Abstract
Background
Genetic factors and previous alcohol experience influence alcohol consumption in both humans and rodents. Specifically, a prior experience with ethanol increases ethanol intake in both ethanol-preferring C57BL/6 (C57) and ethanol non-preferring DBA/2 (DBA) mice. Whereas the ventral tegmental area (VTA) importantly regulates dopamine levels and ethanol intake, it is unknown whether ethanol experience differentially alters synaptic properties of VTA dopamine neurons in ethanol-preferring and non-preferring mice.
Methods
The properties of excitatory and inhibitory inputs and the ability to elicit long-term potentiation (LTP) were assessed with whole-cell patch-clamp recordings in VTA dopamine neurons from C57 and DBA mice 24 hours after a single ethanol (2 g/kg, IP) or equivalent saline injection.
Results
Ethanol exposure increased γ-aminobutyric acid (GABA) release onto VTA dopamine neurons in DBA mice, as previously observed in C57 mice. However, a single ethanol exposure reduced α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) and N-methyl-D-aspartate receptor (NMDAR) function and LTP in VTA dopamine neurons from DBA but not C57 mice.
Conclusions
A single ethanol exposure selectively reduced glutamate receptor function in VTA dopamine neurons from the ethanol non-preferring DBA strain but enhanced GABA signaling in both C57 and DBA strains. These results support the notion that VTA dopamine neurons are a central target of ethanol-induced neural plasticity, which could contribute to ethanol consumption. Furthermore, these findings highlight the possible need for specialized therapeutic interventions for alcoholism based on individual intrinsic differences.
Keywords: C57, DBA, dopamine, ethanol, plasticity, VTA
One major focus in alcoholism research is to identify neural adaptations responsible for the pathological consequences of alcohol abuse. Determining the causes of alcoholism and establishing effective treatment options is complex, because genetic factors (1) and previous experience with alcohol (2) can contribute to alcoholism. Similar to humans (1-3), rodent strains exhibit varying degrees of ethanol preference (4-7), which can be affected by prior ethanol experience (8-10). Many studies have examined differences between ethanol-preferring C57BL/6 (C57) and ethanol non-preferring DBA/2 (DBA) mouse strains in ethanol-related behaviors (4,5,11-14). For example, C57 mice prefer orally administered ethanol relative to DBA mice (4,5), although intravenous ethanol intake is similar between the strains (12). Ethanol produces greater conditioned taste aversion in DBA mice (12,13), which could influence the motivation for ethanol due to taste. Nonetheless, a single ethanol injection enhances ethanol consumption in both mouse strains (9). Thus, different plastic mechanisms could underlie the increased ethanol intake in ethanol-preferring and non-preferring mouse strains.
Ethanol can affect a number of molecular targets throughout the brain (15,16), but the ventral tegmental area (VTA), a major dopamine-producing brain structure, is thought to be especially important in the development of ethanol-related behaviors (3). For example, the VTA is required for the development of ethanol conditioned place preference (17), and rodents self-administer ethanol directly into the VTA (18,19). In addition, oral self-administration of ethanol is enhanced by systemic administration of dopamine receptor agonists and attenuated by dopamine receptor antagonists (20). Furthermore, ethanol intake is attenuated by strong VTA inactivation (21,22) or infusions of dopamine receptor antagonists into the nucleus accumbens (23,24), a region receiving strong VTA input. Interestingly, VTA dopamine neurons from C57 mice are less sensitive to excitatory effects of ethanol compared with DBA mice in vitro (25). Thus, the VTA and dopamine likely play an important role in many although perhaps not all (26) ethanol-related behaviors.
Whereas dopamine receptor activation augments ethanol consumption during self-administration, early withdrawal from ethanol is associated with a lower functionality of the mesolimbic dopaminergic system in human alcoholic subjects, which might arise from reduced glutamatergic input to dopamine neurons (27). A reduced dopaminergic function is observed in rodents after early (28-30 [but see 31]) and more chronic withdrawal after ethanol treatment (30,32,33), consistent with humans. These findings concur with the hypothesis that enhanced drug seeking during early withdrawal can result from a decrease in function in some brain circuits important for motivation and reinforcement (15,34). Furthermore, experience with addictive drugs, including ethanol, can alter the synaptic properties of VTA dopamine neurons, which is likely involved with many drug-related behaviors (35-40). Because a single exposure to ethanol increases ethanol consumption in rodents (9) and lower functionality of the dopamine system can be associated with increases in ethanol intake (6,7,27,30,34), we hypothesized that a single ethanol injection would reduce activity of the dopamine system, perhaps by altering the synaptic properties of VTA dopamine neurons and thus promote drinking in order to restore normal dopaminergic function (30). Here, we explored how a single in vivo exposure to ethanol and 24-hour withdrawal affected inhibitory and excitatory synaptic properties as well as the ability to elicit long-term potentiation (LTP) in VTA dopamine neurons from ethanol-preferring C57 and ethanol non-preferring DBA mice.
Methods and Materials
Electrophysiology
All procedures conformed to National Institutes of Health and Ernest Gallo Clinic and Research Center standards. We prepared horizontal VTA brain slices (170–230 μm) from P21–P25 C57 (Charles River, Hollister, California) or DBA (Jackson, Bar Harbor, Maine) mice anaesthetized with halothane until loss of righting reflex. We prepared brain slices 24 hours after mice received a single IP injection of saline or ethanol as described previously (38). Solutions were saturated with 95% oxygen–5% carbon dioxide. Brain slices were cut in a chilled solution containing, in mmol/L: 87 sodium chloride (NaCl), 2.5 potassium chloride (KCl), 1.25 sodium dihydrogen phosphate (NaH2PO4), 25 NaHCO3, .5 calcium chloride (CaCl2), 7 magnesium chloride (MgCl2), and 75 sucrose or 126 NaCl, 1.6 KCl, 1.2 NaH2PO4, 1.2 MgCl2, .625 CaCl2, 18 NaHCO3, and 11 glucose. Slices recovered >1 hour at 32°C in artificial cerebral spinal fluid, at 295–305 mOsm, and contained, in mmol/L: 126 NaCl, 2.5 or 1.6 KCl, 1.1 NaH2PO4, 1.4 MgCl2, 2.4 CaCl2, 11 d-glucose, and 26 NaHCO3.
We performed whole cell patch-clamp recordings with artificial cerebral spinal fluid (approximately 33°C) continuously perfused at approximately 2.0 mL/min and made recordings with 2–6 MΩ electrodes with an Axopatch 1D amplifier with filtering at 2 KHz and digitizing at 10 KHz. Igor Pro (Wavemetrics, Lake Oswego, Oregon) was used for data acquisition. Dopamine neurons were identified by the presence of Ih, which is found in >98% of mouse VTA dopamine neurons (41). The γ-aminobutyric acid (GABA)A inhibitory post-synaptic currents (IPSCs) were recorded with an internal solution containing 144 mmol/L KCl, 1 mmol/L CaCl2, 3.45 mmol/L potassium-1,2-bis(2-aminophenoxy)-ethane-N,N,N,N-tetraacetic acid, and 10 mmol/L N-2-hydroxyethylpiperazine-N’-2-ethane sulfonic acid (HEPES). Excitatory postsynaptic current (EPSC) recordings and LTP experiments used an internal solution containing, in mmol/L: 130 potassium hydroxide, 105 methanesulfonic acid, 17 hydrogen chloride, 20 HEPES, glycolbis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid (EGTA), and 2.8 NaCl. Voltage clamp recordings of miniature EPSCs (mEPSCs) used an internal solution containing: 117 mmol/L cesium methanesulfonate, 20 mmol/L HEPES, .4 mmol/L EGTA, 2.8 mmol/L NaCl, 5 mmol/L tetraethylammonium chloride. Internal recording solutions were 7.2–7.4 pH and 275–285 mOsm and contained 2.5 mg/mL magnesium adenosine triphosphate and .25 mg/mL magnesium guanidine triphosphate.
A bipolar stimulating electrode was placed 100–300 μm rostral to the recording electrode for stimulating IPSCs, EPSCs, and excitatory post-synaptic potentials (EPSPs) at .1 Hz. The GABAA IPSCs were recorded in the presence of 2-amino-5-phosphonopentanoic acid (AP5; 100 μmol/L), 6-cyano-2,3-dihy-droxy-7-nitro-quinoxaline (CNQX; 10 μmol/L), strychnine (1 μmol/L), and eticlopride (100 nmol/L) to block NMDAR, AMPAR, glycine, and dopamine D2 receptors, respectively. To minimize D2 receptor-mediated effects during IPSCs (42), we included eticlopride for IPSC experiments. For EPSC and LTP experiments and bath application of glutamate receptor agonists, the GABAA blocker picrotoxin (100 μmol/L) was added. We held neurons at −70 mV for experiments with LTP, IPSCs, EPSCs, and AMPA bath application and at +40 mV for experiments with the AMPAR/NMDAR and NMDA bath application. Cyclothiazide (100 μmol/L) was added 10 min before and during AMPA application to prevent desensitization of AMPARs that could confound accurate measurements of receptor currents (43). To calculate the AMPAR/NMDAR, an average of 12 EPSCs was computed before and after NMDAR block with AP5 (50 μmol/L). The NMDAR responses were calculated by subtracting the average response in the presence of AP5 (AMPAR only) from the current before AP5 addition. The AMPAR EPSC peak was divided by the NMDAR EPSC peak to yield the AMPAR/NMDAR.
We calculated paired-pulse ratios (PPR), with an interstimulus interval of 50 msec, as the ratio between the second and first IPSC or EPSC averaged over 10 min. The LTP was induced by pairing a set of five EPSPs with depolarizing current pulses sufficient to generate an action potential (2 msec, 1–2 nA) at 10 Hz. The EPSP and current-pulse pairings were repeated 20 times with 5 sec between each set. Spontaneous miniature EPSCs or IPSCs in the presence of 500 μmol/L lidocaine or 500 nmol/L tetrodotoxin (Alomone Labs, Jerusalem, Israel) were collected for 5 min or up to 300 detected events with Clampex (Axon Instruments, Cupertino, California), filtered at 1 KHz, and analyzed with Mini Analysis program (Synaptosoft, Decatur, Georgia). Each event was visually inspected to prevent noise disturbance of the analysis.
Results are presented as the mean ± SEM. Significance was determined between groups with unpaired Student t test. We used a minimum of three mice/condition. The AMPA, NMDA, and AP5 were purchased from Tocris (Ellisville, Missouri), TTX from (Alomone Labs), and all other chemicals from Sigma-Aldrich (St. Louis, Missouri).
Results
Increased Probability of GABA Release After Ethanol Exposure in DBA Mice
A single ethanol exposure augments ethanol consumption in both C57 and DBA mice (9) and is associated with an increase in spontaneous and stimulated GABA release onto VTA dopamine neurons in C57 mice (38). However, it is unknown whether synaptic changes occur on dopamine neurons from DBA mice 24 hours after a single ethanol injection (2 g/kg, IP) relative to similarly treated saline-injected mice.
Therefore, in DBA mice, we first examined miniature IPSCs (mIPSCs) on VTA dopamine neurons, which reflect the spontaneous GABA input onto the recorded neuron (44). Ethanol exposure significantly increased mIPSC event frequency in ethanol-injected mice (saline: .56 ± .10 Hz, n = 6; ethanol: 1.25 ± .24 Hz, n = 6; p < .05, Figures 1A–1C), which indicates that ethanol treatment affected GABA release on VTA dopamine neurons. No change was detected in mIPSCs amplitude between saline-injected (26.0 ± 1.4 pA, n = 6) and ethanol-injected (23.7 ± 1.7 pA, n = 6, Figures 1A, 1D, and 1E) DBA mice, suggesting that ethanol exposure did not alter post-synaptic GABA receptor function. Also, the PPR of IPSCs (IPSC2/IPSC1), which can indicate changes in stimulated GABA release (38), was reduced in ethanol-injected DBA mice (saline: .95 ± .09, n = 12; ethanol: .72 ± .07, n = 15; p < .05, Figures 1F and 1G). These results are similar to those observed in C57 mice (38), although unlike the C57 strain, GABAB receptor antagonists (100 μmol/L CGP-36742) did not modulate the PPR of IPSCs in saline- or ethanol-exposed DBA mice (% of baseline PPR after CGP: saline 87.4 ± 4.1%, n = 7; ethanol 105.1 ± 12.6%, n = 8). Nonetheless, these results demonstrate that a single ethanol exposure increased both spontaneous and stimulated GABA release on VTA dopamine neurons in DBA mice, consistent with our hypothesis that ethanol exposure might enhance ethanol preference by reducing the excitatory capacity of dopamine neurons.
Figure 1.

A single ethanol injection increased the probability of γ-aminobutyric acid release on ventral tegmental area dopamine neurons in ethanol non-preferring DBA/2 (DBA) mice. (A) Example miniature inhibitory post-synaptic currents (IPSCs) from mice receiving an ethanol (2 g/kg, IP) or equivalent saline injection 24 hours prior. Representative cumulative probability distributions are shown for the miniature IPSC (mIPSC) frequency (B) and amplitude (D) in saline- and ethanol-injected mice. The average mIPSC frequency (C) was increased in ethanol-treated relative to saline-treated mice, whereas there was no change in the average mIPSC amplitude (E). The paired-pulse ratio (with a 50-msec interstimulus interval) of IPSCs was reduced in ethanol-treated mice compared with saline-treated mice in a representative (F) and average of all recorded neurons (G).*p < .05 between treatment groups.
Ethanol Exposure Alters Spontaneous Excitatory Input on VTA Dopamine Neurons in DBA But Not C57 Mice
Although a single ethanol injection enhanced inhibitory input onto VTA dopamine neurons in both C57 (38) and DBA mice (Figure 1), it is unknown whether a single in vivo ethanol exposure affects excitatory input onto these neurons in either mouse strain after 24-hour withdrawal. Thus, we examined the effect of an ethanol injection in C57 and DBA mice on spontaneous miniature EPSCs (mEPSCs), which are mediated by AM-PAR currents at the holding potential of −70 mV (43).
In C57 mice, ethanol exposure did not alter either the frequency (saline: 1.95 ± .33 Hz, n = 8; ethanol: 1.72 ± .27, n = 6, p > .05, Figures 2A–2C) or amplitude of mEPSCs (saline: 10.85 ± .68 pA; ethanol: 11.93 ± .56 pA, p > .05, Figures 2A, 2D, and 2E) on VTA dopamine neurons, suggesting no change in glutamate release or post-synaptic AMPAR currents (45). In addition, the PPR of EPSCs (EPSC2/EPSC1) was unaffected by ethanol pretreatment (saline: .84 ± .07, n = 8; ethanol: .86 ± .06, n = 7, p > .05, Figures 2F and 2G), suggesting that ethanol exposure did not alter glutamate inputs onto VTA dopamine neurons of C57 mice.
Figure 2.

A single ethanol injection did not affect miniature excitatory post-synaptic current (mEPSC) or excitatory paired-pulse ratio (PPR) on ventral tegmental area dopamine neurons in ethanol-preferring C57BL/6 (C57) mice. (A) Example mEPSCs from mice receiving an ethanol or saline injection 24 hours prior. Representative cumulative probability distributions are shown for the mEPSC frequency (B) and amplitude (D) in saline- and ethanol-injected mice. There was no change in either the average mEPSC frequency (C) or amplitude (E). The PPR of EPSCs was not different between saline- and ethanol-treated mice in a representative (F) and average of all recorded neurons (G). p > .05 between treatment groups.
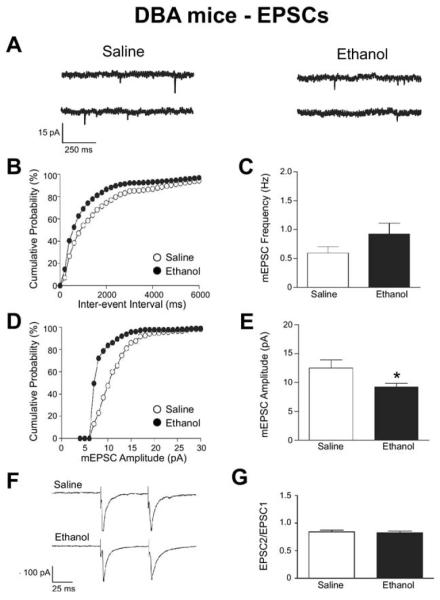
In DBA mice, ethanol treatment significantly reduced the amplitude of mEPSCs in VTA dopamine neurons (saline: 12.5 ± 1.4 pA, n = 7; ethanol: 9.2 ± .6 pA, n = 8; p < .05, Figures 3A, 2D, and 2E), indicating a reduction in post-synaptic AMPAR currents by ethanol exposure. In contrast, no changes by ethanol exposure in DBA mice were evident in mEPSC frequency (saline: .59 ± .11 Hz, n = 7; ethanol: .92 ± .19 Hz, n = 8, p > .05, Figures 3A–3C) or PPR (saline: .84 ± .03, n = 7; ethanol: .83 ± .03, n = 6, p > .05, Figures 3F and 3G), suggesting that a prior ethanol exposure did not affect spontaneous or stimulated glutamate release on dopamine neurons in DBA mice. Interestingly, the frequency of detected mEPSC events was lower in saline-treated DBA relative to C57 mice (p < .01, Figures 1 and 2). Regardless, these results suggest that ethanol exposure in DBA mice reduced post-synaptic AMPAR currents but not glutamate release on VTA dopamine neurons, with no changes in glutamatergic inputs in similarly treated C57 mice.
Figure 3.
A single ethanol injection reduced the probability of spontaneous glutamate release on ventral tegmental area dopamine neurons in DBA mice. (A) Example mEPSCs from mice receiving an ethanol or saline injection 24 hours prior. Representative cumulative probability distributions are shown for the mEPSC frequency (B) and amplitude (D) in saline- and ethanol-injected mice. The average mEPSC frequency (C) was decreased in ethanol-treated relative to saline-treated mice, whereas there was no change in the average mEPSC amplitude (E). The PPR of EPSCs was not different between saline- and ethanol-treated mice in a representative (F) and average of all recorded neurons (G).*p < .05 between treatment groups. Abbreviations as in Figures 1 and 2.
Ethanol Exposure Depresses the AMPAR/NMDAR in VTA Dopamine Neurons From DBA But Not C57 Mice
To further explore how 24-hour withdrawal after a single ethanol experience affected glutamate receptor currents in VTA dopamine neurons, we examined the AMPAR/NMDAR, which is a commonly used measure of excitatory synaptic strength (35,36,39,45,46). Because a single ethanol injection reduced AMPAR mEPSCs amplitude in VTA dopamine neurons of DBA but not C57 mice, we expected the AMPAR/NMDAR would also be reduced by ethanol experience in DBA but not in C57 mice. Consistent with our prediction, acute ethanol withdrawal did not alter the AMPAR/NMDAR in C57 mice (saline: .41 ± .05, n = 7; ethanol: .43 ± .07, n = 6, p > .05, Figures 4A and 4B), but significantly reduced the AMPAR/NMDAR in DBA mice (saline: .49 ± .03, n = 12; ethanol: .37 ± .03, n = 16, p < .05, Figures 4C and 4D). These results further support that ethanol selectively alters VTA synaptic glutamatergic function in DBA but not in C57 mice.
Figure 4.

A single ethanol injection reduced the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR)/N-methyl-D-aspartate receptor (NMDAR) in DBA mice, with no effect in C57 mice. The AMPAR/NMDAR was calculated by taking an average of 12 EPSCs at +40 mV before and after application of the NMDAR blocker AP5 (50 μmol/L). Thus, the current in the presence of AP5 (AMPAR only) would be subtracted from the total current before AP5, yielding the NMDAR component of the evoked current. The peak of the AMPAR EPSC was then divided by the peak of the NMDAR EPSC to yield an AMPAR/NMDAR. Examples (A,C) and grouped data (B,D) showing depressed AMPAR/NMDARs in ethanol-exposed DBA mice but not in ethanol-exposed C57 mice. *p < .05 between treatment groups. Other abbreviations as in Figures 1 and 2.
Ethanol Exposure Alters Glutamate Receptor Currents in VTA Dopamine Neurons From DBA But Not C57 Mice
A single ethanol injection reduced the amplitude of AMPAR mEPSCs in VTA dopamine neurons of DBA mice (Figures 3D and 3E), which perhaps suggests a reduction in post-synaptic AMPAR function. To directly ascertain the effect of previous ethanol exposure on post-synaptic glutamate currents, we bath-applied specific glutamate receptor agonists to dopamine neurons from saline- and ethanol-injected mice. The AMPA-induced current (2.5 μmol/L, 30 sec, held at −70 mV; see Methods) was not altered by ethanol exposure in C57 mice (saline: −724.3 ± 120.1 pA, n = 6; ethanol: −640.0 ± 116.1 pA, n = 6; Figures 5A and 5B), consistent with our results from the AMPAR mEPSC experiments (Figures 2D and 2E). There were also no differences in the NMDA-induced current between treatment groups in C57 mice in response to a 30-sec application of 10 μmol/L NMDA when holding the neuron at +40 mV (saline: 310.7 ± 96.2 pA, n = 6; ethanol: 308.2 ± 72.3 pA, n = 6, Figures 5C and 5D).
Figure 5.

A single ethanol injection did not affect AMPAR or NMDAR function on ventral tegmental area dopamine neurons in C57 mice. (A) The time course of current change by a 30-sec application of 2.5 μmol/L AMPA in the presence of 100 μmol/L cyclothiazide at −70 mV in saline- and ethanol-injected C57 mice. (B) The maximal AMPA-induced change in current in saline- and ethanol-injected mice. (C) The time course of current change by a 30-sec application of 10 μmol/L NMDA at +40 mV in both saline- and ethanol-injected C57 mice. (D) The maximal NMDA-induced change in current in both saline- and ethanol-injected mice. p > .05 between treatment groups. Other abbreviations as in Figures 1, 2, and 3.
Interestingly, ethanol treatment did not alter AMPA-induced currents in DBA mice (saline: −468.8 ± 48.7 pA, n = 6; ethanol: −540.1 ± 73.2, n = 6; Figures 6A and 6B). Because reduced mEPSC amplitudes in ethanol-exposed DBA mice (Figures 3D and 3E) could indicate decreased synaptic AMPAR function, the lack of an effect on AMPA-generated currents suggests that bath applied AMPA could primarily activate extrasynaptic AMPARs (47). It should be noted that there was a non-significant trend for a greater AMPA current in saline-exposed C57 relative to DBA mice (p = .08). Additionally, ethanol exposure in DBA mice significantly reduced the NMDA-induced currents (saline: 404.2 ± 41.6 pA, n = 6; ethanol: 185.6 ± 48.9 pA, n = 6, p < .05, Figures 6C and 6D). Together, our results indicate that withdrawal from a single ethanol injection alters AMPAR and NMDAR function in VTA dopamine neurons in DBA mice, whereas no such changes are observed in C57 mice.
Figure 6.

A single ethanol injection reduced NMDAR function on ventral tegmental area dopamine neurons in DBA mice. (A) The time course of current change by a 30-sec application of 2.5 μmol/L AMPA in the presence of 100 μmol/L cyclothiazide at −70 mV in saline- and ethanol-injected DBA mice. (B) The maximal AMPA-induced change in current in saline- and ethanol-injected mice. (C) The time course of current change by a 30-sec application of 10 μmol/L NMDA at +40 mV in both saline- and ethanol-injected DBA mice. (D) The maximal NMDA-induced change in current in both saline- and ethanol-injected mice. **p < .01 between treatment groups. Other abbreviations as in Figures 1, 2, and 3.
Ethanol Exposure Alters Synaptic Plasticity in VTA Dopamine Neurons From DBA But Not C57 Mice
The effects of abused drugs on the synaptic plasticity of VTA dopamine neurons might be involved in the development of drug-related behaviors (40,48), and in vivo drug exposure alters the capacity of VTA dopamine neurons to express LTP (35-37,39), a synaptic process thought to play a role in learning and memory (40,49,50). The LTP of excitatory synapses on VTA dopamine neurons requires NMDARs (37,51), and we interestingly found that ethanol exposure selectively reduced NMDAR function in DBA mice (Figures 6C and 6D). Therefore, we hypothesized that a single injection of ethanol 24 hours prior would not alter the induction of LTP in C57 mice but would attenuate LTP in DBA mice.
Long-term potentiation of EPSPs was generated in VTA dopamine neurons in C57 mice receiving either a single injection of saline or ethanol (saline: 124.4 ± 12.3% of baseline EPSP, n = 9; ethanol: 120.0 ± 10.1% of baseline EPSP, n = 6, Figures 7A–7C). However, ethanol treatment significantly reduced LTP in DBA mice (saline: 135.4 ± 9.9% of baseline EPSP, n = 6; ethanol: 111.4 ± 4.9% of baseline EPSP, n = 7, p < .05, Figures 7B and7D). Thus, the reduced NMDAR function after ethanol exposure in DBA mice impaired LTP generation, highlighting the physiological relevance of the selective ethanol-induced alteration in VTA glutamatergic function in DBA mice.
Figure 7.
A single ethanol injection impaired long-term potentiation (LTP) generation in DBA mice, with no effect in C57 mice. The LTP was induced by pairing a set of five EPSPs with depolarizing current pulses (2 msec, 1–2 nA) at 10 Hz. The EPSP and current-pulse pairings were repeated 20 times with 5 sec between each set. (A) The time course of the average change in EPSPs (left) and EPSPs of a representative neuron (right) from saline- and ethanol-injected C57 mice. (C) The average percentage change in EPSPs 30–35 min after induction in C57 mice injected with saline or ethanol. (B) The time course of the average change in EPSPs (left) and EPSPs of a representative neuron (right) from saline- and ethanol-injected DBA mice. (D) The average percentage change in EPSPs 30–35 min after induction in DBA mice injected with saline or ethanol. Upward arrow (A,B) signifies time of LTP induction. *p < .05 between treatment groups. Other abbreviations as in Figures 1 and 2.
Discussion
In this study, we examined the effect of withdrawal after a single ethanol injection on synaptic inputs onto VTA dopamine neurons from ethanol-preferring C57 mice and ethanol non-preferring DBA mice. We observed that, similar to C57 mice (38), ethanol-treated DBA mice exhibited increased spontaneous and stimulated GABA release on dopamine neurons. Interestingly, acute withdrawal from ethanol treatment reduced AMPAR and NMDAR function and impaired the ability to generate LTP in VTA dopamine neurons from DBA mice, with no observed changes in glutamatergic function in C57 mice. Because increased ethanol consumption after acute withdrawal is associated with decreased dopamine system function (27,30,34), the enhanced ethanol consumption after acute withdrawal from ethanol exposure in C57 and DBA mouse strains might result in part from a lower functionality of the dopamine system induced by the differential synaptic changes on VTA dopamine neurons that we report here and in our previous work (38). Furthermore, we hypothesize that augmented inhibitory GABAergic input onto dopamine neurons might be sufficient to increase ethanol intake in the ethanol-preferring C57 strain (38), whereas both enhanced GABAergic and reduced excitatory synaptic strength onto dopamine neurons are necessary for promoting ethanol consumption in the ethanol non-preferring DBA strain.
Surprisingly, very little was known about the effect of ethanol on excitatory synaptic input on VTA dopamine neurons, although it was recently suggested that this might be altered in human alcoholic subjects in withdrawal (27). First, we found that ethanol exposure in DBA mice reduced synaptic AMPAR function in dopamine neurons, indicated by a decreased amplitude of AMPAR mEPSCs. The AMPAR/NMDAR, which is a measure of excitatory synaptic strength (35,36,39,45), was attenuated in ethanol-injected DBA mice, likely resulting from the decreased AMPAR function we identified in our mEPSC experiments, although AMPAR/NMDAR alterations could also arise from changes in NMDAR function or from functional changes in both AMPAR and NMDARs. As a final assessment of the effect of ethanol experience on AMPAR function in the VTA, we bath-applied AMPA to dopamine neurons and unexpectedly found no difference between saline- and ethanol-treated DBA mice. This finding suggests that currents generated by AMPA bath application might primarily reflect extra-synaptic receptors (47), although we cannot rule out a possible compensatory increase in extrasynaptic AMPAR as well as a decrease in synaptic AMPAR after ethanol exposure in DBA mice, and this remains a question for future study.
We next examined NMDAR function after acute withdrawal from ethanol treatment and found that the NMDA-induced current was significantly attenuated in ethanol-treated DBA mice, suggesting a reduced NMDAR function. The NMDARs are critical for many forms of LTP (37,51,52), a synaptic phenomenon thought to contribute to learning, memory, and substance abuse (40,49,50). Accordingly, we also observed a reduced expression of LTP in VTA dopamine neurons from ethanol-exposed DBA mice but not in C57 mice. Because the NMDA-induced current was reduced in ethanol-treated DBA mice, we might have expected that this would translate into a larger AMPAR/NMDAR, the opposite of what we observed. However, as noted previously, bath application of glutamate receptor agonists can activate synaptic and extrasynaptic receptors (47), whereas the AMPAR/NMDAR reflects activation of synaptic glutamate receptors (45). Finally, it should be noted that the reduced function of glutamate receptors we report could reflect either a reduced ionic conductance and/or number of receptors expressed on the cell surface. Nonetheless, our results suggest that both AMPAR and NMDAR function are inhibited after a single ethanol exposure selectively in DBA mice.
Human and rodent studies demonstrate a reduced function of the dopamine system during withdrawal from alcohol (27-30), and drug-seeking can result from decreased function in some brain circuits important for reinforcement and motivation (15). Because the VTA and dopamine are important for reinforcing behaviors and motivation (53,54), we hypothesize that a reduced function of the dopamine system underlies the increased ethanol consumption after withdrawal from ethanol exposure in C57 and DBA mice (9). Also, because ethanol increases VTA dopamine neuron firing (25), increased ethanol consumption during early withdrawal might serve to restore normal levels of dopamine function (15,30). Although our study examined the differential synaptic changes after a single ethanol exposure that is associated with increased ethanol consumption, we should note that several distinct mechanisms could contribute to the many cellular and behavioral differences that have been identified between C57 and DBA strains (9,12,14,55). Thus, differential ethanol consumption between C57 and DBA mice is likely regulated in a complex manner by prior ethanol experience (9), differences in metabolism (14,55), taste (14), and the baseline properties of dopamine neurons including the differences in mEPSC frequency we report here and in the excitability by ethanol application (25). We did not examine firing properties here, although AMPARs, NMDARs, and GABA receptors can significantly regulate dopamine neuron firing activity (31,56-59). Possible differences in the regulation of intrinsic firing activity after ethanol treatment represent an interesting future direction, as will the dose-response effect of ethanol exposure and the time course of the observed plastic changes on VTA dopamine neurons.
Many have suggested that increased AMPAR function in VTA dopamine neurons might contribute to an enhanced motivational importance of drugs or drug-related cues (35,36,39,60), although increased excitatory synaptic input onto dopamine neurons does not always associate with the development of drug-dependent behaviors (46,61,62). We believe that the behavioral consequence of changes in glutamate receptor function varies, depending upon the behavioral conditions and the timing of the observation; therefore, drug seeking is likely not a unitary phenomenon (63). For example, antagonizing dopamine signaling attenuates ethanol consumption (20,21,23,24), whereas lower dopamine system function during acute withdrawal is associated with increased ethanol intake (30). Here, we observed a decreased AMPAR/NMDAR after acute withdrawal from ethanol exposure only in DBA mice, in agreement with reduced mEPSC amplitudes and also with the hypothesis that withdrawal from ethanol reduces dopaminergic function (15). We note that a previous study found an increased AMPAR/NMDAR ratio in C57 mice 24 hours after ethanol exposure (60), which was not observed here. This previous study used a 20% ethanol solution relative to the 10% ethanol solution in our current findings, which could have elevated the AMPAR/NMDAR through stress (60) associated with the irritant effect of ethanol.
Alcoholism research attempts to identify the neural adaptations associated with the disease, whereby highlighting potential therapeutic targets. Because alcoholism involves both genetic and environmental components (1,2), it is often difficult to determine the critical neural regions associated with alcoholism, especially because the primary effects of ethanol are widespread throughout the central nervous system (15,16), although a number of studies have identified a role for the VTA and dopamine in ethanol-related behaviors (3,17-19,21-23,64). Many lines of evidence suggest that lower function of the dopamine system is associated with increased alcohol consumption in both rodents and humans (6,7,27,30,34), perhaps reflecting the drive to overcome a low dopamine state during early withdrawal (15). Thus, we speculate that the changes in both GABA and glutamatergic VTA function in DBA mice that we observed might contribute to the ability of a single ethanol exposure to increase ethanol consumption in this normally ethanol non-preferring mouse strain, whereas only changes in GABA release are required to enhance intake in the ethanol-preferring C57 mouse strain (9,38). These results not only demonstrate that VTA dopamine neurons are a central target of ethanol-induced synaptic alterations but also suggest that these neuroadaptations depend on preference to ethanol, which highlights the possibility that differential therapeutic interventions might be warranted for treating human alcoholic subjects.
Acknowledgments
Support was provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (AB), and the National Institutes of Health, R01 DA016782-04 (AB), F31 DA21464-01 (MW), T32 AA009455 (MW), and the Department of the Army Grant #DAMD17-01-10736 (AB). The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, Maryland 21702-5014, is the awarding and administering acquisition office. The content of the information represented does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. The authors report no biomedical financial interests or conflicts of interest.
We thank Lisa G. Daitch for proofreading the manuscript and for the critical input of all members of the Bonci lab.
Footnotes
The authors MJW and DRS contributed equally to this work.
References
- 1.True WR, Xian H, Scherrer JF, Madden PA, Bucholz KK, Heath AC, et al. Common genetic vulnerability for nicotine and alcohol dependence in men. Arch Gen Psychiatry. 1999;56:655–661. doi: 10.1001/archpsyc.56.7.655. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: Results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- 3.Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Belknap JK, Richards SP, O’Toole LA, Helms ML, Phillips TJ. Short-term selective breeding as a tool for QTL mapping: Ethanol preference drinking in mice. Behav Genet. 1997;27:55–66. doi: 10.1023/a:1025615409383. [DOI] [PubMed] [Google Scholar]
- 5.Meliska CJ, Bartke A, McGlacken G, Jensen RA. Ethanol, nicotine, amphetamine, and aspartame consumption and preferences in C57BL/6 and DBA/2 mice. Pharmacol Biochem Behav. 1995;50:619–626. doi: 10.1016/0091-3057(94)00354-8. [DOI] [PubMed] [Google Scholar]
- 6.Quintanilla ME, Bustamante D, Tampier L, Israel Y, Herrera-Marschitz M. Dopamine release in the nucleus accumbens (shell) of two lines of rats selectively bred to prefer or avoid ethanol. Eur J Pharmacol. 2007;573:84–92. doi: 10.1016/j.ejphar.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 7.Ramachandra V, Phuc S, Franco AC, Gonzales RA. Ethanol preference is inversely correlated with ethanol-induced dopamine release in 2 substrains of C57BL/6 mice. Alcohol Clin Exp Res. 2007;31:1669–1676. doi: 10.1111/j.1530-0277.2007.00463.x. [DOI] [PubMed] [Google Scholar]
- 8.Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- 9.Camarini R, Hodge CW. Ethanol preexposure increases ethanol self-administration in C57BL/6J and DBA/2J mice. Pharmacol Biochem Behav. 2004;79:623–632. doi: 10.1016/j.pbb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: An overview. Addict Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- 11.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 12.Grahame NJ, Cunningham CL. Intravenous ethanol self-administration in C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 1997;21:56–62. [PubMed] [Google Scholar]
- 13.Risinger FO, Brown MM, Doan AM, Oakes RA. Mouse strain differences in oral operant ethanol reinforcement under continuous access conditions. Alcohol Clin Exp Res. 1998;22:677–684. doi: 10.1111/j.1530-0277.1998.tb04311.x. [DOI] [PubMed] [Google Scholar]
- 14.Risinger FO, Cunningham CL. Genetic differences in ethanol-induced hyperglycemia and conditioned taste aversion. Life Sci. 1992;50:PL113–118. doi: 10.1016/0024-3205(92)90463-y. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Le Moal M. Addiction and the brain antireward system. Ann Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 16.Ron D, Jurd R. The “ups and downs” of signaling cascades in addiction. Sci STKE 2005. 2005:re14. doi: 10.1126/stke.3092005re14. [DOI] [PubMed] [Google Scholar]
- 17.Bechtholt AJ, Cunningham CL. Ethanol-induced conditioned place preference is expressed through a ventral tegmental area dependent mechanism. Behav Neurosci. 2005;119:213–223. doi: 10.1037/0735-7044.119.1.213. [DOI] [PubMed] [Google Scholar]
- 18.Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 19.Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- 20.Cohen C, Perrault G, Sanger DJ. Preferential involvement of D3 versus D2 dopamine receptors in the effects of dopamine receptor ligands on oral ethanol self-administration in rats. Psychopharmacology. 1998;140:478–485. doi: 10.1007/s002130050792. [DOI] [PubMed] [Google Scholar]
- 21.Hodge CW, Haraguchi M, Erickson H, Samson HH. Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodd ZA, Oster SM, Ding ZM, Toalston JE, Deehan G, Bell RL, et al. The reinforcing properties of salsolinol in the ventral tegmental area: Evidence for regional heterogeneity and the involvement of serotonin and dopamine. Alcohol Clin Exp Res. 2008;32:230–239. doi: 10.1111/j.1530-0277.2007.00572.x. [DOI] [PubMed] [Google Scholar]
- 23.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: Further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 25.Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- 26.Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmacology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, et al. Profound decreases in dopamine release in striatum in detoxified alcoholics: Possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain Res. 1998;803:144–152. doi: 10.1016/s0006-8993(98)00654-4. [DOI] [PubMed] [Google Scholar]
- 29.Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: Electrophysiological and biochemical evidence. Proc Natl Acad SciUSA. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, et al. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. J Neurosci. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- 32.Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- 33.Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biol Psychiatry. 2007;61:93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 34.George SR, Fan T, Ng GY, Jung SY, O’Dowd BF, Naranjo CA. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: Reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273:373–379. [PubMed] [Google Scholar]
- 35.Bellone C, Luscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 36.Chen BT, Bowers MS, Martin M, Hopf FW, Gilroy AM, Chou JK, et al. Cocaine but not natural reward self-administration nor passive cocaine injection produces persistent LTP in the VTA. Neuron. 2008;59:288–297. doi: 10.1016/j.neuron.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 40.Kauer JA. Learning mechanisms in addiction: Synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu Rev Physiol. 2004;66:447–475. doi: 10.1146/annurev.physiol.66.032102.112534. [DOI] [PubMed] [Google Scholar]
- 41.Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotrop-in-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Awatramani GB, Turecek R, Trussell LO. Staggered development of GABAergic and glycinergic transmission in the MNTB. J Neurophysiol. 2005;93:819–828. doi: 10.1152/jn.00798.2004. [DOI] [PubMed] [Google Scholar]
- 45.Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 46.Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: Electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adesnik H, Nicoll RA, England PM. Photoinactivation of native AMPA receptors reveals their real-time trafficking. Neuron. 2005;48:977–985. doi: 10.1016/j.neuron.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 48.Kim JA, Pollak KA, Hjelmstad GO, Fields HL. A single cocaine exposure enhances both opioid reward and aversion through a ventral tegmental area-dependent mechanism. Proc Natl Acad Sci U S A. 2004;101:5664–5669. doi: 10.1073/pnas.0401373101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, et al. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 51.Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport. 1999;10:221–226. doi: 10.1097/00001756-199902050-00004. [DOI] [PubMed] [Google Scholar]
- 52.Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu Rev Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- 54.Salamone JD, Correa M. Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 55.Eriksson CJ, Atkinson N, Petersen DR, Deitrich RA. Blood and liver acetaldehyde concentrations during ethanol oxidation in C57 and DBA mice. Biochem Pharmacol. 1984;33:2213–2216. doi: 10.1016/0006-2952(84)90656-7. [DOI] [PubMed] [Google Scholar]
- 56.Chandler LJ, Carpenter-Hyland E, Hendricson AW, Maldve RE, Morrisett RA, Zhou FC, et al. Structural and functional modifications in glutamateric synapses following prolonged ethanol exposure. Alcohol Clin Exp Res. 2006;30:368–376. doi: 10.1097/01.ALC.0000167959.84516.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komendantov AO, Komendantova OG, Johnson SW, Canavier CC. A modeling study suggests complementary roles for GABAA and NMDA receptors and the SK channel in regulating the firing pattern in midbrain dopamine neurons. J Neurophysiol. 2004;91:346–357. doi: 10.1152/jn.00062.2003. [DOI] [PubMed] [Google Scholar]
- 58.Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- 59.Seutin V, Johnson SW, North RA. Apamin increases NMDA-induced burst-firing of rat mesencephalic dopamine neurons. Brain Res. 1993;630:341–344. doi: 10.1016/0006-8993(93)90675-d. [DOI] [PubMed] [Google Scholar]
- 60.Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 61.Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: A trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- 62.Wanat MJ, Bonci A. Dose-dependent changes in the synaptic strength on dopamine neurons and locomotor activity after cocaine exposure. Synapse. 2008;62:790–795. doi: 10.1002/syn.20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: Links to drug addiction. Curr Opin Neurobiol. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 64.Hodge CW, Haraguchi M, Chappelle AM, Samson HH. Effects of ventral tegmental microinjections of the GABAA agonist muscimol on self-administration of ethanol and sucrose. Pharmacol Biochem Behav. 1996;53:971–977. doi: 10.1016/0091-3057(95)02146-9. [DOI] [PubMed] [Google Scholar]