Abstract
Purpose
Germline mutations in BRCA genes are associated with breast and ovarian cancer susceptibility. Because infertility is associated with breast and ovarian cancer risks, we hypothesized that the mutations in the BRCA gene may be associated with low response to fertility treatments.
Methods
We performed ovarian stimulation in 126 women with breast cancer by using letrozole and gonadotropins for the purpose of fertility preservation by embryo or oocyte cryopreservation. As surrogates of ovarian reserve, the oocyte yield and the incidence of low response were compared with ovarian stimulation according to BRCA mutation status.
Results
Of the 82 women who met the inclusion criteria, 47 women (57%) had undergone BRCA testing, and 14 had a mutation in BRCA genes, of which two were of clinically undetermined significance. In BRCA mutation–positive patients, low ovarian response rate was significantly higher compared with BRCA mutation–negative patients (33.3 v 3.3%; P = .014) and with BRCA-untested women (2.9%; P = .012). All BRCA mutation–positive low responders had BRCA1 mutations, but low response was not encountered in women who were only BRCA2 mutation positive. Compared with controls, BRCA1 mutation– but not BRCA2 mutation–positive women produced lower numbers of eggs (7.4 [95% CI, 3.1 to 17.7] v 12.4 [95% CI, 10.8 to 14.2]; P = .025) and had as many as 38.3 times the odds ratio of low response (95% CI, 4.1 to 353.4; P = .001).
Conclusion
BRCA1 mutations are associated with occult primary ovarian insufficiency. This finding may, at least in part, explain the link between infertility and breast/ovarian cancer risks.
INTRODUCTION
Through recombination with undamaged, homologous DNA strands, BRCA genes play an essential role in double-strand DNA break (DSB) repair.1 Mutations in either gene (ie, BRCA1 or BRCA2) are associated with breast and ovarian cancer susceptibility, and these mutations are inherited in an autosomal dominant fashion.2 We have recently developed a method of ovarian stimulation that uses the aromatase inhibitor letrozole (ie, controlled ovarian stimulation treatment with letrozole supplementation study; COST-LESS) for women with breast cancer who wish to preserve their fertility by oocyte or embryo cryopreservation before undergoing chemotherapy. In this protocol, administration of letrozole concurrently with gonadotropins reduces estrogen exposure significantly compared with standard ovarian stimulation regimens.3–5 During the aforementioned study we repeatedly encountered young patients with breast cancer who have no history of infertility and who unexpectedly have had low response to ovarian stimulation. Because low response to ovarian stimulation is associated with diminished oocyte reserve, occult primary ovarian insufficiency, and—although unconfirmed—increased DNA errors in oocytes, we hypothesized that the mutations in the BRCA gene may also be associated with primary occult ovarian insufficiency and infertility, as determined by low ovarian response to in vitro fertilization treatments.6,7
METHODS
Data for this study were generated from a secondary analysis of the prospective, controlled COST-LESS study, which involved women with breast cancer who underwent oocyte or embryo cryopreservation for fertility preservation. Details of the COST-LESS protocol have been published.3,5 Briefly, all women received letrozole 5 mg/d, starting on menstrual cycle day 2. Follicle-stimulating hormone (FSH) was added 2 days later at 150 to 300 IU/d on the basis of age and body mass index (BMI). Because patients were referred shortly after diagnosis of breast cancer and because BRCA testing was ordered by the referring oncologists, BRCA status was known in only one patient at the time of ovarian stimulation. The investigators, thus, were blinded to BRCA status of all but one patient. Because an association between BRCA mutations and ovarian response was not known during the COST-LESS study, BRCA status did not play a role in ovarian stimulation decisions in that patient.
A widely accepted definition of low ovarian response, retrieval of four or fewer oocytes in women younger than 38 years, was utilized in this study.8,9 Women older than 38 years and women with prior ovarian surgery or with infertility treatments were excluded from consideration. BRCA testing was performed at commercial clinical laboratories, and the decision to perform such testing was made by the patient's oncologist, who was not involved with ovarian stimulation.
Statistical Analysis
Statistical analysis was performed with the SPSS 15.0 for Windows package (SPSS, Chicago, IL). Continuous data were analyzed with an independent t test if a normal distribution was likely and with log-converted data if the distribution was skewed. Log-converted data are presented as the harmonic mean and 95% CIs of the mean. Normally distributed data is presented as means ± standard deviations. To examine the association of BRCA mutation and low ovarian response, cross tabulations and Pearson's χ2 test were used. We performed linear and logistic regression analyses to adjust for age. Various models also were employed to take into account total FSH dose and BMI. Statistical significance was reached at P < .05. The fit of logistic models was assessed with the Hosmer-Lemeshow test. Differences in continuous data were presented as mean differences and 95% CIs. Differences in categoric data (2 × 2) were expressed in terms of odds ratios (ORs) and 95% CIs.
RESULTS
One hundred twenty-six patients underwent ovarian stimulation for fertility preservation via embryo or oocyte cryopreservation according to the COST-LESS protocol. Of those, 82 patients met the study criteria. Of the excluded, 12 (27%) of 44 patients were tested for BRCA, and only one was BRCA mutation positive. She was excluded because of a history of prior chemotherapy.
Of the 82 women who met inclusion criteria, 47 (57%) had undergone BRCA testing. Of those, 14 (30%) had a mutation in BRCA genes, and 33 (70%) did not. Of those 14 women, nine had a mutation in the BRCA1 gene, four had a mutation in the BRCA2 gene, and one had mutations in both genes (Table 1). Of the 14 BRCA mutations, two were of clinically undetermined significance; thus, the primary analysis was performed with 12 BRCA mutation–positive patients. Mean ages of untested, BRCA mutation–negative and –positive women were similar (Table 2). Low ovarian response rate was significantly higher in BRCA mutation–positive patients (four [33.3%] of 12) compared with BRCA mutation–negative patients (one [3.3%] of 33; P = .014) and BRCA-untested women (one [2.9%] of 35; P = .012). All BRCA mutation–positive low responders had BRCA1 mutations, and one patient also had a mutation in BRCA2. Low response was not encountered in women who were only BRCA2 mutation positive. When analysis was controlled for age, a BRCA mutation of known significance was associated with 28.7 times the normal OR of low response (95% CI, 1.8 to 447; P = .016) compared with BRCA mutation–negative women. When compared with the combined group of BRCA mutation–negative and -untested women, the OR was 24.7 (95% CI, 1.9 to 208; P = .003).
Table 1.
Mutation Types and Number of Oocytes Retrieved in BRCA Mutation–Positive Women Ordered by Age
| Patient No. | Age (years) | No. of Total Oocytes | BRCA | Mutation |
|---|---|---|---|---|
| 1 | 28 | 15 | 2 | 9637del4 |
| 2 | 30 | 27 | 1 | 187delAG |
| 3 | 30 | 5 | 1 | k1109n 3446A>C* |
| 4 | 31 | 17 | 1 | W1815X 5563G->A |
| 5 | 32 | 8 | 1 | 187delAG |
| 6 | 32 | 30 | 1 | 187delAG |
| 7 | 32 | 6 | 2 | IVS22-1del3insAA |
| 8 | 33 | 3 | 1 | 187delAG |
| 9 | 34 | 10 | 2 | 6174delT |
| 10 | 35 | 34 | 1 | M1083VG 3366A>G* |
| 11 | 35 | 3 | 1 and 2 | 1(185delAG), 2(6174delT) |
| 12 | 36 | 3 | 1 | 3889delAG |
| 13 | 37 | 3 | 1 | 187delAG |
| 14 | 37 | 8 | 2 | 6174delT |
Variant of unknown significance.
Table 2.
Age, FSH, and Oocyte Number Comparisons Among BRCA Mutation–Negative, –Positive, and –Untested Women
| Variable |
BRCA Mutation Status |
P | ||||
|---|---|---|---|---|---|---|
| All Positive (n = 12) | All Negative (n = 33)* | Untested(n = 35) | BRCA1 Positive (n = 8)† | All Negative and Untested (n = 68)‡ | ||
| Age, years | NS | |||||
| Mean | 33.1 | 32.8 | 33.0 | 33.9 | 32.9 | |
| SD | 2.8 | 2.9 | 2.9 | 2.7 | 2.9 | |
| Day-2 FSH, mU/mL | NS | |||||
| 5.7 | 7.1 | 6.4 | 6.2 | 6.7 | ||
| 3.0 | 2.7 | 2.3 | 3.4 | 2.5 | ||
| Oocytes | ||||||
| Mean | 7.9 | 11.3 | 13.5 | 7.4 | 12.4 | |
| 95% CI§ | 4.6 to 13.8 | 9.1 to 14.1 | 11.4 to 16.0 | 3.1 to 17.7 | 10.8 to 14.2 | |
Abbreviations: FSH, follicle-stimulating hormone; NS, not significant; SD, standard deviation.
P positive v negative = .025.
P BRCA1 mutation–positive v –negative and untested combined = .03.
P positive v negative and untested combined = .003.
Analysis was performed after log conversion because of non-normal distribution. Thus, 95% CIs were used instead of SDs.
Mean oocyte numbers were significantly lower in BRCA mutation–positive women than in BRCA mutation–negative women (7.9 [95% CI, 4.6 to 13.8] v 11.3 [95% CI, 9.1 to 14.1]; P = .025). On analysis of variance, BRCA-negative and -untested groups were similar and, thus, merged to increase statistical power (Table 2). When deleterious BRCA1 mutation–positive women were compared with the combined group of BRCA mutation–negative and -untested women, mean oocyte numbers were significantly lower in BRCA1 mutation–positive women (7.4 [95% CI, 3.1 to 17.7] v 12.4 [95% CI, 10.8 to 14.2]; P = .03; (Table 2).
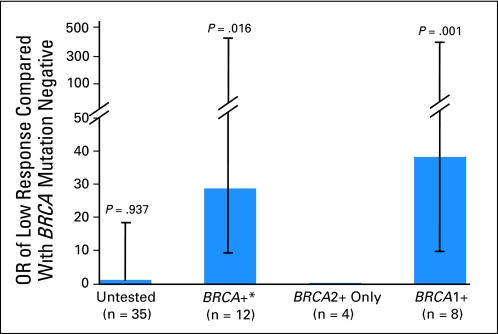
Analysis of BRCA subtypes revealed that BRCA1, but not BRCA2, mutations were associated with low response with an OR of 38.3 (95% CI, 4.1 to 353.4; P = .001). Inclusion of two nondeleterious BRCA1 mutations in this analysis reduced the OR but tightened the 95% CI (OR, 25.5; 95% CI, 3.2 to 204.2; P = .002). In BRCA1 mutation–positive patients, no specific deletion appeared to be associated with low response, but the sample size was too small to reach statistically sound conclusions. (Fig 1, OR data).
Fig 1.
Odds ratios (ORs) of low ovarian response in women with BRCA mutations. (*) Only mutations with proven clinical significance for breast and ovarian cancer risk are included.
DISCUSSION
On the basis of previous studies and large clinical experience with in vitro fertilization, low response to ovarian stimulation is one of the strongest indications of diminished ovarian reserve and infertility.10 By utilizing the largest and only prospective database available for women with breast cancer undergoing ovarian stimulation, we described here for the first time, to our knowledge, that mutations in the BRCA1 gene are associated with low response to ovarian stimulation and, by inference, infertility risks. We therefore hypothesize that, because DNA repair is deficient in patients with BRCA mutations, oocytes may be more prone to DNA damage. Follicles can reside in the ovary for decades, their oocytes potentially accumulating lethal DNA damage.6 Research in nonreproductive cell types demonstrate that when DNA damage is severe and cannot be repaired, apoptotic pathways are activated.11–14 Thus oocytes with deficient BRCA function may be prematurely eliminated by a similar mechanism, resulting in early depletion of egg reserve and, as a consequence, primary ovarian insufficiency.
Primary ovarian insufficiency is a continuum that ranges from occult insufficiency that is only detectable by laboratory markers to low response to fertility drugs to its overt form, which presents with clinical symptoms.15–17 We demonstrated that BRCA mutations are associated with occult primary ovarian insufficiency.
As in the case of occult primary ovarian insufficiency, although mutations in BRCA1 and BRCA2 are associated with ovarian cancer risk, the risk is considerably higher for BRCA1 mutations. The latter observation, thus, may suggest a common pathway in the development of diminished ovarian reserve and ovarian cancer in BRCA1 mutation–positive women. Because occult primary ovarian insufficiency is associated with female infertility, BRCA mutations may contribute to the long-known association between breast as well as ovarian cancer risks.18,19
It is estimated that, in the general population, one in every 1,000 women is BRCA mutation positive, and this incidence is as high as 2.5% in certain ethnic groups, such as people with Jewish-Ashkenazi origin.2,20 Regardless of underlying mechanisms of occult primary ovarian insufficiency in BRCA1 mutation–positive women, our findings may have profound implications for the future fertility of a large number of women in the general population. In this study, we did not measure serum ovarian reserve markers, such as antimullerian hormone, but we used response to ovarian stimulation as a surrogate of ovarian reserve. The presumed lower ovarian reserve, which was based on the low oocyte yield, may place BRCA1 mutation–positive women with breast cancer also at higher risk for chemotherapy-induced ovarian failure. This underscores the importance of fertility preservation in BRCA mutation–positive women with cancer.21 We do not know to what extent BRCA mutations affect the fertility of women who have not developed cancer; thus, widespread fertility preservation of BRCA mutation–positive women cannot be recommended at this time. Similarly, we do not yet know to what extent variations in the sequence of the BRCA gene contribute to ovarian dysfunction in the general population.
Nevertheless, considering that 1% of females suffer from primary ovarian insufficiency, that the underlying mechanism is unknown in 90%, and that an even a larger fraction of infertile women may suffer from occult primary ovarian insufficiency, discovery of susceptibility genes for premature ovarian insufficiency, such as BRCA1, will have positive implications for understanding the link between infertility and breast/ovarian cancer risks.17,22
An association between BRCA mutations and diminished oocyte reserve or occult primary ovarian insufficiency is biologically plausible on the basis of the previous laboratory and clinical data. BRCA1 expression is reduced in oocytes of aging mice, and RNAi-mediated reduction of BRCA1 in oocytes from young female mice perturbs oocyte spindle formation, which indicates that intact BRCA1 function is essential for oocyte survival.23 It has been demonstrated recently that BRCA1 localizes to unsynapsed chromosomes at the pachytene stage in human oocytes, so it may play a similar role in humans. The DSB repair pathway not only involves BRCA genes but also coordinates activity of at least six other genes, all linked to Fanconi anemia (FA).11,24 In FA, another disease caused by mutations in DSB repair genes, women experience early menopause; Fanconi gene–mutated mice display premature reproductive aging, and their ovarian primordial follicle reserves are severely reduced.14,25 Interestingly, the activated version of FANCD2, a gene involved in FA, targets BRCA1, and disruption of BRCA1 results in the disruption of DNA damage–inducible FANCD2-containing subnuclear foci. Although homozygous BRCA knockout mice are generally not viable, spermatogenesis in BRCA1 mutant mice was impaired, but ovarian follicle numbers or function was not quantified.26
We could not detect an association between BRCA2 mutations and the probability of low ovarian response. However, because of the smaller number of patients with those mutations, an impact of BRCA2 mutations on ovarian function cannot be ruled out. In fact, in mice with a truncating BRCA2 mutation, both testis and ovaries were devoid of germ cells and were hypoplastic, which resulted in infertility.27,28 Also, BRCA1 mutations did not always result in clinically overt low response, which suggests that BRCA1 may be, as is the case in breast and ovarian cancer, only a susceptibility gene for oocyte death. Accumulation of additional environmental factors with age may determine whether germline mutations in BRCA will result in occult primary ovarian insufficiency. In fact, in this study, all BRCA mutation–positive low responders were 33 years of age or older, which indicated that the degree of occult primary ovarian insufficiency may only become clinically significant in older women. This, coupled with the fact that many of the women in their late thirties are undergoing risk-reducing oophorectomies, may explain why the association of occult ovarian insufficiency and BRCA mutation status was not discovered previously. In addition, before our introduction of ovarian stimulation with aromatase inhibitors in women with breast cancer, most women would not have been given the option of ovarian stimulation for embryo or oocyte freezing; thus, an association between breast cancer and occult primary ovarian insufficiency could not have been recognized.
The proportion of women with BRCA mutations in this study may be perceived as higher than expected. Besides the young age of patients in our study, this can be additionally explained by the fact that a large population of people in our geographic area are of Ashkenazi-Jewish origin.20 It is also possible that BRCA mutation–positive women are more likely to remain childless because of the effects of these mutations on fertility; therefore, they will be more likely to need fertility preservation when faced with the prospects of chemotherapy-induced ovarian failure.
More speculatively, in majority of oligo-azospermic men, no underlying cause can be identified currently.29 Given that sperm production is altered in both FA and BRCA mutant rodent models), it is conceivable that BRCA mutations may be responsible for male-factor infertility in some of these men.
In conclusion, we showed a novel association between low response to ovarian stimulation and BRCA1 mutations, which suggests a possible link between DSB repair gene function, infertility, and breast/ovarian cancer risks. The analysis of the BRCA gene in women with infertility and low response to ovarian stimulation may be worthwhile, especially when there is family history of breast and/or ovarian cancer. Larger studies are warranted to investigate the impact of BRCA mutations on fertility in general population.
Footnotes
Supported in part by Grant No. HD53112 from National Institute of Child Health and Human Development and National Cancer Institute (K.O.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Kutluk Oktay
Financial support: Kutluk Oktay
Administrative support: Kutluk Oktay
Provision of study materials or patients: Kutluk Oktay
Collection and assembly of data: Kutluk Oktay, Ja Yeon Kim, Samir N. Babayev
Data analysis and interpretation: Kutluk Oktay, Ja Yeon Kim, David Barad, Samir N. Babayev
Manuscript writing: Kutluk Oktay, Ja Yeon Kim, Samir N. Babayev
Final approval of manuscript: Kutluk Oktay, Ja Yeon Kim, David Barad, Samir N. Babayev
REFERENCES
- 1.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- 2.Ford D, Easton DF, Peto J. Estimates of the gene frequency of BRCA1 and its contribution to breast and ovarian cancer incidence. Am J Hum Genet. 1995;57:1457–1462. [PMC free article] [PubMed] [Google Scholar]
- 3.Azim AA, Costantini-Ferrando M, Lostritto K, et al. Relative potencies of anastrozole and letrozole to suppress estradiol in breast cancer patients undergoing ovarian stimulation before in vitro fertilization. J Clin Endocrinol Metab. 2007;92:2197–2200. doi: 10.1210/jc.2007-0247. [DOI] [PubMed] [Google Scholar]
- 4.Oktay K, Buyuk E, Libertella N, et al. Fertility preservation in breast cancer patients: A prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J Clin Oncol. 2005;23:4347–4353. doi: 10.1200/JCO.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 5.Oktay K, Hourvitz A, Sahin G, et al. Letrozole reduces estrogen and gonadotropin exposure in women with breast cancer undergoing ovarian stimulation before chemotherapy. J Clin Endocrinol Metab. 2006;91:3885–3890. doi: 10.1210/jc.2006-0962. [DOI] [PubMed] [Google Scholar]
- 6.Keefe DL, Franco S, Liu L, et al. Telomere length predicts embryo fragmentation after in vitro fertilization in women: Toward a telomere theory of reproductive aging in women. Am J Obstet Gynecol. 2005;192:1256–1260. doi: 10.1016/j.ajog.2005.01.036. discussion 60-61. [DOI] [PubMed] [Google Scholar]
- 7.Magli MC, Gianaroli L, Munne S, et al. Incidence of chromosomal abnormalities from a morphologically normal cohort of embryos in poor-prognosis patients. J Assist Reprod Genet. 1998;15:297–301. doi: 10.1023/A:1022596528036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Surrey ES, Bower J, Hill DM, et al. Clinical and endocrine effects of a microdose GnRH agonist flare regimen administered to poor responders who are undergoing in vitro fertilization. Fertil Steril. 1998;69:419–424. doi: 10.1016/s0015-0282(97)00575-x. [DOI] [PubMed] [Google Scholar]
- 9.Ubaldi FM, Rienzi L, Ferrero S, et al. Management of poor responders in IVF. Reprod Biomed Online. 2005;10:235–246. doi: 10.1016/s1472-6483(10)60946-7. [DOI] [PubMed] [Google Scholar]
- 10.Kwee J, Elting ME, Schats R, et al. Ovarian volume and antral follicle count for the prediction of low and hyper-responders with in vitro fertilization. Reprod Biol Endocrinol. 2007;5:9. doi: 10.1186/1477-7827-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia-Higuera I, Taniguchi T, Ganesan S, et al. Interaction of the Fanconi anemia proteins and BRCA1 in a common pathway. Mol Cell. 2001;7:249–262. doi: 10.1016/s1097-2765(01)00173-3. [DOI] [PubMed] [Google Scholar]
- 12.Hofmann ER, Milstein S, Boulton SJ, et al. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr Biol. 2002;12:1908–1918. doi: 10.1016/s0960-9822(02)01262-9. [DOI] [PubMed] [Google Scholar]
- 13.Skalka AM, Katz RA. Retroviral DNA integration and the DNA damage response. Cell Death Differ. 2005;12(suppl 1):971–978. doi: 10.1038/sj.cdd.4401573. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi T, D'Andrea AD. Molecular pathogenesis of Fanconi anemia: Recent progress. Blood. 2006;107:4223–4233. doi: 10.1182/blood-2005-10-4240. [DOI] [PubMed] [Google Scholar]
- 15.Albright FSP, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. Am J Med Sci. 1942;204:625–648. [Google Scholar]
- 16.Welt CK. Primary ovarian insufficiency: A more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 17.Nelson LM. Clinical practice: Primary ovarian insufficiency. N Engl J Med. 2009;360:606–614. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci. 2008;1127:27–30. doi: 10.1196/annals.1434.011. [DOI] [PubMed] [Google Scholar]
- 19.Jensen A, Sharif H, Olsen JH, et al. Risk of breast cancer and gynecologic cancers in a large population of nearly 50,000 infertile Danish women. Am J Epidemiol. 2008;168:49–57. doi: 10.1093/aje/kwn094. [DOI] [PubMed] [Google Scholar]
- 20.Warner E, Foulkes W, Goodwin P, et al. Prevalence and penetrance of BRCA1 and BRCA2 gene mutations in unselected Ashkenazi Jewish women with breast cancer. J Natl Cancer Inst. 1999;91:1241–1247. doi: 10.1093/jnci/91.14.1241. [DOI] [PubMed] [Google Scholar]
- 21.Sonmezer M, Oktay K. Fertility preservation in young women undergoing breast cancer therapy. Oncologist. 2006;11:422–434. doi: 10.1634/theoncologist.11-5-422. [DOI] [PubMed] [Google Scholar]
- 22.Gleicher N, Barad D. Unexplained infertility: Does it really exist? Hum Reprod. 2006;21:1951–1955. doi: 10.1093/humrep/del135. [DOI] [PubMed] [Google Scholar]
- 23.Pan H, Ma P, Zhu W, et al. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev Biol. 2008;316:397–407. doi: 10.1016/j.ydbio.2008.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Cruz R, Roig I, Robles P, et al. ATR, BRCA1 and gammaH2AX localize to unsynapsed chromosomes at the pachytene stage in human oocytes. Reprod Biomed Online. 2009;18:37–44. doi: 10.1016/s1472-6483(10)60422-1. [DOI] [PubMed] [Google Scholar]
- 25.Wong JC, Alon N, McKerlie C, et al. Targeted disruption of exons 1 to 6 of the Fanconi anemia group A gene leads to growth retardation, strain-specific microphthalmia, meiotic defects and primordial germ cell hypoplasia. Hum Mol Genet. 2003;12:2063–2076. doi: 10.1093/hmg/ddg219. [DOI] [PubMed] [Google Scholar]
- 26.Cressman VL, Backlund DC, Avrutskaya AV, et al. Growth retardation, DNA repair defects, and lack of spermatogenesis in BRCA1-deficient mice. Mol Cell Biol. 1999;19:7061–7075. doi: 10.1128/mcb.19.10.7061. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27.Connor F, Bertwistle D, Mee PJ, et al. Tumorigenesis and a DNA repair defect in mice with a truncating Brca2 mutation. Nat Genet. 1997;17:423–430. doi: 10.1038/ng1297-423. [DOI] [PubMed] [Google Scholar]
- 28.Sharan SK, Pyle A, Coppola V, et al. BRCA2 deficiency in mice leads to meiotic impairment and infertility. Development. 2004;131:131–142. doi: 10.1242/dev.00888. [DOI] [PubMed] [Google Scholar]
- 29.Rowe PJ. Cambridge, MA: Cambridge University Press; 2000. WHO Manual for the Standardized Investigation, Diagnosis, and Management of the Infertile Male. [Google Scholar]