Abstract
Recent research has indicated that the antimicrobial chemical triclocarban (TCC) represents a new type of endocrine disruptor, amplifying the transcriptional activity of steroid hormones and their receptors while itself exhibiting little affinity for these receptors. The effects of TCC were studied in the freshwater mudsnail Potamopyrgus antipodarum. Specimens were exposed to concentrations ranging from 0.05 to 10.5 μg/L dissolved TCC and were removed and dissected, and embryos contained within the brood pouch were counted and classified as shelled or unshelled after2 and 4weeksof exposure. After 4 weeks, environmentally relevant TCC concentrations of 1.6 to 10.5 μg/L resulted in statistically significant increases in the number of unshelled embryos, while 0.2, 1.6, and 10.5 μg/L exposures significantly increased numbers of shelled embryos. The lowest observed effect concentration (LOEC) was 0.2 μg/L, the no observed effect concentration (NOEC) was 0.05 μg/L, and the EC10 and EC50 for unshelled effects were 0.5 μg/L and 2.5 μg/L, respectively. Given the widespread occurrence of TCC in the environment and effects shown at environmentally relevant concentrations, these results indicate that TCC may be causing reproductive effects in the environment. Furthermore, the present study indicates that environmental risk from a new class of EDCs is both qualitatively and quantitatively similar to risk from existing classes of EDCs.
Keywords: Emerging contaminants, Reproduction, Endocrine disruptors, Personal care products, Snails
Introduction
Many synthetic organic chemicals have been classified as endocrine disrupting chemicals (EDCs) due to their ability to interact with and alter endocrine systems and cause adverse health effects in organisms or their offspring. In humans, there is evidence and concern that these chemicals may be contributing to various types of cancer[1, 2], abnormal timing of the onset of puberty[3], and fetal abnormalities[4, 5]. In wildlife, effects range from feminization, hermaphroditism, and intersexuality [6, 7] to impacts on fertility and fecundity [8, 9] to behavioral effects [10], and in some cases complete collapse of populations has been documented [11, 12].
There is a large body of literature concerning EDCs with estrogenic or androgenic potential [13]. Many of these studies address the question of whether a single chemical alone acts as an endocrine disruptor, generally as an agonist or antagonist to one of the steroid hormone receptors but also through non-receptor mediated modes of action. New research suggests that the chemical triclocarban (TCC; Figure 1) , long suspected to interfere with reproduction in rats and rabbits [14], exhibits a novel form of endocrine disruption. Triclocarban alone exhibits little or no activity towards steroid hormone receptors but amplifies transcriptional activity of steroid sex hormones in the estrogen and androgen receptors, both in human cell lines[15]. In vivo, when added to a diet containing a high amount of testosterone, it significantly increased male sex organ weight relative to control diets, or those diets containing testosterone or TCC alone in castrated rats [16].
Figure 1.
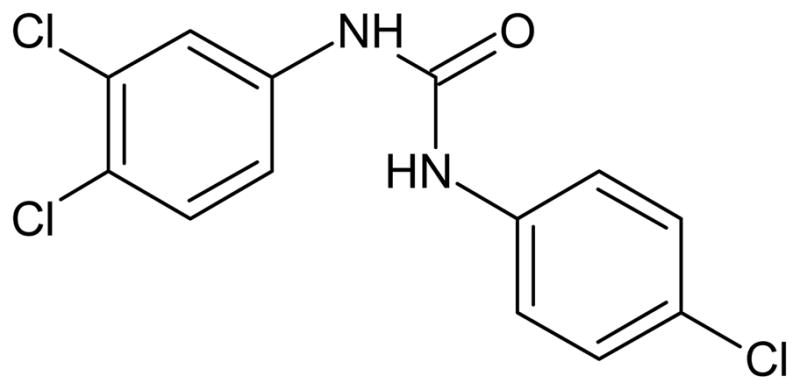
Chemical structure of triclocarban (TCC) .
Triclocarban was introduced to commerce in the United States in 1957, and has been routinely added to cosmetics and personal care products since then. Annual production in the United States is estimated at between 500,000 and 1,000,000 lbs (225,000 to 450,000 kgs) per year[17]. Triclocarban is incompletely removed in wastewater treatment plants. Most partitions into sludge, but some is also discharged in effluent accompanying steroid hormones and other EDCs[18, 19], causing a potential risk to aquatic organisms downstream. Itis estimated to be detectable in 60% of US streams with mean and median concentrations of 213 and 109 ng/L, respectively[18].
Given its characteristics as a new kind of endocrine disruptor and its widespread occurrence in the aquatic environment, there is a need to determine if TCC poses a demonstrated risk to aquatic species. The test species for these experiments is the freshwater mudsnail Potamopyrgus antipodarum (Gastropoda, Prosobranchia, Hydrobiidae) , commonly called the New Zealand Mudsnail, which has previously been used in a whole organism bioassay for estrogenic and androgenic endocrine disrupting effects [20]. Native to New Zealand, it was introduced to Europe in the mid 1800s [21] and North America in the late 1980s [22]. Unlike its native range where males are present and it reproduces sexually, in its invasive range, the species is almost exclusively female and is ovoviviparous and parthenogenetic [22].
The objectives of the present study are to determine: (1) whether environmentally relevant concentrations of TCC impact reproductive output in P. antipodarum, and if so (2) whether TCC causes effects that are distinguishable from the effects of traditional estrogen receptor agonists in vivo. The hypothesis is that TCC will increase reproductive output in a dose-dependent manner. The reasoning behind this is that TCC acts by enhancing the transcriptional activity of endogenous estrogens present in the female, and this should lead to increased numbers of embryos within the brood pouch, as has been found in experiments with other exogenous estrogenic EDCs[23].
Methods and materials
Chemicals
Sea salt was obtained from Aquarium Pharmaceuticals (Chalfont, PA). Calcium carbonate, sodium bicarbonate, reagent alcohol, acetonitrile, methanol, ethyl acetate, acetone, and acetic acid were all obtained from Fisher Scientific (Pittsburgh, PA) and were the highest grade available. Triclocarban (3,4,4’-Trichlorocarbanilide, 99% purity) was obtained from Aldrich (St. Louis, MO). Deuterated triclocarban (TCC-d7) andcarbon-13 labeled triclocarban (13C6-TCC) were obtained from Cambridge Isotope laboratories (Andover, MA) .
Experimental methods
Specimens of the freshwater mudsnail Potamopyrgus antipodarum were collected from Putah Creek near Winters, CA on October 17, 2008 under the supervision of California Department of Fish and Game staff. Aquatic vegetation was collected in D-nets and snails were removed and kept in river water in 1 gallon buckets for transfer to the laboratory. Within 2 hours, snails were transferred into 10 gallon aquaria filled with artificial freshwater (Milli-Q water plus added salts at a rate of 5 g CaCO3, 5 g Sea Salt, and 0.5 g NaHCO3 per 10 gallons of water) .
After 3 to 5 days acclimation to laboratory conditions, 60 individuals with shell lengths greater than 3.0 mm were transferred to each individual 1-L jar filled with 800 mlof artificial freshwater. The jars were aerated through glass pipettes to keep dissolved oxygen near saturation.
Each jar was spiked with a solution of TCC dissolved in reagent alcohol to achieve five nominal target aqueous TCC concentrations (0.045, 0.14, 0.45, 1.4, 4.5, and 14.0 ug/L, in triplicate). Reagent alcohol always represented less than 0.003% of the final volume of solution. The control blank triplicates were also spiked with an equivalent volume of pure reagent alcohol. A pilot study was conducted that contained both water only and solvent controls, and results showed no significant differences in number of embryos between the treatments, suggesting that ethanol had no effect on embryo numbers. Therefore, no water only control was used during the duration of this experiment. The pilot study also indicated that large amounts of TCC (i.e. >10%of the total mass in the jar) were partitioned into the snail biomass within 5 days. In order to keep aqueous TCC concentrations relatively constant, water was replaced and re-spiked at the initial concentration level in each jar every 3 days.
The experiments were conducted at 14 ± 0.7° C under a light:dark rhythm of 16:8 hours. Snails were fed ground TetraMin every day or every other day at an approximate rate of 0.1 mg per day. At t=0, 2, and 4 weeks, 15 specimens were removed from each jar and narcotized for 1 hour in 2.5% MgCl2 solution. Photographs of the specimens were taken and the length of the shell measured using image processing software. Shells were cracked in a vice and dissection took place under a dissecting microscope. To measure reproductive output, embryos were counted, making a distinction between those with shells (i.e. older, more developed embryos) and those without (i.e. newer embryos). The identical procedure was performed at the same time points on 15 individuals that had been kept in 10 L aquaria, and was repeated for snails kept in aquaria at 6 and 8 weeks as well. Mortality in the treatments was recorded every 3 days and dead snails were removed.
All data were analyzed using JMP 8.0 (SAS institute, Cary, NC). Using absolute embryo numbers, means and standard errors were calculated, followed by one way ANOVA (n=3) and comparison of treatment means to the control using Dunnett’s method (α=0.05). All ANOVA assumptions were verified through standard tests of residuals. Using % responses relative to the control, non-linear regressions were calculated using a three-parameter logistic model and were used to calculate an EC10 and EC50 for each, where EC10 and EC50 are the concentrations causing a 10% and 50% increase in embryo numbers relative to the solvent control, respectively. All effects are referenced to time-weighted mean concentrations as determined analytically rather than nominal concentrations.
Water chemistry
At day 1, 4, 10, 16, and 25, water samples were taken from one randomly chosen replicate of each of the exposure concentrations immediately before and shortly after the water renewal and re-spiking procedure. These samples were measured for dissolved oxygen and pH, then analyzed for dissolved TCC concentration. pH was measured using a Mettler Toledo (Columbus, OH) pH meter. Dissolved oxygen was measured using a YSI (Yellow Springs, OH) dissolved oxygen meter. At the same intervals, additional samples were taken to measure nitrite/nitrate and ammonia using Aquarium Pharmaceuticals (Chalfont, PA) test kits.
For the determination of dissolved TCC, samples were first acidified to pH 2 using hydrochloric acid. Deuterated surrogate (TCC-d7) was spiked into the samples, followed by solid phase extraction. The extraction was carried out on Waters (Milford, MA) OASIS HLB 6cc disposable cartridges on a Supelco (St. Louis, MO) Visiprep DL manifold. Each cartridge was conditioned with 5 ml75:25 ethyl acetate/acetone mixture followed by 5 ml methanol and then 5 ml Milli-Q water. Samples of 10 ml were loaded at a rate of between 1 and 2 ml/min and then dried for 10minutes at 40 mmHg. Cartridges were eluted with 8 ml of 75:25 ethyl acetate/acetone. Eluates were then evaporated to dryness under a gentle stream of nitrogen at 65° C. Finally, extracts were redissolved in 200μlor 1 ml of 90:10 acetonitrile/Milli-Q water containing 13C6-TCC as an internal standard.
Extracts were analyzed via liquid chromatography with tandem mass spectrometry. Injection volume was 10 μl, and separation was achieved on a Phenomenex (Torrance, CA) Prodigy ODS 100A 100 x 2.0 mm column at 40° C. The binary mobile phase consisted of 0.5 ml/min of A: 90:10 Milli-Q:acetonitrile with 2 mM acetic acid and B: 50:50 methanol:acetonitrile with 10 mM acetic acid. The gradient was as follows: 20% B rising to 80% B over 16.5 minutes, then rising to 100% B over 2.5 minutes, followed by 1 minute at 100% B. Detection was achieved using an Agilent (Santa Clara, CA) 1100 series LC/MSD ion trap with electrospray ionization in negative mode and multiple reaction monitoring. The drying gas flow rate was 12 l/min, the drying gas temperature was 350° C, and the nebulizer pressure was 35psi. All other instrument parameters were optimized for the detection of TCC.
The response was corrected by recovery of the surrogate TCC-d7and normalized to the response of the internal standard 13C6-TCC. Calibration was via seven external standards which were analyzed before and after every set of samples and the linear regression fit to the averages of each pair of responses. A summary of the chemicals analyzed is shown in Table 1. All data were analyzed using Bruker Daltonik Data Analysis v.2.1 software (Bremen, Germany) .
Table 1.
Selected Analytical Parameters
| Chemical | Triclocarban (TCC) | Deuterated Triclocarban (TCC-d7) | C13 labeled Triclocarban (13C6-TCC) |
|---|---|---|---|
| Purpose | Analyte | Surrogate | Internal Standard |
| Molecular Weight | 315.6 | 322.6 | 321.5 |
| Precursor ion | 313 | 320 | 319 |
| Fragment | 160 | 163 | 160 |
| Instrument Detection Limit | 0.35 μg/L | 0.10μg/L | n/a |
| Instrument Quantitation Limit | 0.80 μg/L | 0.25 μg/L | n/a |
| Limit of Detection | 10 ng/L | 2.9 ng/L | n/a |
| Limit of Quantitation | 23 ng/L | 7.0 ng/L | n/a |
Results and Discussion
Dissolved oxygen was always above 95% saturation and pH was 7.9 ± 0.4. Nitrite, nitrate, and total ammonia were always below the detection limits of the tests, which were 0.1 mg/L, 5 mg/L, and 0.5 mg/L, respectively. Dissolved TCC concentrations decreased by 5–50% over the course of each 3–9 day period between analyses. The rate of disappearance decreased over each interval. Preliminary experiments indicated that significant amounts of TCC partitioned into snail biomass within days, so the decreasing rate of TCC disappearance is likely because the applied concentrations were approaching equilibrium with the TCC that had partitioned into the biomass of the snails. Even so, time weighted mean concentrations in general showed good agreement with nominal concentrations; the concentrations (in μg/L) were determined to be 0.05 (0.04 nominal) , 0.22 (0.14) , 0.47 (0.45) , 1.6 (1.4) , 4.1 (4.5) , and 10.5 (14.0). The highest measured concentration (10.5) was probably lower than the nominal value (14.0) because of insolubility of TCC in the alcohol stock or the water. Halden and Paull predict a solubility of TCC in water of 0.65–1.55 mg/L at 25°C [18]; however, experience in our lab and several others’ indicates a much lower actual solubility. In addition, this experiment was conducted at 14° C where the solubility would be expected to be lower than literature values referenced to 25° C. Calculated recoveries for the analytical procedure averaged 72% with a standard deviation of 18%.
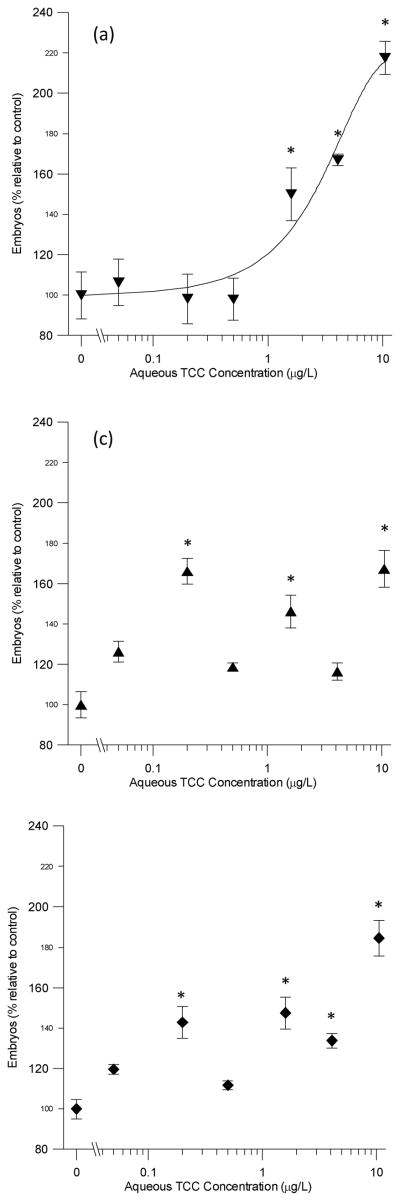
After 2weeks exposure, the number of embryos showed no significant differences from the controls. After4 weeks, significant increases were found for numbers of unshelled, shelled, and total embryos, as shown in Figure 2.Exposures of 1.6, 4.1, and 10.5 μg/L exhibited significantly elevated numbers of unshelled embryos, reaching217% of the control numbers. Exposures of 0.2, 1.6, and 10.5 μg/L resulted in significantly more shelled embryos, up to 167% of the control numbers. Total embryos were significantly greater than controls in snails exposed to 0.2, 1.6, 4.1, and 10.5 μg/L, up to 184% of the controls. The lowest observed effects concentration (LOEC) was therefore 0.2 μg/L, and the no observed effects concentration (NOEC) 0.05 μg/L. The EC10 and EC50 for unshelled effects were 0.5 and 2.5 μg/L, respectively (r2=0.59). The regressions did not show good fit with the unusual dose-response curves for shelled and total embryo numbers, and therefore an EC10 and EC50 were notcalculated. The most logical reason why numbers of unshelled embryos showed the greatest increase is because by 4 weeks, most if not all unshelled embryos have likely been formed since the start of the exposure, and therefore best represent effects of exposure conditions.
Figure 2.
Effects of dissolved Triclocarban (TCC) exposures on embryo numbers of Potamopyrgus antipodarum at 4 weeks in percentage of the solvent control (mean ± standard error of the mean, n = 15) for (a) unshelled embryos, (b) shelled embryos, and (c) total embryos. Logistic regression line for unshelled embryos is shown. *Significantly greater than solvent controls at p<0.05.
No effects on shell length were detected. Shell length was determined not to be a cofactor. If the exposures had been extended to 6 or 8 weeks, it would be possible that effects on embryo numbers would have been seen at even lower exposures, following the trend that was seen in [23]. Exposures were limited to 4 weeks for the sake of expedience and because preliminary experiments indicated that 4 weeks was sufficient to detect the effects. Mortality was less than 10% in all exposures except onejar of 0.5 μg/L and one jar of 4.1 μg/L, for which mortality reached 20% and 17% by the end of the 4 weeks, respectively. These jars had visible fungal growth on their bottoms at between 3 and 4 weeks, likely due to overfeeding, and this most likely led to higher mortality.
Embryo numbers in all treatments, including the controls, decreased substantially during the course of the experiment (see Figure 3). Levels found in snails housed in the aquarium declined in a similar fashion, but at a slower rate. The results of a prior pilot experiment that took place several months earlier indicate a similar decline, suggesting that transferring the organisms to laboratory conditions caused their reproduction to slow down during the course of the experiment. Anecdotal evidence from other labs suggests this to be a common effect of bringing wild-caught mudsnails into the lab.
Figure 3.
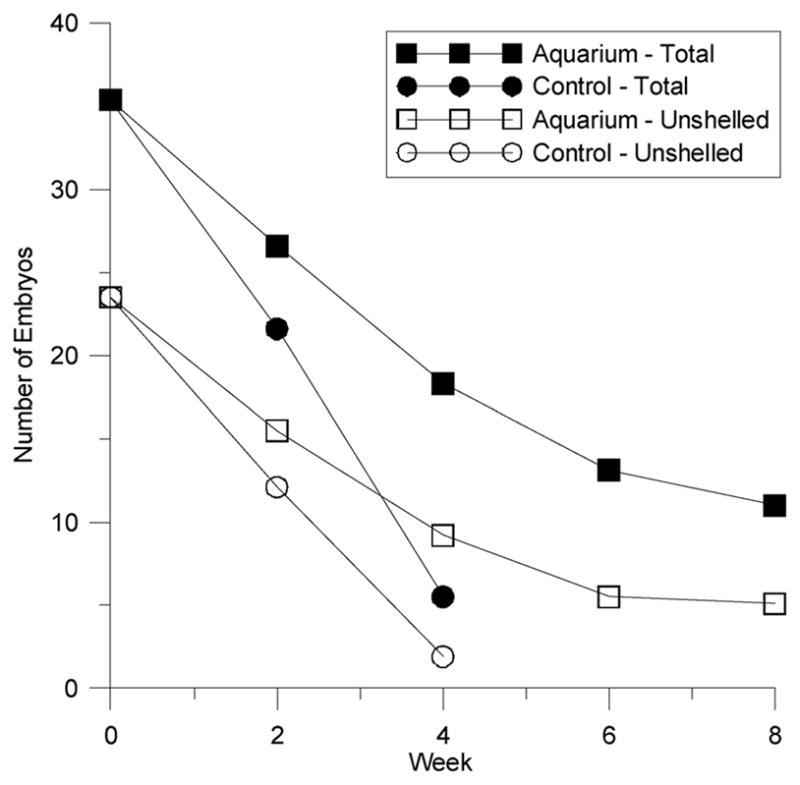
Embryo numbers in the control and in an aquarium declined substantially over the duration of the experiment. The decline in the aquarium was slower and not as pronounced, and leveled off at 8 weeks.
The specific ecological impact of the effects seen in this experiment is not clear, but it is likely that if they are occurring in the environment, populations would be impacted. As Duft points out, increases in embryo production during seasonal minima in the reproductive cycle means more juveniles entering the environment at times when the environment is unfavorable for survival. Furthermore, limitations in the overall energy budget may then contribute to lower fecundity and lower survival rates in the seasonal maxima [23]. Multi-month exposures to examine effects of TCC on survival and multi-generational exposures to examine effects of populations in microcosms would enhance our understanding of the expected effects in the environment.
While many studies have examined acute and chronic effects of TCC on aquatic organisms, few have found effects at such low levels. The NOEC for chronic toxicity to Daphnia magna has been reported at 0.5 to 1.0 μg/L. The most sensitive sensitive endpoint for TCC on aquatic organisms found in the literature is a NOEC of 0.101 μg/L for decreased numbers of young in Americamysis bahia, a saltwater crustacean. The most sensitive study results for molluscs found in the literature were reduced viability (to 20% of control) in clam larvae at 10 μg/L TCC, and decreased larval length as low as 5 μg/L [24], 50 times higher than the LOEC of 0.2 μg/L found in the present study. While TCC concentrations downstream of wastewater treatment plants have only rarely been found to be above 5 μg/L, levels above 0.2 μg/L are quite common. It is estimated that 30–40% of samples described in the literature [18, 25] are above the LOEC for the present study.
Others have found similar effects as the present study on embryo production in P. antipodarum when exposed to the known environmental estrogens bisphenol A (BPA) , octylphenol (OP) , nonylphenol (NP) , and ethynylestradiol (EE2) in both sediment and water [20, 23]. In water, no observed effects concentrations (NOEC) have been determined to be 1 μg/L for BPA and OP and 5 μg/L for NP [20]. The mechanism of action (MOA) of TCC on P. antipodarum is not known, but it is possible that it acts similarly to experiments done with the mammalian estrogen receptor in vitro [16] — that is, amplifying the binding affinity and consequently increasing the transcriptional activity of naturally present estrogen to the estrogen receptor. However, some evidence indicates that estrogenic compounds act via a different route than binding to the vertebrate-like estrogen receptor in mollusks[26]. In fact, it is not clear at this point what the precise MOA of estrogen analogues are in mollusks. While the possibility exists that TCC shares a common MOA in mollusks with already identified estrogenic EDCs, molecular evidence from vertebrate studies suggest that the MOAs differ. If this is true, this experiment shows that chemicals with different mechanisms of action produce nearly identical results in vivo. It also highlights the need for both in vitro and in vivo studies, especially for chemical-by-chemical screening programs. In vivo studies may not distinguish between different mechanisms of EDC, while in vitro studies based on the current single chemical testing paradigm (e.g., Tier 1 of the USEPA’s Endocrine Disruptor Screening Program) may miss potentially hazardous EDCs.
The present study represents a first step in characterizing risk to aquatic organisms of a new class of EDC. By showing that TCC, a chemical that exhibits little to no affinity for the estrogen receptor alone, causes reproductive effects that match those caused by known estrogen receptor agonists, the present study indicates that a chemical not currently addressed by the current paradigm in EDC screening methodologies can exhibit equally problematic environmental risk.
Acknowledgments
The authors would like to thank Mary Giudice for her assistance in dissections; TemitopeOgunyoku and Peter Green for their assistance in chemical analyses; the California Department of Fish and Game for assistance in collection of specimens; and Martina Duft for information on culturing the test species.
This research has been funded by the National Institute of Environmental Health Sciences (NIEHS) under grant number P42 ES004699, and is based upon work supported under a National Science Foundation Graduate Research Fellowship. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of NIEHS or the National Science Foundation.
References
- 1.Fenton SE. Endocrine-disrupting compounds and mammary gland development: Early exposure and later life consequences. Endocrinology. 2006;147 (6):S18–S24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- 2.Rayner JL, Enoch RR, Fenton SE. Adverse effects of prenatal exposure to atrazine during a critical period of mammary gland growth. Toxicol Sci. 2005;87 (1):255–266. doi: 10.1093/toxsci/kfi213. [DOI] [PubMed] [Google Scholar]
- 3.Blanck HM, Marcus M, Tolbert PE, Rubin C, Henderson AK, Hertzberg VS, Zhang RH, Cameron L. Age at menarche and tanner stage in girls exposed in utero and postnatally to polybrominated biphenyl. Epidemiology. 2000;11 (6):641–647. doi: 10.1097/00001648-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Hokanson R, Hanneman W, Hennessey M, Donnelly KC, McDonald T, Chowdhary R, Busbee DL. DEHP, bis (2) -ethylhexyl phthalate, alters gene expression in human cells: possible correlation with initiation of fetal developmental abnormalities. Hum Exp Toxicol. 2006;25 (12):687–695. doi: 10.1177/0960327106071977. [DOI] [PubMed] [Google Scholar]
- 5.Mankame T, Hokanson R, Fudge R, Chowdhary R, Busbee D. Alteration of gene expression in human cells treated with the agricultural chemical diazinon: possible interaction in fetal development. Hum Exp Toxicol. 2006;25 (5):225–233. doi: 10.1191/0960327106ht622oa. [DOI] [PubMed] [Google Scholar]
- 6.Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens) : Laboratory and field evidence. Environ Health Perspect. 2003;111 (4):568–575. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jobling S, Nolan M, Tyler CR, Brighty G, Sumpter JP. Widespread sexual disruption in wild fish. Environ Sci Technol. 1998;32 (17):2498–2506. [Google Scholar]
- 8.Duft M, Schulte-Oehlmann U, Tillmann M, Markert B, Oehlmann J. Toxicity of triphenyltin and tributyltin to the freshwater mudsnail Potamopyrgus antipodarum in a new sediment biotest. Environ Toxicol Chem. 2003;22 (1):145–152. [PubMed] [Google Scholar]
- 9.Hansen FT, Forbes VE, Forbes TL. Effects of 4-n-nonylphenol on life-history traits and population dynamics of a polychaete. Ecol Appl. 1999;9 (2):482–495. [Google Scholar]
- 10.Bell AM. An endocrine disruptor increases growth and risky behavior in threespined stickleback (Gasterosteus aculeatus) Horm Behav. 2004;45:108–114. doi: 10.1016/j.yhbeh.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Matthiessen P, Gibbs P. Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ Toxicol Chem. 1998;17 (1):37–43. [Google Scholar]
- 12.Kidd KA, Blanchfield PJ, Mills KH, Palace VP, Evans RE, Lazorchak JM, Flick RW. Collapse of a fish population after exposure to a synthetic estrogen. Proceedings of the National Academy of Sciences of the UnitedStates of America. 2007;104 (21):8897–8901. doi: 10.1073/pnas.0609568104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guillette LJ. Endocrine disrupting contaminants -Beyond the dogma. Environ Health Perspect. 2006;114 (S1):9–12. doi: 10.1289/ehp.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolen GA, Dierckman TA. Reproduction and teratogenic studies of a 2:1 mixture of 2,4,4'-trichlorocarbanilide and 3-trifluoromethyl-4,4'-dichlorocarbanilide in rats and rabbits. Toxicol Appl Pharmacol. 1979;51 (3):417–25. doi: 10.1016/0041-008x(79)90366-1. [DOI] [PubMed] [Google Scholar]
- 15.Ahn KC, Zhao B, Chen J, Cherednichenko G, Sanmarti E, Denison MS, Lasley B, Pessah IN, Kultz D, Chang DPY, Gee SJ, Hammock BD. In vitro biologic activities of the antimicrobials triclocarban, its analogs, and triclosan in bioassay screens: Receptor-based bioassay screens. Environ Health Perspect. 2008;116 (9):1203–1210. doi: 10.1289/ehp.11200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JG, Ahn KC, NAG, Ahmed MI, Suleba AJ, Zhao L, Gee SJ, Hammock BD, BLL Triclocarban enhances testosterone action: A new type of endocrine disruptor? Endocrinology. 2008;149 (3):1173–1179. doi: 10.1210/en.2007-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.TCC Consortium. Report No. 201-14186A. 2002. High Production Volume (HPV) Chemical Challenge Program Data Availability and Screening Level Assessment for Triclocarban, CAS#: 101-20-2. [Google Scholar]
- 18.Halden RU, Paull DH. Co-occurance of triclocarban and triclosan in U.S. water resources. Environ Sci Technol. 2005;39:1420–1426. doi: 10.1021/es049071e. [DOI] [PubMed] [Google Scholar]
- 19.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, Buxton HT. Pharmaceuticals, hormones, and other organic wastewater contaminants in US streams, 1999– 2000: a national reconnaissance. Environ Sci Technol. 2002;36:1202–1211. doi: 10.1021/es011055j. [DOI] [PubMed] [Google Scholar]
- 20.Duft M, Schmitt C, Bachmann J, Brandelik C, Schulte-Oehlmann U, Oehlmann J. Prosobranch snails as test organisms for the assessment of endocrine active chemicals--an overview and a guideline proposal for a reproduction test with the freshwater mudsnail Potamopyrgus antipodarum. Ecotoxicology. 2007;16:169–182. doi: 10.1007/s10646-006-0106-0. [DOI] [PubMed] [Google Scholar]
- 21.Ponder WF. Potamopyrgus antipodarum--A molluscan coloniser of Europe and Australia. J Molluscan Stud. 1988;54:271–285. [Google Scholar]
- 22.United States Geological Survey Nonindiginous Aquatic Species. Potamopyrgus antipodarum Fact Sheet. 2008. [Google Scholar]
- 23.Duft M, Schulte-Oehlmann U, Weltje L, Tillmann M, Oehlmann J. Stimulated embryo production as a parameter of estrogenic exposure via sediments in the freshwater mudsnail Potamopyrgus antipodarum. Aquat Toxicol. 2003;64 (4):437–449. doi: 10.1016/s0166-445x(03)00102-4. [DOI] [PubMed] [Google Scholar]
- 24.Davis HC, Hidu H. Effects ofpesticides on embryonic development of clams and oysters and on survival and growth of the larvae. Fisheries Bulletin. 1969;67 (2):393–404. [Google Scholar]
- 25.Sapkota A, Heldler J, Halden RU. Detection of triclocarban and two co-contaminating chlorocarbanilides in US aquatic environments using isotope dilution liquid chromatography tandem mass spectrometry. Environ Res. 2007;103 (1):21–29. doi: 10.1016/j.envres.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Matthiessen P. An Assessment of Endocrine Disruption in Mollusks and the Potential for Developing Internationally Standardized Mollusk Life Cycle Test Guidelines. Integr Environ Assess Manage. 2009;4 (3):274–284. doi: 10.1897/IEAM_2008-003.1. [DOI] [PubMed] [Google Scholar]