Abstract
Purpose
To determine whether the use of a goals-of-care video to supplement a verbal description can improve end-of-life decision making for patients with cancer.
Methods
Fifty participants with malignant glioma were randomly assigned to either a verbal narrative of goals-of-care options at the end of life (control), or a video after the same verbal narrative (intervention) in this randomized controlled trial. The video depicts three levels of medical care: life-prolonging care (cardiopulmonary resuscitation [CPR], ventilation), basic care (hospitalization, no CPR), and comfort care (symptom relief). The primary study outcome was participants' preferences for end-of-life care. The secondary outcome was participants' uncertainty regarding decision making (score range, 3 to 15; higher score indicating less uncertainty). Participants' comfort level with the video was also measured.
Results
Fifty participants were randomly assigned to either the verbal narrative (n = 27) or video (n = 23). After the verbal description, 25.9% of participants preferred life-prolonging care, 51.9% basic care, and 22.2% comfort care. In the video arm, no participants preferred life-prolonging care, 4.4% preferred basic care, 91.3% preferred comfort care, and 4.4% were uncertain (P < .0001). The mean uncertainty score was higher in the video group than in the verbal group (13.7 v 11.5, respectively; P < .002). In the intervention arm, 82.6% of participants reported being very comfortable watching the video.
Conclusion
Compared with participants who only heard a verbal description, participants who viewed a goals-of-care video were more likely to prefer comfort care and avoid CPR, and were more certain of their end-of-life decision making. Participants reported feeling comfortable watching the video.
INTRODUCTION
Cancer is a common potentially life-threatening disease and is the second leading cause of death in both the United States1 and Europe.2 Previous studies suggest that patients with cancer wish to be involved in making decisions regarding their medical care at the end of life.3 Advance care planning (ACP) is the process by which patients and their physicians establish future goals of their end-of-life care, which offers patients the opportunity to define their goals and expectations.4,5
Only a minority of patients with cancer complete ACP documents.6,7 This lack of planning can have negative consequences including emotional distress for patients and caregivers, and medical care that is inconsistent with patients' wishes.8–10 Unfortunately, even when ACP discussions are initiated, they are often ineffective because of poor patient-physician communication and patients' lack of sufficient medical knowledge to engage in these discussions.11–15
Currently, most physicians engage in ACP discussions using verbal descriptions of possible goals-of-care options, such as cardiopulmonary resuscitation (CPR) and mechanical ventilation. These interventions may be difficult for patients to imagine using verbal descriptions alone. Video images have been shown to improve understanding of complex health information and inform decision making.16–18 A video supplementing verbal discussions has been shown to improve participants' understanding of the hypothetical health state of advanced dementia, increase their preference for comfort measures when the end of life is near, and decrease their uncertainty regarding decision making.16–18 However, prior studies have only been performed in people with dementia or in those who do not suffer from the actual disease. These studies have not included discussions of treatment options and decisions.
To extend prior work on the role of video in ACP, we conducted a randomized controlled trial of a video that depicts actual treatment options for various goals of care versus a verbal description alone among patients in the advanced stages of cancer, specifically malignant glioma. We hypothesized that participants randomly assigned to the video arm would be more likely to indicate a preference for comfort measures near the end of life, have improved knowledge of the various goals-of-care options, and have less uncertainty about their decision.
METHODS
Participants
Fifty consecutive patients with a diagnosis of malignant glioma were recruited from the outpatient oncology clinics at Massachusetts General Hospital from July 1, 2008, through March 31, 2009. All returning patients with a diagnosis of malignant glioma were approached by their primary oncologist during their scheduled clinic visit and asked if they would be interested in participating in the study. Patients with malignant glioma were chosen for this study due to their overall poor prognosis.19 We chose this disease rather than other cancer types with poor prognosis (eg, pancreatic, lung) due to the wider age-range seen in patients with malignant glioma, increasing the generalizability of our findings.20 If participants were interested in participating, they were then assessed for eligibility. Eligibility criteria included the ability to communicate in English, the ability to provide informed consent, and a Folstein Mini-Mental Status Examination (MMSE) score greater than or equal to 24.21
For eligible participants interested in the study, the oncologists introduced the concept of advance directives, end-of-life care planning, and defining goals-of-care preferences as an introductory discussion to the study.
Study Design and Randomization
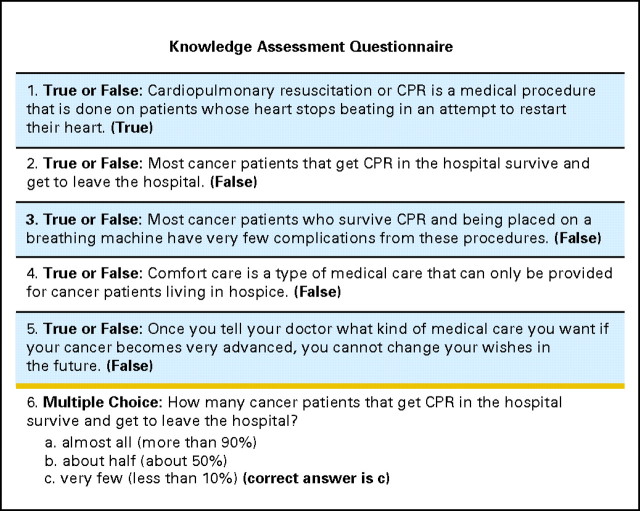
All interviews were conducted by two members of the research team (A.E.J., L.M.P.). After informed consent was obtained, participants underwent MMSE to ensure that they met the eligibility criteria (Fig 1). Eligible participants underwent a baseline assessment including collection of sociodemographic information, a knowledge assessment of the goals-of-care options, and individual preferences for CPR. The sociodemographic assessment included age, self-reported race, sex, religion, educational status, marital status, self-rated health status on a Likert scale (ie, excellent, very good, good, fair, or poor), and self-reported completion of advance directives (health-care proxy or living will). The knowledge assessment consisted of six questions, one multiple choice and five true/false questions that were designed to assess the participants' understanding of different levels of medical care in the advanced stages of cancer (Fig 2). Scores ranged from 0 to 6, with higher scores indicating more knowledge of the goals of care. Participants were also asked whether or not they would want CPR attempted in the advanced stages of cancer.
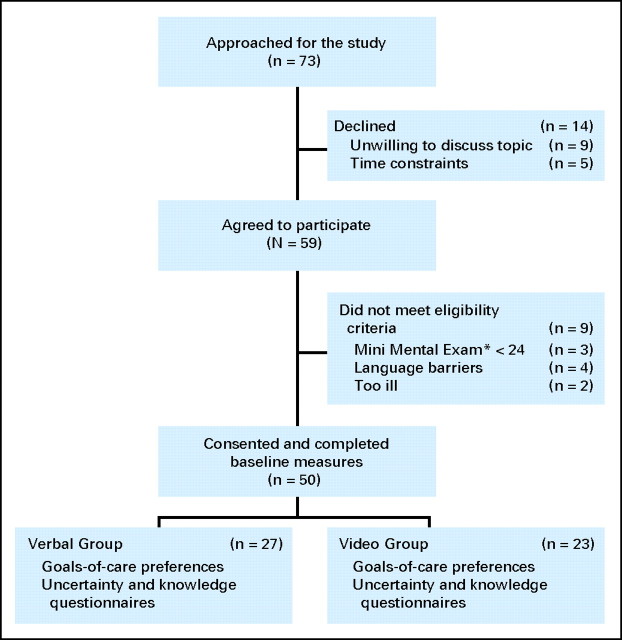
Fig 1.
CONSORT diagram of study process and participants' flow. (*) Mini Mental Exam, The Folstein Mini-Mental Status Examination (range, 0 to 30).
Fig 2.
Knowledge assessment questionnaire used at baseline and post-interventions for both the verbal and video groups (score range, 0 to 6).
Participants were then randomly assigned to either the control group (verbal narrative alone) or to the intervention group (video after verbal narrative) based on a list generated by a computer randomization scheme. Individual assignments were concealed in numbered envelopes, half of which were made available to each interviewer.
Participants randomly assigned to the verbal control group listened to a verbal narrative describing three levels of medical care in advanced cancer: life-prolonging care, basic medical care, and comfort care. The levels of medical care were identified by reviewing the goals-of-care literature and then reviewed and edited by 10 oncologists in an iterative process. Definitions for each of the levels were provided as follows: life-prolonging care aims to prolong life at any cost and includes all potentially indicated medical care including CPR, intubation, mechanical ventilation, and care in the intensive unit. Basic medical care aims to maintain physical and mental function and includes treatments such as hospitalization, intravenous fluids, and antibiotics, but excludes CPR, intubation, mechanical ventilation, and care in the intensive unit. Comfort care aims to maximize comfort and alleviate suffering, and would usually include medications to relieve symptoms but would not normally include hospitalization unless necessary to provide comfort (Appendix, online only).
Participants randomly assigned to the video group listened to the same verbal narrative as the control group followed by a 6-minute video presenting the three levels of medical care, shown on a portable computer. The definitions of the medical care levels were identical to the ones in the verbal narrative, but included visual images of the goals of care described. In the video, life-prolonging care images included: an intensive care unit with a ventilated patient being tended to by respiratory therapists; a simulated code with clinicians illustrating CPR and intubation; and various intravenous medications including vasopressors administered through a venous catheter. Visual images to depict basic medical care included: a patient getting antibiotics via a peripheral intravenous catheter; scenes from a typical medical ward service; and a patient wearing a nasal cannula. The video depiction of comfort care included: a patient on home hospice care receiving pain medications; a patient with a nasal cannula comfortable on oxygen at home; and a medical attendant assisting a patient with self-care.
The video's design, content, and structure were reviewed and edited for appropriateness and accuracy by 10 oncologists, three critical care intensivists, three palliative care physicians, and three medical ethics experts using an iterative process. The video was filmed without the use of prompts or stage directions to convey a candid realism in the style known as cinema verite.22 All filming and editing was done by the investigative team (A.E.V.) following previously published filming criteria.23
All participants were then asked to select which level of care they would prefer if their cancer became very advanced (life-prolonging, basic, or comfort care). They were also asked specifically whether they would want CPR attempted. Advanced cancer was defined to participants as being “very sick” in a situation where they may or may not be able to speak for themselves. All participants then underwent a decision conflict assessment regarding uncertainty, consisting of three Likert-type questions from the Decisional Conflict Scale.24 The uncertainty subset of the Decisional Conflict Scale is a well-validated and commonly used tool to assess uncertainty.24 The responses were converted to a numerical scale from 0 to 15, with 0 representing complete uncertainty and 15 perfect certainty. A higher Decisional Conflict score indicates a decrease in participants' overall uncertainty. We then asked the same six knowledge assessment questions that were asked at baseline.
Finally, for those participants randomly assigned to the video group, a 4-point Likert scale was used to assess perceived value of the video by asking participants whether they were comfortable watching the video, whether they found the video helpful in their understanding of the goals-of-care options, and whether they would recommend it to others. All data were collected in a private quiet room by a trained member of the research team, following a structured script.
Statistical Analysis
Data were analyzed based on the decision-making modality to which each participant was randomly assigned. The primary outcome measure was postintervention preference for care in advanced cancer categorized as four options (life-prolonging care, basic medical care, comfort care, and uncertain). Secondary outcomes included individual preferences for CPR before and after intervention in both the verbal and video groups; change in knowledge scores after receiving the verbal narrative or the video; and the level of uncertainty (decisional conflict scores) between verbal and video interventions.
All participants' characteristics were summarized using proportions for categoric variables and means (standard deviation) for continuous variables. Outcomes with 95% CI were reported. Preferences for postintervention care (life-prolonging, basic care, and comfort care) and CPR preferences were compared between the two groups using exact χ2 tests while the change in CPR preference before and after the intervention within each group were compared using McNemar tests. Changes in knowledge scores from before to after the intervention and decisional conflict scores were compared between the two groups using two-sample t-tests.
All reported P values were two sided, with P < .05 considered statistically significant. With a target of 25 patients in each group, the power of the study was estimated to be more than 90% to detect a 50% difference in the preference for comfort care between the two groups. Data were analyzed using SAS software, version 9.1 (SAS Institute Inc, Cary, NC).
RESULTS
Study Participants
A total of 73 patients were approached for the study (Fig 1). There were 14 patients who declined to participate, but they did not differ significantly from the recruited participants in terms of age, sex, or race/ethnicity. The most common reasons given for not participating were unwillingness to discuss the topic and time constraints. Nine patients were ineligible due to a MMSE score lower than 24, language barriers, or being too ill to participate in the discussion.
A total of 50 participants were randomly assigned to the verbal control group (n = 27) or the video intervention group (n = 23). Baseline characteristics of the participants are presented in Table 1. Despite random assignment, there were some baseline differences in the two groups, including a higher mean age, higher percentage of males, and a lower percentage of married participants in the video group. The two groups did not differ significantly in terms of their preferences for CPR or baseline knowledge scores before random assignment (Table 1).
Table 1.
Baseline Characteristics of Participants in the Verbal and Video Groups
| Characteristic | Verbal |
Video |
Total |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| No. of patients | 27 | 23 | 50 | |||
| Mean age, years | 51 | 56 | 54 | |||
| Standard deviation | 12 | 7 | 10 | |||
| Range | 32-72 | 47-77 | 32-77 | |||
| Female sex | 13 | 48.1 | 9 | 39.1 | 22 | 44.0 |
| White race | 24 | 88.9 | 22 | 95.7 | 46 | 92.0 |
| Education | ||||||
| Elementary | 0 | 0.0 | 1 | 4.3 | 1 | 2.0 |
| High school | 5 | 18.5 | 5 | 21.7 | 10 | 20.0 |
| Some college | 4 | 14.8 | 2 | 8.7 | 6 | 12.0 |
| College graduate | 10 | 37.0 | 8 | 34.8 | 18 | 36.0 |
| Post-graduate | 8 | 29.6 | 7 | 30.4 | 15 | 30.0 |
| Religious affiliation | ||||||
| Christian (non-Catholic) | 10 | 37.0 | 10 | 43.5 | 20 | 40.0 |
| Catholic | 7 | 25.9 | 8 | 34.8 | 15 | 30.0 |
| Jewish | 1 | 3.7 | 0 | 0.0 | 1 | 2.0 |
| Other | 9 | 33.3 | 5 | 21.7 | 14 | 28.0 |
| Marital status | ||||||
| Single | 3 | 11.1 | 2 | 8.7 | 5 | 10.0 |
| Married | 23 | 85.2 | 18 | 78.3 | 41 | 82.0 |
| Divorced | 1 | 3.7 | 3 | 13.0 | 4 | 8.0 |
| Advance directive* | ||||||
| Yes | 20 | 74.1 | 18 | 78.3 | 38 | 76.0 |
| Health status | ||||||
| Excellent/very good/good | 20 | 74.0 | 19 | 82.6 | 39 | 78.0 |
| Fair/poor | 7 | 26.0 | 4 | 17.4 | 11 | 22.0 |
| Type of brain tumor | ||||||
| Anaplastic astrocytoma | 9 | 33.3 | 4 | 17.4 | 13 | 26.0 |
| Glioblastoma | 17 | 63.0 | 19 | 82.6 | 36 | 72.0 |
| Anaplastic oligodendroglioma | 1 | 3.7 | 0 | 0.0 | 1 | 2.0 |
| Desire for CPR at baseline | ||||||
| Yes | 8 | 29.6 | 8 | 34.8 | 16 | 32.0 |
| No | 14 | 51.9 | 11 | 47.8 | 25 | 50.0 |
| Uncertain | 5 | 18.5 | 4 | 17.4 | 9 | 18.0 |
| Mean knowledge score at baseline† | 3.78 | 3.39 | 3.60 | |||
| Standard deviation | 1.37 | 1.23 | 1.31 | |||
| Range | 0-6 | 1-6 | 0-6 | |||
Abbreviation: CPR, cardiopulmonary resuscitation.
Advance directive included those participants having designated a health-care proxy or having completed a living will, or both.
Knowledge score range (0 to 6); higher score indicates more knowledge.
Postintervention Goals-of-Care Preferences
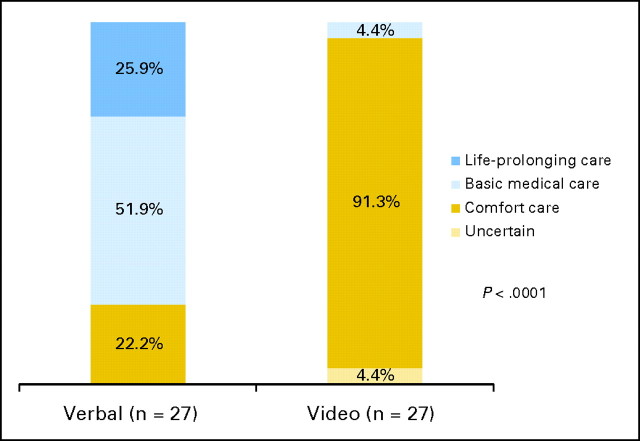
When asked to define their preferences regarding end-of-life care if their cancer became very advanced, among the 27 participants randomly assigned to the verbal control group, seven preferred life-prolonging care (25.9%), 14 preferred basic medical care (51.9%), and six preferred comfort care (22.2%; 95% CI, 8.6% to 42.3%). Among the 23 participants randomly assigned to the video intervention group, none preferred life-prolonging-care, one preferred basic medical care (4.4%), 21 preferred comfort care (91.3%; 95% CI, 72.0% to 98.9%), and one was uncertain of his preferences (4.4%; P < .0001; Fig 3).
Fig 3.
Participants' goals-of-care preferences for advanced cancer in the verbal and video groups.
CPR Preferences
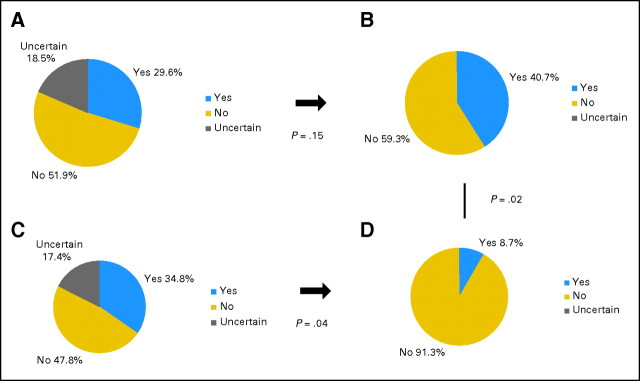
Figure 4 depicts CPR preferences for the two groups at baseline and after random assignment. Among the 27 participants in the verbal control group, eight indicated a willingness to undergo CPR (29.6%; 95% CI, 13.8% to 50.2%), 14 declined (51.9%), and five were uncertain at baseline (18.5%; Fig 4A). After the verbal narrative, the preference distribution was quite similar: 11 expressed a willingness to undergo CPR (40.7%; 95% CI, 22.4% to 61.2%) while 16 declined (59.3%; P = .15; Fig 4B). Among the 23 participants in the video group, eight indicated a willingness to undergo CPR (34.8%; 95% CI, 16.4%–57.3%), 11 declined (47.8%), and four were uncertain at baseline (17.4%; Fig 4C). After the intervention in the video group, only two expressed a willingness to undergo CPR (8.7%; 95% CI, 1.1% to 28.0%), and 21 declined CPR (91.3%; P = .04 for the change; Fig 4D). The distribution of postintervention CPR preferences between the two groups was significantly different (P = .02).
Fig 4.
Preferences for cardiopulmonary resuscitation at baseline and after the interventions in the verbal and video groups.
Uncertainty and Knowledge Scores
The mean uncertainty score (range, 3 to 15) was significantly higher in the video group compared with the verbal group (13.7 [95% CI, 12.8 to 14.6] v 11.5 [95% CI, 10.5 to 12.6], respectively; P = .002). Mean knowledge score (range, 0 to 6) was slightly lower in the video intervention group prerandom assignment compared with the verbal group 3.4 (95% CI, 2.9 to 3.9) versus 3.8 (95% CI, 3.2 to 4.3) respectively (P = .30), but higher postrandom assignment compared with those in the control group 5.3 (95% CI, 4.7 to 5.8) versus 4.6 (95% CI, 4.1 to 5.1) respectively (P = .08). The mean increase in knowledge score in the video intervention group was 1.9 (95% CI, 1.3 to 2.4) compared to 0.9 (95% CI, 0.4 to 1.3) in the verbal control group (P = .004).
Comfort With the Video
The video decision support tool was highly acceptable to participants in the intervention group: 82.6% (95% CI, 61.2% to 95.1%) of participants were “very comfortable” watching the video and 17.4% were “somewhat comfortable”; 78.3% found it to be “very helpful” (95% CI, 56.3% to 92.5%), 17.4% found it “somewhat helpful,” and 4.4% found it “a little helpful”; and 82.6% stated they would “definitely recommend” it to other patients with cancer facing similar decisions (95% CI, 61.2% to 95.1%), while 17.4% stated that they would “probably recommend” the video. There were no adverse events in either group.
DISCUSSION
This study presents an innovative video approach to ACP for patients with cancer. When faced with the possibility of their cancer progressing, participants with malignant glioma who viewed a video of the various goals-of-care options in addition to listening to a verbal description were more likely to prefer comfort measures and avoid CPR, were more knowledgeable regarding the subject matter, and were more certain of their decision when compared to patients only hearing a verbal narrative. In addition, the participants who viewed the video were comfortable watching the images, found the video to be helpful, and stated that they would recommend it to other patients with cancer.
To the best of our knowledge, this study represents the first randomized controlled trial looking at the usefulness of a video to facilitate ACP discussions for patients with cancer. Our findings are consistent with previous investigations looking at the utility of a video depicting the health state of advanced dementia to assist healthy older people in deciding on their preferences for end-of-life care if they were to develop advanced dementia. In the dementia studies, patients viewing the video showed improvement in their overall knowledge, had decreased uncertainty about their decision, and were also more likely to prefer comfort care.16–18 This work extends and builds on these studies by demonstrating the efficacy of the video support tool to discuss ACP for other life-threatening illnesses, such as cancer, and to visualize not just disease states but also possible treatment options. Most importantly, this work succeeds in showing the efficacy of this approach in patients with advanced cancer, where medical decision making is less hypothetical.
Our findings are also consistent with prior studies of ACP in cancer. Previous work has demonstrated that end-of-life discussions are associated with avoidance of CPR and mechanical ventilation near death and with earlier hospice referrals.25,26 These findings suggest that when patients have a better understanding of their goals-of-care options and the likely outcomes, they tend to opt for less aggressive medical care at the end of life, which is consistent with our results.
Physicians have consistently reported difficulty approaching end-of-life discussions and providing prognostic information regarding utilization of CPR in patients with advanced cancer.3,8,9 Physicians often underestimate the emotional resilience of patients and their desire to be involved in this decision-making process.3,8,9 Our participants have further confirmed this resilience.
Our study has several important limitations. First, the research staff was not blinded to the random assignment, which could have introduced bias. We utilized structured interviews and outcome measures, and a verbal script that was followed verbatim to reduce this bias. Second, this is a small pilot study with a sample size of 50 participants with malignant glioma, who were primarily white, well-educated, and drawn from clinics at one teaching hospital. Thus, our findings are not generalizable to minority groups, less-educated patients, patients in other geographical areas, and patients with other cancers. Third, despite random assignment, there were some baseline differences between the two groups that could have confounded our results, which can be expected in a relatively small sample. Fourth, the repetition of the verbal narrative in the video group (listening to the verbal narrative followed by the video with an identical narrative) could have potentially influenced the knowledge assessment results, but it would not fully explain the difference in distribution of preferences between the two groups. The purpose of the video is to reinforce the physician-patient discussion (simulated by the initial oncologist's introductory discussion of the topic and the verbal narrative). Hence, we considered this repetition an integral aspect of the intervention. Finally, an emotional response to the video could have influenced participants' preferences. To ensure that the video was not biased toward any particular perspective, the video content underwent extensive scrutiny by numerous oncologists, intensivists, palliative care physicians, and ethicists with particular expertise in this field. Participants' comfort level with the video is also reassuring against this possibility.
Previous uses of video decision support tools have traditionally focused on helping patients make treatment or screening decisions.27 Our use of video brings a novel approach to initiating end-of-life discussions. Video images could help patients visualize hypothetical goals-of-care options, discuss the likely outcomes, and make an informed decision regarding what is concordant with their preferences. Future work should focus on conducting larger randomized trials on the efficacy of the video in different patient populations with various cancers and various levels of prognostic uncertainty, determining the optimal timing for patients with cancer to view the videos, and integrating the underlying illness trajectory and prognosis in this discussion.
Involving patients with cancer in ACP empowers them by respecting their autonomy and offering them the relevant medical information to make informed decisions. Using video images to educate patients on various end-of-life interventions and outcomes is palatable to patients, leads to more informed decision making, and may potentially lead to higher quality end-of-life care.
Acknowledgment
We thank the patients and families who agreed to be filmed for this study, and Aretha Delight Davis, Jeffrey Rothschild, Chuck Pozner, and the STRATUS Center for Medical Simulation.
Appendix
Verbal narrative.
I would like to talk to you about decision you will have to make because you have an illness that is not curable. I would like to ask you about what kind of treatments you want in the event that you ended up in a situation where you became very sick. Keep in mind, in these situations you may or may not be able to tell your doctors what you want.
You might find it difficult to discuss this, but as it turns out, different people have different ideas about what is best for them. If we talk about your choices ahead of time, you can tell your doctors now what you would want later on. People sometimes change their minds about these decisions, and that is normal. But it's a good idea to start thinking now about what you would want if you became very sick. Here is one way to help you start thinking about your choices.
Basically, there are three general approaches to medical care if you became very sick. They are: life-prolonging care, basic medical care, and comfort care. Let's review these three general approaches together.
The first approach is life-prolonging care. With this approach, the main goal is to prolong life. This means that your doctors would do everything they could to keep you alive. They would do cardiopulmonary resuscitation or CPR. CPR tries to get your heart to beat again if it stops. This involves pressing on your chest and sometimes using an electric shock in an attempt to restart your heart. Most of the time in patients with advanced cancer, CPR does not work.
If you want life-prolonging approach, you would also agree to have a ventilator or breathing machine if you need it to keep breathing. This involves putting a tube down your throat into your lungs and connecting you to a machine that pushes air into your lungs. You cannot eat or talk while on this machine. If you are on a ventilator, you will likely need very strong medicines. These medicines are usually given in the intensive care unit or ICU. With the life-prolonging approach, you choose to have these done in exchange for the small possibility of a longer life. In most patients with advanced cancer, life-prolonging procedures do not return you to your previous state of health.
The second choice is basic medical care. With this approach, prolonging life is still important, but not if it means having CPR or being placed on a breathing machine. People who choose basic medical care would like medicines for treatable problems that may arise, for instance an infection in the lungs or in the urine. This approach lets you have many different treatments including hospital care, antibiotics, other medicines, and fluids through a vein. People who choose basic medical care do not want procedures such as CPR or breathing machines. They would choose to avoid these procedures even if without them they might die.
The third choice is comfort care. The main goal of this approach is to maximize comfort and relieve your symptoms. Treatments are only used if they help control uncomfortable symptoms such as pain, trouble breathing, or feeling sick to your stomach. The main goal is not to prolong life, it is to maximize comfort. People who choose this approach are treated at home, in hospice, or sometimes, in a nursing home. Comfort care does not include CPR, breathing machines, hospitalization, or antibiotics. People who choose comfort care want to avoid these procedures even though, without them, they might die. The goal of this approach is to maximize comfort and relieve symptoms.
Footnotes
Supported by the Foundation for Informed Medical Decision Making and NIH-NIA K24AG033640. The funding agencies had no role in the design or conduct of the study; the collection, analysis, or interpretation of the data; or the preparation, review, or approval of the article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00704886.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Areej El-Jawahri, Lisa M. Podgurski, April F. Eichler, Jennifer S. Temel, Susan L. Mitchell, Michael J. Barry, Angelo E. Volandes
Financial support: Michael J. Barry, Angelo E. Volandes
Administrative support: Areej El-Jawahri, Angelo E. Volandes
Provision of study materials or patients: April F. Eichler, Scott R. Plotkin
Collection and assembly of data: Areej El-Jawahri, Lisa M. Podgurski
Data analysis and interpretation: Areej El-Jawahri, Yuchiao Chang, Angelo E. Volandes
Manuscript writing: Areej El-Jawahri, Lisa M. Podgurski, April F. Eichler, Scott R. Plotkin, Jennifer S. Temel, Susan L. Mitchell, Yuchiao Chang, Michael J. Barry, Angelo E. Volandes
Final approval of manuscript: Areej El-Jawahri, Lisa M. Podgurski, April F. Eichler, Scott R. Plotkin, Jennifer S. Temel, Susan L. Mitchell, Yuchiao Chang, Michael J. Barry, Angelo E. Volandes
REFERENCES
- 1.Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- 3.Back AL, Anderson WG, Bunch L, et al. Communication about cancer near the end of life. Cancer. 2008;113:1897–1910. doi: 10.1002/cncr.23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emanuel LL, Danis M, Pearlman RA, et al. Advance care planning as a process: Structuring the discussions in practice. J Am Geriatr Soc. 1995;43:440–446. doi: 10.1111/j.1532-5415.1995.tb05821.x. [DOI] [PubMed] [Google Scholar]
- 5.Gillick MR. A broader role for advance medical planning. Ann Intern Med. 1995;123:621–624. doi: 10.7326/0003-4819-123-8-199510150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Emanuel LL, Barry MJ, Stoeckle JD, et al. Advance directives for medical care: A case for greater use. N Engl J Med. 1991;324:889–895. doi: 10.1056/NEJM199103283241305. [DOI] [PubMed] [Google Scholar]
- 7.Lamont EB, Siegler M. Paradoxes in cancer patients' advance care planning. J Palliat Med. 2000;3:27–35. doi: 10.1089/jpm.2000.3.27. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins NA, Ditto PH, Danks JH, et al. Micromanaging death: Process preferences, values, and goals in end-of-life medical decision making. Gerontologist. 2005;45:107–117. doi: 10.1093/geront/45.1.107. [DOI] [PubMed] [Google Scholar]
- 9.Emanuel EJ, Emanuel LL. The economics of dying: The illusion of cost savings at the end of life. N Engl J Med. 1994;330:540–544. doi: 10.1056/NEJM199402243300806. [DOI] [PubMed] [Google Scholar]
- 10.Fagerlin A, Schneider CE. Enough: The failure of the living will. Hastings Cent Rep. 2004;34:30–42. [PubMed] [Google Scholar]
- 11.The SUPPORT Principal Investigators. SUPPORT: A controlled trial to improve care for seriously ill hospitalized patients: The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT) JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 12.Hofmann JC, Wenger NS, Davis RB, et al. Patient preferences for communication with physicians about end-of-life decisions: SUPPORT Investigators: Study to Understand Prognoses and Preference for Outcomes and Risks of Treatment. Ann Intern Med. 1997;127:1–12. doi: 10.7326/0003-4819-127-1-199707010-00001. [DOI] [PubMed] [Google Scholar]
- 13.Covinsky KE, Fuller JD, Yaffe K, et al. Communication and decision-making in seriously ill patients: Findings of the SUPPORT project: The Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments. J Am Geriatr Soc. 2000;48:S187–S193. doi: 10.1111/j.1532-5415.2000.tb03131.x. [DOI] [PubMed] [Google Scholar]
- 14.Tulsky JA, Fischer GS, Rose MR, et al. Opening the black box: How do physicians communicate about advance directives? Ann Intern Med. 1998;129:441–449. doi: 10.7326/0003-4819-129-6-199809150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Roter DL, Larson S, Fischer GS, et al. Experts practice what they preach: A descriptive study of best and normative practices in end-of-life discussions. Arch Intern Med. 2000;160:3477–3485. doi: 10.1001/archinte.160.22.3477. [DOI] [PubMed] [Google Scholar]
- 16.Volandes AE, Lehmann LS, Cook EF, et al. Using video images of dementia in advance care planning. Arch Intern Med. 2007;167:828–833. doi: 10.1001/archinte.167.8.828. [DOI] [PubMed] [Google Scholar]
- 17.Volandes AE, Barry MJ, Chang Y, et al. Improving decision making at the end of life with video images. Med Decis Making. doi: 10.1177/0272989X09341587. in press. [DOI] [PubMed] [Google Scholar]
- 18.Volandes AE, Paasche-Orlow MK, Barry MJ, et al. Video decision support tool for advance care planning in dementia: Randomised controlled trial. BMJ. 2009;338:b2159. doi: 10.1136/bmj.b2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glare P, Christakis NA. New York, NY: Oxford University Press; 2008. Prognosis in advanced cancer. [Google Scholar]
- 20.Ohgaki H, Kleihues P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005;109:93–108. doi: 10.1007/s00401-005-0991-y. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Whitehouse PJ. Cognitive impairment of Alzheimer disease. Neurobehav Toxicol Teratol. 1983;5:631–634. [PubMed] [Google Scholar]
- 22.Grant BK, Sloniowski J. Detroit, MI: Wayne State University Press; 1998. Documenting the documentary: Close readings of documentary film and video. [Google Scholar]
- 23.Gillick MR, Volandes AE. The psychology of using and creating video decision aids for advance care planning. In: Lynch TE, editor. Psychology of Decision Making in Medicine and Health Care. New York, NY: Nova Science Publishers; 2007. pp. 193–206. [Google Scholar]
- 24.O'Connor AM. Validation of a decisional conflict scale. Med Decis Making. 1995;15:25–30. doi: 10.1177/0272989X9501500105. [DOI] [PubMed] [Google Scholar]
- 25.Wright AA, Zhang B, Ray A, et al. Associations between end-of-life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300:1665–1673. doi: 10.1001/jama.300.14.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prigerson HG. Socialization to dying: Social determinants of death acknowledgement and treatment among terminally ill geriatric patients. J Health Soc Behav. 1992;33:378–395. [PubMed] [Google Scholar]
- 27.O'Connor AM, Stacey D, Entwistle V, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2003:CD001431. doi: 10.1002/14651858.CD001431. [DOI] [PubMed] [Google Scholar]