Abstract
Purpose
Persistence of ligand-mediated androgen receptor signaling has been documented in castration-resistant prostate cancers (CRPCs). Abiraterone acetate (AA) is a potent and selective inhibitor of CYP17, which is required for androgen biosynthesis in the testes, adrenal glands, and prostate tissue. This trial evaluated the efficacy and safety of AA in combination with prednisone to reduce the symptoms of secondary hyperaldosteronism that can occur with AA monotherapy.
Patients and Methods
Fifty-eight men with progressive metastatic CRPC who experienced treatment failure with docetaxel-based chemotherapy received AA (1,000 mg daily) with prednisone (5 mg twice daily). Twenty-seven (47%) patients had received prior ketoconazole. The primary outcome was ≥ 50% prostate-specific antigen (PSA) decline, with objective response by Response Evaluation Criteria in Solid Tumors (RECIST) criteria, and changes in Eastern Cooperative Oncology Group (ECOG) performance status (PS) and circulating tumor cell (CTC) numbers. Safety was also evaluated.
Results
A ≥ 50% decline in PSA was confirmed in 22 (36%) patients, including 14 (45%) of 31 ketoconazole-naïve and seven (26%) of 27 ketoconazole-pretreated patients. Partial responses were seen in four (18%) of 22 patients with RECIST-evaluable target lesions. Improved ECOG PS was seen in 28% of patients. Median time to PSA progression was 169 days (95% CI, 82 to 200 days). CTC conversions with treatment from ≥ 5 to < 5 were noted in 10 (34%) of 29 patients. The majority of AA-related adverse events were grade 1 to 2, and no AA-related grade 4 events were seen.
Conclusion
AA plus prednisone was well tolerated, with encouraging antitumor activity in heavily pretreated CRPC patients. The incidence of mineralocorticoid-related toxicities (hypertension or hypokalemia) was reduced by adding low-dose prednisone. The combination of AA plus prednisone is recommended for phase III investigations.
INTRODUCTION
Prostate cancer progression despite castrate levels of testosterone is associated with a rising prostate-specific antigen (PSA) level and represents a transition to the lethal phenotype of the disease to which most patients eventually succumb. The rise in PSA, an indication that androgen receptor (AR) signaling has been reactivated, is the result of selective and/or adaptive changes in the AR itself that are oncogenic for growth, of which AR overexpression is the most common.1 Tumor levels of ligand have received less attention. In 1984, Geller et al2 documented elevated levels of androgens in tissue homogenates of the prostates of men who were progressing on medical or surgical castration. Adrenal androgen synthesis was the postulated source, although a failure to completely suppress intratumoral androgens could not be excluded. The finding of increased intratumoral androgens was subsequently confirmed in microdissected locally recurrent and castration-resistant primary and metastatic lesions.3–6 In 2004, Holzbeierlein et al7 first reported a microarray analysis of newly diagnosed primary and metastatic and separately posthormone-treated primary and progressive castration-resistant prostate cancers (CRPCs) that showed an up to five-fold induction of several enzymes involved in steroid hormone production. The finding, subsequently confirmed by others,8,9 suggested the possibility of intracrine signaling as an additional mechanism of AR reactivation.
Abiraterone (CB7630, Cougar Biotechnology, Los Angeles, CA) is a novel, selective, irreversible, and potent inhibitor of 17-[alpha]-hydroxylase/17,20-lyase (CYP17) enzymatic activity that has recently been demonstrated to further reduce testosterone levels in the blood to undetectable range (< 1 ng/dL),10,11 and is suggested to reduce de novo intratumor androgen synthesis.12 Significant antitumor effects were seen in patients with progressive CRPC who had not received prior chemotherapy, where secondary hormonal therapies are traditionally used.11,13 Ketoconazole, a weak inhibitor of adrenal steroid synthesis that suppresses many cytochrome P450 enzymes and other enzymes involved in the conversion of cholesterol to pregnenolone, has also shown activity in the same clinical context14,15 but is associated with significant toxicities.16–18
Recognizing that PSA levels also rise in patients who are progressing after treatment with cytotoxic agents, we postulated that these tumors might also be sensitive to a potent AR signaling inhibitor. In a separate trial, we showed significant antitumor effects of abiraterone acetate (AA) monotherapy in this patient group, emphasizing further how considering these tumors as hormone-refractory can deny patients effective therapies.19 The adverse events seen with AA monotherapy were largely those associated with secondary hyperaldosteronism, including hypokalemia, fluid retention, and hypertension that required treatment with the selective aldosterone inhibitor eplerenone or low-dose steroids to suppress the hypothalamic-pituitary-adrenal axis, blunting the feedback rise in adrenocorticotropic hormone to reduce production of adrenal steroids with mineralocorticoid activity.11,20 Anticipating phase III development, we designed this study to confirm the antitumor activity of AA in patients with CRPC after failure of docetaxel-based chemotherapy using the proposed registration regimen of AA at 1,000 mg daily in combination with prednisone at 5 mg twice daily, and separately, to begin to address the influence on outcome of the number and type of prior hormone treatments, particularly ketoconazole.21 Importantly, the evaluation of this steroid combination was further supported by our work indicating that low-dose steroids can reverse clinical AA resistance and decrease steroid precursors upstream of CYP17 that can activate AR signaling. Recognizing that PSA changes may not be a reliable indicator of the antitumor effects of an AR signaling–directed therapy and that more informative indicators of clinical benefit represent a critical unmet need for the management of CRPC, we also evaluated pre- and post-therapy circulating tumor cell (CTC) number using an analytically valid assay that is cleared by the US Food and Drug Administration for use as an aid to monitoring treatment and prognosis in patients with breast,22 colorectal,23 and prostate cancer.24
PATIENTS AND METHODS
Patients
Castrate men (serum testosterone < 50 ng/dL [< 2.0 nmol/L]) with metastatic prostate cancer who had experienced treatment failure with androgen deprivation therapy and docetaxel-based chemotherapy were eligible. Disease progression was defined as documented PSA progression according to Prostate Specific Antigen Working Group 1 criteria25 and a PSA > 5 ng/mL, or objective progression by Response Evaluation Criteria in Solid Tumors (RECIST) criteria26 for patients with measurable disease. Patients had to have received prior chemotherapy with docetaxel, and treatment with up to two prior chemotherapy regimens was permitted. Prior treatment with ketoconazole was allowed and was recorded separately. Eligibility criteria also included Eastern Cooperative Oncology Group (ECOG) performance status (PS) of ≤ 2, (Karnofsky PS ≥ 50%), normal serum potassium, and adequate hematologic, hepatic, and renal function. With the exception of luteinizing hormone-releasing hormone agonists, a minimum of 4 weeks must have elapsed from discontinuation of prior prostate cancer therapies and a minimum of 6 weeks for antiandrogen therapy. Patients were excluded if they had brain metastases or spinal cord compression, active autoimmune disease requiring corticosteroid therapy, uncontrolled hypertension, a depressed cardiac ejection fraction or history of cardiac failure (New York Heart Association class III or IV), or a serious concurrent medical illness. The trial protocol was approved by the institutional review board at each site and was conducted in accordance with the Declaration of Helsinki, current US Food and Drug Administration Good Clinical Practices, and local ethical and legal requirements.
Study Design
After written informed consent was obtained, eligible patients in this multicenter phase II clinical trial received AA at 1,000 mg (four 250-mg tablets daily in the morning after an overnight fast) concurrently with prednisone at 5 mg twice daily. Treatment was administered in 28-day cycles, and up to 12 cycles of therapy were permitted; continuation beyond 12 cycles was allowed on approval by the investigators and sponsor.
Patient Evaluation
Patients were seen and examined at the beginning of every cycle while receiving treatment, and adverse events were recorded using the National Cancer Institute Common Toxicity Criteria, version 3.0. Potential attributions to AA and prednisone were recorded separately. A CBC, chemistry panel, PSA, and androgen levels were evaluated monthly.
Antitumor Outcomes
The primary study objective was determination of the proportion of patients achieving a decline in PSA ≥ 50% according to Prostate Specific Antigen Working Group 1 criteria (PSA response). The maximal and 12-week post-therapy declines in PSA were recorded using waterfall plots. The maximal decline had to be confirmed by a second value obtained ≥ 4 weeks later.25 Patients who had metastatic disease evident on baseline imaging (computed tomography scan, magnetic resonance imaging, or bone scan) had follow-up studies at 3-month intervals. Measurable disease response rate was reported using the RECIST criteria, while post-treatment changes on radionuclide bone scan were reported as stable or progression per investigator's assessment.
Time to PSA progression was calculated for patients with PSA decline ≥ 50% from baseline at the time the PSA increased to 50% above the nadir and was > 5 ng/mL. For those not meeting the PSA decline criteria, time to PSA progression was the time when PSA increased by 25% from baseline.
Other end points recorded were changes in ECOG PS, and pre-and post-therapy CTC counts (number of cells/7.5 mL of blood) were enumerated separately using the CellTracks system (Veridex, Raritan, NJ), as described previously.27,28
Statistical Analysis
The primary end point of the trial was the proportion of patients who achieved a PSA decline of ≥ 50% from baseline that was confirmed by a second value following treatment with AA plus prednisone. The combination would be considered worthy of further study if ≥ 30% of patients met the end point and not worthy of further study if fewer than 15% achieved the end point. With a population of 50 patients, the null hypothesis would be rejected and further study of the combination would be warranted if > 25% of eligible and treated patients had a ≥ 50% decline in PSA (alpha of 6% with 86% power).
RESULTS
Patients
Between June 2007 and November 2007, 58 men with CRPC were enrolled across seven study centers: six in the United States and one in the United Kingdom. Patient demographics and baseline characteristics are listed in Table 1. All were heavily pretreated with a number of hormonal therapies that included a median of four (range, one to eight) prior antiandrogens in 52 (91%) and estrogens in nine (16%), while 27 (47%) had prior ketoconazole. While all had been treated with prior docetaxel, 24% had also received a second chemotherapy regimen. Consistent with the advanced state of the population, only 11 (19%) had disease limited to bone, while 13 (22%) had visceral spread, and 34 patients (59%) had soft tissue disease with or without osseous spread. The median PSA level at baseline was 190 ng/mL (range, 10 to 3,846 ng/mL). The median testosterone level was 4.8 ng/dL (range, below limit of detection [0.05] to 30.5 ng/dL).
Table 1.
Patient Baseline Demographics and Clinical Characteristics
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 69.5 | |
| Range | 44-86 | |
| ECOG performance status | ||
| 0 | 24 | 41 |
| 1 | 31 | 53 |
| 2 | 2 | 3 |
| Unknown | 1 | 2 |
| Gleason score | ||
| Median | 7 | |
| Range | 5-10 | |
| Baseline PSA, ng/mL | ||
| Median | 189.6 | |
| Range | 10.1-3,846 | |
| Involved metastatic sites | ||
| Visceral (with or without bone or soft tissue) | 13 | 22 |
| Bone only | 11 | 19 |
| Soft tissue only | 8 | 14 |
| Bone and soft tissue only | 26 | 45 |
| Prior hormonal therapies | ||
| LHRH agonists | 57 | 98 |
| Orchiectomy | 3 | 5 |
| Antiandrogens | 53 | 91 |
| Diethylstilbestrol | 8 | 14 |
| Steroids | 21 | 36 |
| Dexamethasone | 5 | 9 |
| Other | 20 | 34 |
| Ketoconazole | 27 | 47 |
| Prior lines of chemotherapy | ||
| 1 | 44 | 76 |
| > 1 | 14 | 24 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen; LHRH, luteinizing hormone–releasing hormone.
Antitumor Effects
PSA.
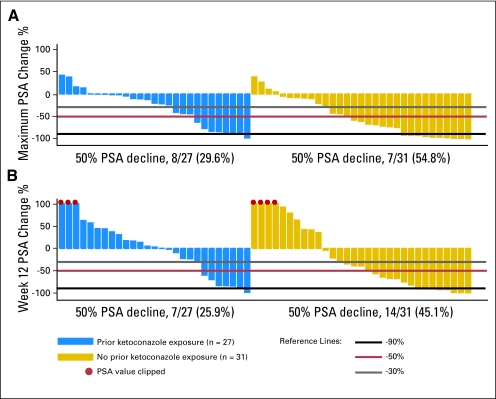
A waterfall plot of the maximal PSA decline and 12-week post-therapy PSA changes is shown in Figure 1. Overall, the proportion of patients achieving a 50% or greater maximal decline in PSA was 43% (25 of 58; 95% CI, 30% to 55%), including 30% (8 of 27; 95% CI, 13% to 47%) of those who had received previous ketoconazole and 55% (17 of 31; 95% CI, 37% to 73%) of those who were ketoconazole-naïve. At the 12-week assessment, the overall proportion of patients achieving a ≥ 50% decline in PSA was 36% (21 of 58; 95% CI, 24% to 48%), including 26% (7 of 27; 95% CI, 9% to 435%) of those who had received previous ketoconazole and 45% (14 of 31; 95% CI, 27% to 63%) of those who were ketoconazole-naive. The differences in the proportion showing declines in PSA were not statistically significant. Overall, confirmed PSA responses of ≥ 30%, ≥ 50%, and ≥ 90% decline were observed in 47%, 36%, and 16% of patients, respectively. In the prior ketoconazole versus ketoconazole-naïve patients, the confirmed PSA decline of ≥ 30%, ≥ 50%, and ≥ 90% were, respectively, nine (33%) versus 18 (58%), seven (26%) versus 14 (45%), and zero (0%) versus nine (29%) patients.
Fig 1.
Changes in prostate-specific antigen (PSA) levels with abiraterone acetate plus prednisone. Waterfall plots of (A) maximum PSA change and (B) change at week 12. Patients with prior ketoconazole exposure appear on the left side of the panel in blue and those without prior ketoconazole exposure appear on the right side of the panel in gold.
Soft tissue.
Twenty-two of 28 patients with baseline scans had target lesions assessed by computed tomography imaging; of these 22 patients, partial responses were seen in four (18%), stable disease lasting more than 3 months in 13 (59%), and disease progression in five (23%).
Osseous disease.
Bone scans provided objective radiologic response information for 27 evaluable patients of the 34 patients with baseline scans. By investigator assessment, 16 (59%) patients had stable disease and 11 (41%) patients experienced disease progression.
Clinical benefit and PS.
Changes in ECOG PS from baseline were evaluated as an indication of clinical benefit (Table 2). Overall, 16 patients (28%) had an improvement in ECOG PS, with 14 patients improving from PS 1 to PS 0, and one patient each improving from PS 2 to PS 1 or PS 0. Four patients (7%) had a worsening PS, with two patients each changing from baseline PS 0 to PS 1 or PS 1 to PS 2. PS remained stable among the remaining 37 (64%) patients.
Table 2.
ECOG Performance Score Change From Baseline (N = 58)
| Baseline ECOG Performance Score | No. of Patients | Postbaseline ECOG Performance Score |
|||||
|---|---|---|---|---|---|---|---|
| 0 |
1 |
2 |
|||||
| No. of Patients | % | No. of Patients | % | No. of Patients | % | ||
| 0 | 24 | 22 | 2 | — | |||
| 1 | 31 | 14 | 15 | 2 | |||
| 2 | 2 | 1 | 1 | — | |||
| Total* | 37 | 64 | 18 | 31 | 2 | 3 | |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
One patient did not have baseline ECOG recorded.
Time to progression.
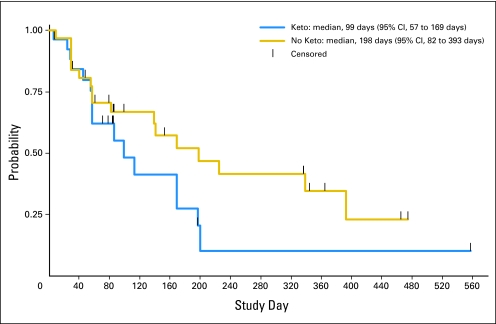
The overall median time to PSA progression was 169 days (95% CI, 86 to 200 days); based on prior exposure to ketoconazole, the median time to PSA progression was 198 days (95% CI, 82 to 393 days) among ketoconazole-naïve patients and 99 days (95% CI, 57 to 169 days) among ketoconazole-treated patients (Fig 2).
Fig 2.
Time to prostate-specific antigen (PSA) progression with abiraterone acetate and prednisone in patients with and without prior ketoconazole (Keto) exposure.
CTCs.
Cell counts were available for all 38 patients treated at the Memorial Sloan-Kettering Cancer Center and four patients treated at the Royal Marsden Hospital. Overall, 29 (69%) patients had unfavorable baseline counts (≥ 5 cells/7.5 mL blood), of which 10 (34%) converted to a favorable count after treatment (Table 3).
Table 3.
Patients With High CTC Counts at Baseline Stayed on Treatment for a Shorter Time, Unless Counts Dropped With Treatment
| CTC/7.5 mL of Blood | Time on Protocol (weeks) |
||
|---|---|---|---|
| ≤ 12 | 12-24 | > 24 | |
| Baseline < 5 CTC (n = 13) | |||
| Post-treatment CTC ≥ 5 (n = 4) | 1 | 1 | 2 |
| Post-treatment CTC < 5 (n = 9) | 1 | 4 | 4 |
| Baseline ≥ 5 CTC (n = 29) | |||
| Post-treatment CTC ≥ 5 (n = 19) | 9 | 7 | 3 |
| Post-treatment CTC < 5 (n = 10) | 1 | 3 | 6 |
Abbreviation: CTC, circulating tumor cell.
Safety and Tolerability
The combination of AA and prednisone was well tolerated in this heavily pretreated population. Grade 3 or 4 adverse events occurred infrequently (Table 4). No significant hypertension or hypokalemia were noted as clinical signs of mineralocorticoid excess with the prospective addition of prednisone. No patients required treatment with the mineralocorticoid antagonist eplerenone on this trial. Spinal cord compressions were seen in two (3%) patients, which were deemed related to disease progression.
Table 4.
Incidence of Most Frequent (≥ 5%) Treatment-Related Adverse Events (N = 58)
| Adverse Event | Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 No. | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Nausea | 8 | 14 | 0 | 0 | 0 | ||
| Vomiting | 5 | 9 | 2 | 3 | 0 | 0 | |
| Diarrhea | 5 | 9 | 0 | 0 | 0 | ||
| Constipation | 3 | 5 | 0 | 0 | 0 | ||
| Fatigue | 9 | 16 | 9 | 16 | 1 | 2 | 0 |
| Edema peripheral | 4 | 7 | 1 | 2 | 0 | 0 | |
| AST | 3 | 5 | 3 | 5 | 0 | 0 | |
| ALT | 2 | 3 | 1 | 2 | 0 | 0 | |
| Hypokalemia | 3 | 5 | 0 | 0 | 0 | ||
| Dyspnea | 2 | 3 | 4 | 7 | 0 | 0 | |
NOTE: Two patients experienced hypertension, one each of grade 1 and grade 2.
DISCUSSION
This study is the second trial showing the activity of AA in patients with castration-resistant prostate cancer who have progressed on chemotherapy, and the first to explore the registration regimen currently under evaluation in two prospective randomized, phase III, placebo-controlled registration trials with primary end points of survival and progression-free survival (www.clinicaltrials.gov, NCT00638690, and NCT00887198).29,30 Antitumor activity was demonstrated on the basis of post-therapy PSA declines, changes in soft tissue disease by RECIST, the lack of progression in bone, and unfavorable to favorable changes in CTCs assessed with an analytically valid assay cleared by the US Food and Drug Administration for monitoring treatment effects in CRPC. The results show that the decision to treat a patient with progressive castration-resistant disease with chemotherapy does not necessarily mean that the tumor is resistant to further hormone treatments.
Noteworthy in this trial was the lower incidence of hypokalemia, hypertension, and fluid retention (5%, < 5%, and < 10%, respectively) compared with 55%, 17%, and 15%, respectively, relative to our separate study of AA without prednisone.19 The hyperaldosteronism syndrome developed in the absence of concomitant use of low-dose steroids frequently required treatment with either a mineralocorticoid antagonist (eplerenone) or low-dose glucocorticoids. These data, coupled with the high frequency of comorbid conditions in this group and the risk of adrenal insufficiency in patients receiving glucocorticoids with prior docetaxel treatments, provided strong support for the combination of AA plus prednisone as the preferred regimen for further development.
This trial is, to the best of our knowledge, the first to address the effect of prior ketoconazole exposure on outcome, although the time interval between prior ketoconazole therapy and protocol entry was highly variable, given that all of the patients were treated with chemotherapy in this interval. Patients with prior exposure to treatment and possible therapy-related selection pressure with ketoconazole had an inferior percentage of PSA decline by ≥ 50% compared with patients without prior ketoconazole exposure. The time to PSA progression tended to be shorter in patients with prior ketoconazole exposure compared with ketoconazole-naïve patients. Although this difference did not reach statistical significance, these findings were considered in the design of the exclusion criteria for this phase III trial of AA and prednisone in patients with castrate metastatic prostate cancer postdocetaxel treatment failure. Unfortunately, the response to prior treatment with ketoconazole was not known, so the question of cross-resistance could not be fully addressed. These important findings will be addressed in the future in a dedicated trial.13
Given the modest association with changes in PSA and the limitations of imaging distant metastases, there is an unmet medical need to develop tumor-specific markers to aid the selection of targeted therapies and to assess clinical outcome. Using a US Food and Drug Administration-cleared, analytically valid assay for CTC enumeration run in a Clinical Laboratory Improvement Amendments–certified laboratory, we have shown that CTC counts are prognostic pretherapy and the change in CTC number post-therapy is predictive of survival.31–33
The CTC conversion rate after treatment with AA was also associated with an extended time on treatment. The number of patients treated in this study was too small to further explore associations with clinical outcome.
In conclusion, this study showed biochemical, CTC, and radiologic evidence to support the evaluation of this AA and prednisone schedule in patients with progressive CRPC in a randomized study in the postchemotherapy setting with the primary end point of overall survival. Importantly, the trial also includes an evaluation of CTC number as an efficacy response biomarker in the phase III trial of AA plus prednisone versus placebo. Moreover, these data indicate that the discovery and development of drugs targeting steroid receptor signaling remain critically important for improving outcomes of this disease.
Acknowledgment
We thank Barbara Byczek and Christine Gutheil for editorial assistance. M.J. Morris, C.J. Ryan, M.E. Taplin and H.I. Scher are members of the Prostate Cancer Clinical Trials Consortium, a program of the Prostate Cancer Foundation and the Department of Defense Prostate Cancer Research Program.
Footnotes
Supported by Cougar Biotechnology, the Memorial Sloan-Kettering Cancer Center Specialized Program of Research Excellence (SPORE) Grant in Prostate Cancer (P50 CA92629), the Department of Defense Prostate Cancer Research Program (PC051382) and the Prostate Cancer Foundation.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology (ASCO), May 29-June 2, 2009, Orlando, FL, and the 44th Annual Meeting of ASCO, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Thian Kheoh, Cougar Biotechnology (C); Christopher Haqq, Cougar Biotechnology (C); Arturo Molina, Ortho Biotech/Cougar Biotechnology (C) Consultant or Advisory Role: Daniel C. Danila, Cougar Biotechnology (U); Johann S. de Bono, Cougar Biotechnology (U); Matthew R. Smith, Cougar Biotechnology (U); Steve M. Larson, Cougar Biotechnology (C); Howard I. Scher, Cougar Biotechnology (C), Veridex (U) Stock Ownership: Thian Kheoh, Cougar Biotechnology Honoraria: None Research Funding: Mary-Ellen Taplin, Cougar Biotechnology; Howard I. Scher, Cougar Biotechnology, Veridex Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Daniel C. Danila, Johann S. de Bono, Christopher Haqq, Howard I. Scher
Financial support: Christopher Haqq, Arturo Molina
Administrative support: Christopher Haqq, Arturo Molina, Howard I. Scher
Provision of study materials or patients: Daniel C. Danila, Michael J. Morris, Johann S. de Bono, Charles J. Ryan, Matthew R. Smith, Mary-Ellen Taplin, Glenn J. Bubley, Christopher Haqq, Howard I. Scher
Collection and assembly of data: Daniel C. Danila, Johann S. de Bono, Charles J. Ryan, Mary-Ellen Taplin, Thian Kheoh, Christopher Haqq, Arturo Molina, Aseem Anand, Michael Koscuiszka, Howard I. Scher
Data analysis and interpretation: Daniel C. Danila, Michael J. Morris, Samuel R. Denmeade, Mary-Ellen Taplin, Glenn J. Bubley, Thian Kheoh, Christopher Haqq, Arturo Molina, Aseem Anand, Michael Koscuiszka, Steve M. Larson, Lawrence H. Schwartz, Martin Fleisher, Howard I. Scher
Manuscript writing: Daniel C. Danila, Johann S. de Bono, Samuel R. Denmeade, Mary-Ellen Taplin, Glenn J. Bubley, Thian Kheoh, Christopher Haqq, Arturo Molina, Howard I. Scher
Final approval of manuscript: Daniel C. Danila, Michael J. Morris, Johann S. de Bono, Charles J. Ryan, Samuel R. Denmeade, Matthew R. Smith, Mary-Ellen Taplin, Glenn J. Bubley, Thian Kheoh, Christopher Haqq, Arturo Molina, Aseem Anand, Michael Koscuiszka, Steve M. Larson, Lawrence H. Schwartz, Martin Fleisher, Howard I. Scher
REFERENCES
- 1.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: Directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–8261. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 2.Geller J, Albert JD, Nochstein DA, et al. Comparison of prostatic cancer tissue dehydrotestosterone levels at the time of relapse following orchiectomy or estrogen therapy. J Urol. 1984;132:693–696. doi: 10.1016/s0022-5347(17)49829-6. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki K, Nishiyama T, Hara N, et al. Importance of the intracrine metabolism of adrenal androgens in androgen-dependent prostate cancer. Prostate Cancer Prostatic Dis. 2007;10:301–306. doi: 10.1038/sj.pcan.4500956. [DOI] [PubMed] [Google Scholar]
- 4.Titus MA, Schell MJ, Lih FB, et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 5.Nelson PS, Han D, Rochon Y, et al. Comprehensive analyses of prostate gene expression: Convergence of expressed sequence tag databases, transcript profiling and proteomics. Electrophoresis. 2000;21:11–23. doi: 10.1002/(SICI)1522-2683(20000501)21:9<1823::AID-ELPS1823>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 6.Montgomery RB, Mostaghel EA, Vessella R, et al. Maintenance of intratumoral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68:4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol. 2004;164:217–227. doi: 10.1016/S0002-9440(10)63112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stanbrough M, Bubley GJ, Ross K, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 9.Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67:5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 10.Yap TA, Carden CP, Attard G, et al. Targeting CYP17: Established and novel approaches in prostate cancer. Curr Opin Pharmacol. 2008;8:449–457. doi: 10.1016/j.coph.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Attard G, Reid AH, Yap TA, et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 12.Montgomery B, Mostaghel E, Nelson P, et al. Abiraterone suppresses castration resistant human prostate cancer growth in the absence of testicular and adrenal androgens. Am Assoc Cancer Res Special Conference: Advances in Prostate Cancer Research; January 21-24, 2009; San Diego, CA. [Google Scholar]
- 13.Ryan C, Efstathiou E, Smith M, et al. Phase II multicenter study of chemotherapy (chemo)-naive castration-resistant prostate cancer (CRPC) not exposed to ketoconazole (keto), treated with abiraterone acetate (AA) plus prednisone. J Clin Oncol. 2009;27(suppl):245s. abstr 5046. [Google Scholar]
- 14.Figg WD, Liu Y, Arlen P, et al. A randomized, phase II trial of ketoconazole plus alendronate versus ketoconazole alone in patients with androgen independent prostate cancer and bone metastases. J Urol. 2005;173:790–796. doi: 10.1097/01.ju.0000147013.09157.8e. [DOI] [PubMed] [Google Scholar]
- 15.Harris KA, Weinberg V, Bok RA, et al. Low dose ketoconazole with replacement doses of hydrocortisone in patients with progressive androgen independent prostate cancer. J Urol. 2002;168:542–545. [PubMed] [Google Scholar]
- 16.Trump DL, Havlin KH, Messing EM, et al. High-dose ketoconazole in advanced hormone-refractory prostate cancer: Endocrinologic and clinical effects. J Clin Oncol. 1989;7:1093–1098. doi: 10.1200/JCO.1989.7.8.1093. [DOI] [PubMed] [Google Scholar]
- 17.Small EJ, Baron AD, Fippin L, et al. Ketoconazole retains activity in advanced prostate cancer patients with progression despite flutamide withdrawal. J Urol. 1997;157:1204–1207. [PubMed] [Google Scholar]
- 18.Ryan CJ, Halabi S, Ou SS, et al. Adrenal androgen levels as predictors of outcome in prostate cancer patients treated with ketoconazole plus antiandrogen withdrawal: Results from a Cancer and Leukemia Group B study. Clin Cancer Res. 2007;13:2030–2037. doi: 10.1158/1078-0432.CCR-06-2344. [DOI] [PubMed] [Google Scholar]
- 19.Reid AH, Attard G, Danila DC, et al. Significant and sustained anti-tumor activity in post-docetaxel castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. doi: 10.1200/JCO.2009.24.6819. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Attard G, Reid AH, A'Hern R, et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27:3742–3748. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buchanan G, Yang M, Harris JM, et al. Mutations at the boundary of hinge and ligand binding domain of the androgen receptor confer increased transactivation function. Mol Endocrinol. 2001;15:46–56. doi: 10.1210/mend.15.1.0581. [DOI] [PubMed] [Google Scholar]
- 22.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 23.Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 25.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: Recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 28.Shaffer DR, Leversha MA, Danila DC, et al. Circulating tumor cell analysis in patients with progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:2023–2029. doi: 10.1158/1078-0432.CCR-06-2701. [DOI] [PubMed] [Google Scholar]
- 29.Abiraterone Acetate in Castration-Resistant Prostate Cancer Previously Treated With Docetaxel-Based Chemotherapy. http://clinicaltrials.gov/show/NCT00638690.
- 30.Abiraterone Acetate in Asymptomatic or Mildly Symptomatic Patients with Metastatic Castration-Resistant Prostate Cancer. http://clinicaltrials.gov/show/NCT00887198.
- 31.Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res. 2007;13:7053–7058. doi: 10.1158/1078-0432.CCR-07-1506. [DOI] [PubMed] [Google Scholar]
- 32.Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: A reanalysis of IMMC38 trial data. Lancet Oncol. 2009;10:233–239. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olmos D, Arkenau HT, Ang JE, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): A single-centre experience. Ann Oncol. 2009;20:27–33. doi: 10.1093/annonc/mdn544. [DOI] [PubMed] [Google Scholar]