91 | 23 | 193 | 58-1,380 | 79 |
| VCP | 36 | 218 | 46-1,543 | 32 | 124 | 37-1,014 | 59† | 29 | 192 | 37-858 | 79 | 26 | 198 | 32-785 | 84 |
| IL-1RA | |||||||||||||||
| V | 55 | 610 | 150-6,234 | 45 | 599 | 175-11,929 | 101 | 35 | 750 | 148-8,626 | 101 | 31 | 643 | 197-8,121 | 104 |
| CP | 32 | 493 | 188-27,612 | 27 | 338 | 88-18,191 | 66† | 30 | 431 | 108-11,340 | 83 | 23 | 594 | 145-11,139 | 85 |
| VCP | 36 | 600 | 130-11,201 | 32 | 347 | 102-11,005 | 71† | 29 | 507 | 109-10,351 | 80 | 26 | 538 | 95-7,516 | 74 |
| MMP-9 | |||||||||||||||
| V | 55 | 38,592 | 5,742-892,964 | 45 | 35,458 | 2,749-980,916 | 106 | 35 | 39,045 | 5,750-211,208 | 123 | 31 | 32,037 | 2,752-402,460 | 108 |
| CP | 32 | 45,165 | 6,305-2,500,000 | 27 | 24,574 | 1,771-188,733 | 51† | 30 | 28,842 | 2,606-553,724 | 86 | 23 | 30,811 | 5,443-201,182 | 75 |
| VCP | 36 | 30,703 | 6,198-393,092 | 32 | 25,560 | 2,119-122,638 | 80† | 29 | 41,787 | 9,489-174,531 | 128 | 26 | 28,625 | 2,587-196,677 | 106 |
| MCP-1 | |||||||||||||||
| V | 55 | 349 | 132-2,841 | 45 | 370 | 117-7,539 | 109 | 35 | 416 | 141-3,270 | 102 | 31 | 370 | 133-4,743 | 126 |
| CP | 32 | 358 | 140-4,587 | 27 | 576 | 147-3,161 | 136† | 30 | 375 | 66-2,363 | 96 | 23 | 386 | 114-2,243 | 103 |
| VCP | 36 | 387 | 41-5,226 | 32 | 458 | 221-5,732 | 139† | 29 | 451 | 72-5,076 | 102 | 26 | 485 | 143-4,925 | 116 |
| Eotaxin | |||||||||||||||
| V | 55 | 33 | 8-150 | 45 | 33 | 11-235 | 92 | 35 | 32 | 13-224 | 95 | 31 | 35 | 13-178 | 102 |
| CP | 32 | 43 | 9-179 | 27 | 39 | 12-108 | 100 | 30 | 39 | 13-116 | 98 | 23 | 44 | 17-190 | 110 |
| VCP | 36 | 43 | 7-108 | 32 | 45 | 10-102 | 110 | 29 | 47 | 7-114 | 129 | 26 | 53 | 14-121 | 121† |
| G-CSF | |||||||||||||||
| V | 55 | 94 | 1-1,733 | 45 | 77 | 1-3,393 | 100 | 35 | 79 | 1-825 | 111 | 31 | 101 | 6-564 | 116† |
| CP | 32 | 129 | 4-1,717 | 27 | 102 | 8-550 | 94 | 30 | 99 | 1-1,832 | 109 | 23 | 134 | 1-1,625 | 115 |
| VCP | 36 | 92 | 1-3,792 | 32 | 95 | 1-3,252 | 110 | 29 | 92 | 1-3,511 | 91 | 26 | 104 | 1-2,991 | 101 |
| IL-4 | |||||||||||||||
| V | 55 | 24 | 0-750 | 45 | 24 | 0-727 | 100 | 35 | 22 | 0-368 | 109 | 31 | 31 | 0-209 | 100 |
| CP | 32 | 23 | 0-217 | 27 | 17 | 0-267 | 79† | 30 | 22 | 0-276 | 106 | 23 | 24 | 0-727 | 102 |
| VCP | 36 | 19 | 0-652 | 32 | 20 | 0-798 | 99 | 29 | 26 | 0-491 | 109 | 26 | 25 | 0-579 | 105 |
| IL-8 | |||||||||||||||
| V | 55 | 41 | 2-2,644 | 45 | 49 | 2-417 | 115† | 35 | 41 | 6-219 | 131 | 31 | 38 | 11-238 | 123 |
| CP | 32 | 43 | 11-2,300 | 27 | 58 | 10-866 | 92 | 30 | 33 | 2-929 | 68 | 23 | 35 | 7-136 | 99 |
| VCP | 36 | 53 | 4-711 | 32 | 59 | 12-362 | 118 | 29 | 35 | 7-213 | 80 | 26 | 40 | 9-279 | 104 |
| IL-10 | |||||||||||||||
| V | 55 | 86 | 1-2,273 | 45 | 68 | 1-1,669 | 101 | 35 | 71 | 6-924 | 111 | 31 | 63 | 2-1,400 | 117 |
| CP | 32 | 80 | 15-6,176 | 27 | 64 | 10-2,044 | 63† | 30 | 68 | 13-36,940 | 94 | 23 | 64 | 11-36,940 | 93 |
| VCP | 36 | 50 | 9-36,940 | 32 | 54 | 7-36,940 | 106 | 29 | 61 | 6-36,940 | 102 | 26 | 52 | 13-6,870 | 123 |
| IL-13 | |||||||||||||||
| V | 55 | 83 | 2-1,401 | 45 | 90 | 2-1,542 | 100 | 35 | 103 | 2-750 | 100 | 31 | 90 | 2-382 | 107 |
| CP | 32 | 95 | 2-1,598 | 27 | 78 | 2-926 | 81† | 30 | 82 | 2-2,676 | 100 | 23 | 98 | 15-1,791 | 100 |
| VCP | 36 | 82 | 2-2,163 | 32 | 80 | 2-2,701 | 98 | 29 | 74 | 2-2,229 | 100 | 26 | 67 | 2-2,151 | 100 |
| IL-17 | |||||||||||||||
| V | 55 | 95 | 0-2,483 | 45 | 78 | 0-2,694 | 100 | 35 | 63 | 0-2,400 | 100 | 31 | 104 | 0-1,548 | 128† |
| CP | 32 | 93 | 0-1,675 | 27 | 64 | 0-710 | 100 | 30 | 48 | 0-1,742 | 102 | 23 | 116 | 0-1,975 | 100 |
| VCP | 36 | 75 | 0-2,028 | 32 | 69 | 0-2,439 | 100 | 29 | 57 | 0-1,393 | 94 | 26 | 28 | 0-1,959 | 100 |
| IP-10 | |||||||||||||||
| V | 55 | 35 | 5-328 | 45 | 45 | 6-151 | 81 | 35 | 53 | 9-264 | 118 | 31 | 62 | 5-505 | 147 |
| CP | 32 | 36 | 9-889 | 27 | 30 | 12-265 | 105 | 30 | 30 | 6-106 | 96† | 23 | 23 | 8-102 | 60† |
| VCP | 36 | 33 | 6-6,044 | 32 | 33 | 12-530 | 110 | 29 | 33 | 3-1,609 | 94 | 26 | 43 | 3-1,388 | 88 |
| MIP-1α | |||||||||||||||
| V | 55 | 70 | 9-1,786 | 45 | 70 | 2-4,016 | 94 | 35 | 62 | 3-2,945 | 102 | 31 | 56 | 2-2,149 | 99 |
| CP | 32 | 55 | 20-3,376 | 27 | 60 | 2-2,101 | 89† | 30 | 53 | 17-1,427 | 102 | 23 | 77 | 19-1,830 | 101 |
| VCP | 36 | 53 | 2-1,471 | 32 | 41 | 9-1,421 | 90 | 29 | 51 | 2-1,112 | 84 | 26 | 58 | 2-1,014 | 90 |
NOTE. The data presented in this table are based on the raw data prior to log2 transformation.
Abbreviations: CAF, cytokine and angiogenic factor; VEGF, vascular endothelial growth factor; V, vandetanib; CP, carboplatin and paclitaxel; VCP, vandetanib, carboplatin, and paclitaxel; sVEGFR-2, soluble vascular endothelial growth factor receptor 2; IL, interleukin; IL-1RA, interleukin-1 receptor antagonist; MMP-9, matrix metalloproteinase 9; MCP-1, macrophage chemoattractant protein 1; G-CSF, granulocyte colony-stimulating factor; IP-10, interferon gamma–induced protein 10; MIP-1α, macrophage inflammatory protein 1α.
Percent baseline indicates the median of the ratios of CAF concentration at each time point to baseline concentration expressed as a percentage (not the ratio of the median concentration at each time point to the median baseline concentration).
In the Cox proportional hazards model using log2 transformation of data, P < .05 for change in CAF concentration from baseline to time point.
Fig 1.
(A-F) Changes in concentrations of cytokines and angiogenic factors (CAFs) during treatment (% baseline indicates the median of the ratios of CAF concentration at each time point to baseline concentration expressed as a percentage, not the ratio of the median concentration at each time point to the median baseline concentration). These figures are based on raw data. The P values in the text are based on the analysis of log2 transformed data in mixed linear models and adjusted for sex. For figures generated from the log2 transformed data with error bars, please see Figure A1. CP, carboplatin and paclitaxel; VCP, vandetanib, carboplatin, and paclitaxel; VEGF, vascular endothelial growth factor; IL-12, interleukin-12; MMP-9, matrix metalloproteinase-9; sVEGFR-2, soluble vascular endothelial growth factor receptor 2; IL-1RA, interleukin-1 receptor antagonist; MCP-1, macrophage chemoattractant protein 1.
In the vandetanib monotherapy arm, plasma concentrations of VEGF significantly increased (P = .048) and concentrations of sVEGFR-2 significantly decreased (P < .001) from baseline to D43. There were also significant increases in IL-8 (P = .041) at D8 and in granulocyte colony-stimulating factor (P = .03) and IL-17 at D43 (P = .045). There were no significant CAF changes at D22 from baseline.
In the CP arm, there were significant decreases from baseline to D8 in plasma concentrations of IL-12 (P < .001), IL-1RA (P = .009), and MMP-9 (P < .001) and a significant increase in MCP-1 at D8 (P = .013). Other significant CAF changes in the CP arm were decreases in IL-4 (P = .031), IL-10 (P = .043), IL-13 (P = .011), sVEGFR-2 (P = .024), and macrophage inflammatory protein 1α (P = .027) at D8 and a decrease in IP-10 at D22 (P = .036) and D43 (P = .012).
In the VCP arm, there were similarly significant decreases from baseline to D8 in IL-12 (P < .001), IL-1RA (P = .003), and MMP-9 (P = .035) concentrations. There were significant increases in MCP-1 (P = .004) at D8 and eotaxin (P = .016) at D43. No significant CAF changes were detected at D22.
It is notable that even though vandetanib targets the VEGFR and EGFR pathways, no significant changes in epidermal growth factor levels over time were observed in any of the treatment arms.
Correlation Between CAF Changes and PFS
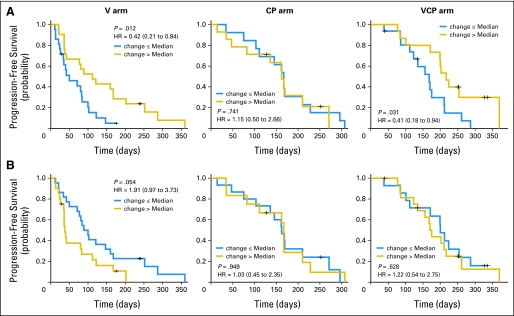
We found correlations between the changes in 14 CAF concentrations during treatment and PFS for individual treatment arms (Table 4). We then further tested whether the correlation between the change in a CAF and PFS differed between the treatment arms; that is, we assessed for interactions between treatment and the change-in-CAF concentration as a continuous variable. Six CAFs had significant interactions (Table 4). We further evaluated these CAFs by comparing patients with CAF changes ≤ or greater than (>) the median degree of change (Fig 2). For example, patients with a greater than median increase in intercellular adhesion molecule 1 (ICAM-1) concentration at D8 had a significantly improved PFS in the vandetanib arm and VCP arm compared with patients with a ≤ median increase, but there were no significant differences in outcome in the CP arm (P for interaction = .021; Fig 2A). Patients with a greater than median increase in VEGF levels had an inferior PFS in the vandetanib arm compared with patients with a ≤ median increase, but there were no significant differences in outcome in the CP or VCP arms (P for interaction = .009; Fig 2B).
Table 4.
Associations Between Change in CAF Plasma Concentrations and PFS
| Time Point and CAF | HR* | 95% CI |
|---|---|---|
| Day 8 | ||
| VCP | ||
| IL-8 | 1.48 | 1.02 to 2.16 |
| IL-12 | 0.61 | 0.40 to 0.94 |
| IP-10 | 0.69 | 0.48 to 0.98 |
| MIP-1α | 0.61 | 0.39 to 0.98 |
| CP | ||
| IL-13 | 0.70 | 0.55 to 0.89 |
| IL-15 | 0.76 | 0.60 to 0.98 |
| IL-17† | 0.75 | 0.60 to 0.95 |
| V | ||
| ICAM-1† | 0.53 | 0.30 to 0.96 |
| VEGF† | 1.93 | 1.06 to 3.52 |
| Osteopontin | 0.88 | 0.78 to 1.00 |
| Day 22 | ||
| CP | ||
| MMP-9 | 1.31 | 1.04 to 1.64 |
| Day 43 | ||
| VCP | ||
| IL-8†‡ | 1.56 | 0.98 to 2.48 |
| IFN-ᆇ | 1.27 | 0.98 to 1.60 |
| CP | ||
| IL-15 | 1.74 | 1.04 to 2.90 |
| V | ||
| sIL-2R† | 0.67 | 0.45 to 0.99 |
| IL-5 | 0.83 | 0.71 to 0.96 |
NOTE. HR < 1.0 indicates that increase in CAF level correlates with improved PFS; HR >1.0 = rise in CAF level correlates with worse PFS.
Abbreviations: CAF, cytokine and angiogenic factor; PFS, progression-free survival; HR, hazard ratio; VCP, vandetanib, carboplatin, and paclitaxel; IL, interleukin; IP-10, interferon gamma–induced protein 10; MIP-1α, macrophage inflammatory protein 1α; CP, carboplatin and paclitaxel; V, vandetanib; ICAM-1, intercellular adhesion molecule 1; VEGF, vascular endothelial growth factor; MMP-9, matrix metalloproteinase 9; IFN-α, interferon alfa; sIL-2R, soluble interleukin-2 receptor.
HR indicates relative increase in risk of progression for a patient with a two-fold increase in CAF concentration from baseline compared with a patient with no increase in CAF concentration.
Treatment × change-in-CAF interaction was significant (P < .05).
All associations with PFS were significant (P < .05), except day 43 IL-8 (P = .059) and IFN-α (P = .068), which had significant interactions.
Fig 2.
Kaplan-Meier curves of progression-free survival (PFS) based on extent of change in (A) intercellular adhesion molecule 1 (ICAM-1) and (B) vascular endothelial growth factor (VEGF) concentrations (≤ median indicates increase ≤ the median increase in concentration of that cytokine and angiogenic factor [CAF]; > median indicates increase > the median increase in concentration of that CAF). Note that the P values are from a log-rank test for the comparison of the Kaplan-Meier curves, whereas the P values shown in the text are from a Cox model with the change of the markers as continuous variables adjusting for sex and smoking status. Change in ICAM-1 at day 8 by treatment interaction, P = .021; change in VEGF at day 8 by treatment interaction, P = .009. V, vandetanib; CP, carboplatin and paclitaxel; VCP, vandetanib, carboplatin, and paclitaxel; HR, hazard ratio.
DISCUSSION
In this exploratory analysis of plasma levels of 35 CAFs during treatment with vandetanib and/or CP chemotherapy for advanced NSCLC, we found that vandetanib and chemotherapy were associated with distinct patterns of CAF changes and that the CAF changes with the VCP combination resembled those with chemotherapy alone. In addition, the changes in specific CAFs that correlated with clinical outcome differed for each treatment arm. Our results are summarized in appendix Table A1 (online only). Interestingly, most of the significant associations between outcome and changes in CAF levels in our study were seen at D8, suggesting that these changes in markers could indicate responsiveness or resistance to therapy earlier than imaging studies.
The finding that chemotherapy and vandetanib treatment are associated with distinct changes in the CAF profile has a number of potentially important implications for biomarker development as well as understanding the biologic effects of these agents. First, it suggests that for each drug (or class of drugs), it may be possible to identify specific CAFs whose changes during treatment may serve as pharmacodynamic and/or efficacy markers. In the case of vandetanib monotherapy, we noted a significant decrease in sVEGFR-2 and increase in VEGF by D43, consistent with a previous report.28 Similar sVEGFR-2 and VEGF changes have been reported in patients with a variety of solid tumor types treated with other VEGFR TKIs and seem to be a pharmacologic class effect.15,16,22,29–32 Of note, in the VCP arm, reciprocal changes in sVEGFR-2 and VEGF were not observed, suggesting that the effect of chemotherapy on CAF changes dominated over that of vandetanib. The underlying molecular mechanisms of these VEGF and sVEGFR-2 changes are not fully understood.17 Ebos et al16 recently showed that the changes in VEGF and sVEGFR-2 in human tumor xenograft-bearing and non–tumor-bearing mice treated with sunitinib occurred through a systemic, multiorgan, tumor-independent mechanism that correlated with the optimal antitumor dose of sunitinib. Therefore, these VEGF and sVEGFR-2 changes have the potential to serve as pharmacodynamic biomarkers to guide optimal biologic dosing.
A number of studies have analyzed for associations between VEGF and/or sVEGFR-2 changes during treatment and clinical outcome, with conflicting findings.18–22,33,34 Although two phase II studies of VEGFR TKIs for renal cell carcinoma (sunitinib21 and pazopanib33) reported associations between tumor response and plasma VEGF/VEGFR-2 changes, there were no correlations detected between patient outcome and VEGF/sVEGFR-2 changes in a large, randomized, phase III study of sorafenib versus placebo for renal cell carcinoma.34 In the current study, there was a trend toward inferior PFS with an increase in VEGF at D8 in the vandetanib monotherapy arm, but there was no association between PFS and a change in VEGF levels in the CP and VCP arms. It is noteworthy that neither changes in VEGF nor sVEGFR-2 correlated with outcome in the chemotherapy-containing arms of this study, suggesting that their potential utility as predictors of clinical benefit may be specific for VEGF pathway inhibitors alone.
The pattern of CAF changes over time in the CP and VCP arms was distinct from that in the vandetanib arm, with several CAFs undergoing maximal changes at D8 and returning toward baseline levels by D22. These included significant decreases in MMP-9, IL-12, and IL-1RA and increase in MCP-1 at D8. The effects of chemotherapy on the circulating concentrations of these four CAFs have not been previously reported. Leukocytes are major sources of IL-12, IL-1RA, and MMP-9, and the decrease of these CAFs at D8 may mirror changes in leukocyte levels with cytotoxic chemotherapy.
The biologic consequences of the distinct treatment-induced CAF changes remain to be determined, but preclinical studies suggest that they may have significant effects on both the host and tumor. For example, paclitaxel-induced increases in the chemokine stromal cell-derived factor-1α were recently found to contribute to the mobilization of circulating endothelial progenitors, increased angiogenesis, and tumor growth in a murine lung cancer model.23 In this study, we report, for the first time to our knowledge, that chemotherapy also induces increases in MCP-1, a known proangiogenic chemokine that regulates VEGF levels and is a key chemoattractant for monocytes.35 The potential role of MCP-1 in chemotherapy-induced mobilization of proangiogenic mononuclear cells merits further investigation.
We also observed that specific CAF changes were associated with PFS during treatment, and moreover, there were a number of significant treatment × change-in-CAF interactions, indicating that the correlation differed depending on the treatment. This highlights the need for treatment-specific markers of benefit and suggests potential mechanisms of therapeutic resistance that merit further investigation. For example, greater increases in IL-8 were associated with inferior PFS in the VCP arm. IL-8–mediated angiogenesis was previously identified as a key compensatory angiogenic pathway in a murine model of colorectal cancer.36 However, an increase in ICAM-1 in the vandetanib arm was predictive of superior PFS, which could perhaps reflect shedding of ICAM-1 secondary to treatment-induced tumor endothelial cell death. It is particularly interesting that an increase in MMP-9 in the CP arm was associated with inferior PFS. A similar association between adverse outcome and increasing serum MMP-9 concentration was reported in a study of 116 consecutive patients treated with gemcitabine and cisplatin for NSCLC.37 MMP-9 has multiple proangiogenic functions, including the degradation of collagen in basement membrane that facilitates endothelial cell migration and liberation of other proangiogenic growth factors, including VEGF.38,39 MMP-9 delivered to the tumor site by proangiogenic bone marrow–derived cells (BMDCs) has been shown to be critical for tumor neovascularization and BMDC recruitment.40–43 In light of these data, the association between an increased risk of tumor progression and an increase in MMP-9 could reflect a greater recruitment of BMDCs to tumor sites in these patients, resulting in tumor neovascularization and growth.
Although other researchers have considered the effects of anticancer treatments on CAFs, they have generally evaluated a more limited number of markers in retrospectively identified cohorts of patients or single-arm clinical trials. To our knowledge, this analysis of CAF changes over time considers the largest number of CAFs to date and is one of only a few to assess correlations between modulation of blood-based biomarkers and patient outcome in a prospective, randomized clinical trial using both standard chemotherapy and a targeted agent. In 113 of the 878 participating patients in the randomized phase II/III Eastern Cooperative Oncology Group 4599 study of CP with or without bevacizumab for the first-line treatment of stage IIIB or IV NSCLC, Dowlati et al44 analyzed the changes during treatment of the following three CAFs: bFGF, ICAM-1, and E-selectin. They found an association between relative stability of E-selectin at week 7 and greater survival benefit from CP plus bevacizumab compared with CP. We found no relationship between E-selectin change/stability and PFS in our study. This discrepancy may reflect differences in the mechanisms of action of bevacizumab and vandetanib, or it could simply be a result of modest patient numbers in both studies.
It is important to note that the CAF analyses reported here are exploratory, and the number of patients is modest. Therefore, it is possible that some of the observed marker changes and their associations with outcome occurred by chance or that we failed to detect clinically relevant changes in markers as a result of a lack of statistical power. Nevertheless, it is notable that we found the same VEGF and sVEGFR-2 changes in the vandetanib arm that have been previously reported with vandetanib and other similar agents.16,28
We have shown that vandetanib and chemotherapy are associated with distinct patterns of CAF changes during treatment. Such patterns of CAF changes may provide insight into the biologic effects of these agents and suggest potential mechanisms of resistance and novel therapeutic combinations that merit further investigation. Furthermore, we have identified changes in CAFs that were associated with PFS benefit. These markers were drug specific because changes in no single marker were associated with outcome in all three treatment arms. On the basis of these findings, additional analyses are planned in ongoing phase III studies of vandetanib in NSCLC to potentially validate these findings and identify other novel markers of activity and clinical benefit.
Acknowledgment
We thank Robert S. Kerbel for his thoughtful comments.
Appendix
Plasma Samples
Plasma samples were not obtained from some participating sites that did not have the necessary preparation or storage facilities. Plasma samples were shipped on dry ice from each participating site to a central contract research organization, where they were stored at −70 to −80°C. All samples were then shipped on dry ice in a single batch to The University of Texas M. D. Anderson Cancer Center, where they were stored at −70 to −80°C until analysis. Before analysis, plasma samples were thawed overnight at 4°C (first thaw), centrifuged at 1,500 × g to remove debris, and aliquoted to multiple tubes for same-day analysis by multiplexed bead suspension arrays or stored at −70 to −80°C for future studies. The samples for enzyme-linked immunosorbent assay were conducted using these aliquots, which were thawed (second thaw) and prepared in the same manner.
Cytokines and Angiogenic Factors
Thirty cytokines and angiogenic factors were analyzed using the Human Cytokine 30-plex panel from Biosource (Camarillo, CA; catalog No. LHC6003). Three cytokines (matrix metalloproteinase-9, intercellular adhesion molecule 1, and E-selectin) were analyzed using a customized three-plex panel derived from the Lincoplex Human Cardiovascular Disease I kit from LINCO Research/Millipore (St Charles, MO; catalog No. HCVD1-67AK). Osteopontin and soluble vascular endothelial growth factor receptor 2 were analyzed using enzyme-linked immunosorbent assay kits from R&D Systems (Minneapolis, MN; catalog Nos. DOST00 and DVR200).
Fig A1.
Changes in plasma levels of cytokines and angiogenic factors during treatment (log2 transformed data). VCP, vandetanib, carboplatin, and paclitaxel; CP, carboplatin and paclitaxel; V, vandetanib; sVEGFR-2, soluble vascular endothelial growth factor receptor 2; B, baseline; IL-12, interleukin-12; MMP-9, matrix metalloproteinase-9; VEGF, vascular endothelial growth factor; interleukin-1 receptor antagonist; MCP-1, macrophage chemoattractant protein 1.
Table A1.
Summary of CAF Changes During Treatment and Associations Between CAF Changes and Outcome
| Change | CAF |
|---|---|
| CAFs that increased during treatment | |
| V | VEGF, IL-8, G-CSF, IL-17 |
| CP | MCP-1 |
| VCP | MCP-1, eotaxin |
| CAFs that decreased during treatment | |
| V | sVEFGR-2 |
| CP | IL-12, IL-1RA, MMP-9, IL-4, IL-10, IL-13, sVEGFR-2, MIP-1α, IP-10 |
| VCP | IL-12, IL-1RA, MMP-9 |
| CAF increases associated with improved PFS outcome | |
| V | ICAM-1, osteopontin, sIL-2R, IL-5 |
| CP | IL-13, IL-15, IL-17 |
| VCP | IL-12, IP-10, MIP-1α |
| CAF increases associated with inferior PFS outcome | |
| V | VEGF |
| CP | MMP-9, IL-15 |
| VCP | IL-8, IFN-α |
| CAF changes predictive of PFS benefit from treatment | |
| V | ICAM-1 (increase → superior PFS); VEGF (increase → inferior PFS) |
| CP | IL-17 (increase → superior PFS) |
| VCP | sIL-2R (increase → superior PFS); IL-8 (increase → inferior PFS); IFN-α (increase → inferior PFS) |
Abbreviations: CAF, cytokine and angiogenic factor; V, vandetanib; VEGF, vascular endothelial growth factor; IL, interleukin; G-CSF, granulocyte colony-stimulating factor; CP, carboplatin and paclitaxel; MCP-1, macrophage chemoattractant protein-1; VCP, vandetanib, carboplatin, and paclitaxel; sVEGFR-2, soluble vascular endothelial growth factor receptor 2; IL-1RA, interleukin-1 receptor antagonist; MMP-9, matrix metalloproteinase 9; MIP-1α, macrophage inflammatory protein 1α; IP-10, interferon gamma–induced protein 10; PFS, progression-free survival; ICAM-1, intercellular adhesion molecule 1; sIL-2R, soluble interleukin-2 receptor; IFN-α, interferon alfa.
Footnotes
Supported in part by an American Society of Clinical Oncology (ASCO) Young Investigator Award (E.O.H.), ASCO Career Development Award (J.V.H.), Specialized Programs of Research Excellence Grant No. P50 CA70907, and AstraZeneca. J.V.H. is a Damon Runyon-Lilly Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation (Grant No. CI 24-04).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Anderson J. Ryan, AstraZeneca (C); Peter Langmuir, AstraZeneca (C) Consultant or Advisory Role: Edward S. Kim, AstraZeneca (C); John V. Heymach, AstraZeneca (C) Stock Ownership: None Honoraria: None Research Funding: Edward S. Kim, AstraZeneca; John V. Heymach, AstraZeneca Expert Testimony: None Other Remuneration: Bruce E. Johnson, Genzyme
AUTHOR CONTRIBUTIONS
Conception and design: Emer O. Hanrahan, Hai T. Tran, Anderson J. Ryan, Bruce E. Johnson, John V. Heymach
Administrative support: Kathryn S. McKee
Provision of study materials or patients: Bruce E. Johnson, John V. Heymach
Collection and assembly of data: Emer O. Hanrahan, Shaoyu Yan, Danny Z. Du, Kathryn S. McKee, Anderson J. Ryan, John V. Heymach
Data analysis and interpretation: Emer O. Hanrahan, Heather Y. Lin, Edward S. Kim, J. Jack Lee, Anderson J. Ryan, Peter Langmuir, Bruce E. Johnson, John V. Heymach
Manuscript writing: Emer O. Hanrahan, Heather Y. Lin, Edward S. Kim, Hai T. Tran, J. Jack Lee, Anderson J. Ryan, Bruce E. Johnson, John V. Heymach
Final approval of manuscript: Emer O. Hanrahan, Heather Y. Lin, Edward S. Kim, Shaoyu Yan, Danny Z. Du, Kathryn S. McKee, Hai T. Tran, J. Jack Lee, Anderson J. Ryan, Peter Langmuir, Bruce E. Johnson, John V. Heymach
REFERENCES
- 1.Folkman J. Tumor angiogenesis: Therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Herbst RS, Onn A, Sandler A. Angiogenesis and lung cancer: Prognostic and therapeutic implications. J Clin Oncol. 2005;23:3243–3256. doi: 10.1200/JCO.2005.18.853. [DOI] [PubMed] [Google Scholar]
- 4.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 5.Benelli R, Lorusso G, Albini A, et al. Cytokines and chemokines as regulators of angiogenesis in health and disease. Curr Pharm Des. 2006;12:3101–3115. doi: 10.2174/138161206777947461. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 8.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 9.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 10.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 13.Demetri G, van Oosterom AT, Garrett C, et al. Improved survival and sustained clinical benefit with SU11248 (SU) in pts with GIST after failure of imatinib mesylate (IM) therapy in a phase III trial. 2006 Gastrointestinal Cancers Symposium; January 26-28, 2006; San Francisco, CA. abstr 8. [Google Scholar]
- 14.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 15.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 16.Ebos JM, Lee CR, Christensen JG, et al. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci U S A. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Longo R, Gasparini G. Challenges for patient selection with VEGF inhibitors. Cancer Chemother Pharmacol. 2007;60:151–170. doi: 10.1007/s00280-006-0403-6. [DOI] [PubMed] [Google Scholar]
- 18.Peña C, Gatzemeier U, Lathia C, et al. Plasma biomarkers in a phase II trial of sorafenib in advanced non-small cell lung cancer. AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics Conference; October 22-26, 2007; San Francisco, CA. abstr B18. [Google Scholar]
- 19.Nikolinakos P, Altorki N, Guarino M, et al. Analyses of plasma cytokine/angiogenic factors (C/AFs) profile during preoperative treatment with pazopanib (GW786034) in early-stage non-small cell lung cancer. J Clin Oncol. 2008;26(suppl):414s. abstr 7568. [Google Scholar]
- 20.Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, et al. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol. 2005;16:558–565. doi: 10.1093/annonc/mdi118. [DOI] [PubMed] [Google Scholar]
- 21.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: Modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaked Y, Henke E, Roodhart JM, et al. Rapid chemotherapy-induced acute endothelial progenitor cell mobilization: Implications for antiangiogenic drugs as chemosensitizing agents. Cancer Cell. 2008;14:263–273. doi: 10.1016/j.ccr.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heymach JV, Paz-Ares L, De Braud F, et al. Randomized phase II study of vandetanib alone or with paclitaxel and carboplatin as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:5407–5415. doi: 10.1200/JCO.2008.17.3138. [DOI] [PubMed] [Google Scholar]
- 25.Heymach JV, Johnson BE, Prager D, et al. Randomized, placebo-controlled phase II study of vandetanib plus docetaxel in previously treated non small-cell lung cancer. J Clin Oncol. 2007;25:4270–4277. doi: 10.1200/JCO.2006.10.5122. [DOI] [PubMed] [Google Scholar]
- 26.Natale RB, Bodkin D, Govindan R, et al. Vandetanib versus gefitinib in patients with advanced non–small-cell lung cancer: Results from a two-part, double-blind, randomized phase II study. J Clin Oncol. 2009;27:2523–2529. doi: 10.1200/JCO.2008.18.6015. [DOI] [PubMed] [Google Scholar]
- 27.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 28.Kiura K, Nakagawa K, Shinkai T, et al. A randomized, double-blind, phase IIa dose-finding study of vandetanib (ZD6474) in Japanese patients with non-small cell lung cancer. J Thorac Oncol. 2008;3:386–393. doi: 10.1097/JTO.0b013e318168d228. [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 30.Elting J, Bigwood D, Brown-Shimer S, et al. Biomarkers associated with clinical outcomes in TARGETs, a phase III single-agent, placebo-controlled study of sorafenib in advanced renal cell carcinoma. 97th Annual Meeting of the American Association for Cancer Research; April 1-5, 2006; Washington, DC. abstr 2909. [Google Scholar]
- 31.Drevs J, Medinger M, Mross K, et al. Phase I clinical evaluation of AZD2171, a highly potent VEGF receptor tyrosine kinase inhibitor, in patients with advanced tumors. J Clin Oncol. 2005;23(suppl):192s. abstr 3002. [Google Scholar]
- 32.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 33.Hutson TE, Davis ID, Machiels JH, et al. Biomarker analysis and final efficacy and safety results of a phase II renal cell carcinoma trial with pazopanib (GW786034), a multi-kinase angiogenesis inhibitor. J Clin Oncol. 2008;26(suppl):261s. abstr 5046. [Google Scholar]
- 34.Bukowski RM, Eisen T, Szczylik C, et al. Final results of the randomized phase III trial of sorafenib in advanced renal cell carcinoma: Survival and biomarker analysis. J Clin Oncol. 2007;25(suppl 18S):240s. abstr 5023. [Google Scholar]
- 35.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 36.Mizukami Y, Jo WS, Duerr EM, et al. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 37.Mihaylova Z, Ludovini V, Gregorg V, et al. Serum level changes of matrix metalloproteinases 2 and 9, vascular endothelial growth factor and epidermal growth factor receptor during platinum-based chemotherapy in advanced non-small cell lung cancer patients. J Buon. 2007;12:105–111. [PubMed] [Google Scholar]
- 38.Cox G, Jones JL, Walker RA, et al. Angiogenesis and non-small cell lung cancer. Lung Cancer. 2000;27:81–100. doi: 10.1016/s0169-5002(99)00096-3. [DOI] [PubMed] [Google Scholar]
- 39.Seandel M, Noack-Kunnmann K, Zhu D, et al. Growth factor-induced angiogenesis in vivo requires specific cleavage of fibrillar type I collagen. Blood. 2001;97:2323–2332. doi: 10.1182/blood.v97.8.2323. [DOI] [PubMed] [Google Scholar]
- 40.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin DK, Shido K, Kopp HG, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dowlati A, Gray R, Sandler AB, et al. Cell adhesion molecules, vascular endothelial growth factor, and basic fibroblast growth factor in patients with non-small cell lung cancer treated with chemotherapy with or without bevacizumab: An Eastern Cooperative Oncology Group Study. Clin Cancer Res. 2008;14:1407–1412. doi: 10.1158/1078-0432.CCR-07-1154. [DOI] [PubMed] [Google Scholar]