Kallikrein-binding protein gene expression is repressed by thyroid hormone (T3), but enhanced by COUP-TF1, normally a repressor of T3-regulated genes.
Abstract
Kallikrein-binding protein (KBP) is a component of the kallikrein-kinin system that mediates vasodilation and inhibits tumor growth by antagonizing vascular endothelial growth factor-mediated angiogenesis. We demonstrate that KBP gene expression is repressed by T3 and modulated by the orphan nuclear receptor, chicken ovalbumin upstream promoter transcription factor 1 (COUP-TF1). In hypothyroid mice, KBP mRNA expression in the testis was increased 2.1-fold compared with euthyroid mice. We have identified two negative thyroid hormone response elements (nTREs) in the mouse KBP gene, nTRE1 located in the 5′ flanking region (−53 to −29) and nTRE2, located in the first intron (104–132). We used functional assays, cofactor knockdown, and chromatin immunoprecipitation assays to characterize nTRE1 and nTRE2 in hepatic (HepG2) and testes (GC-1spg) cell lines. Reporter expression directed by both elements was enhanced with addition of thyroid hormone receptor and repressed with the addition of T3. COUP-TF1 enhanced basal expression of both elements but blunted unliganded thyroid hormone receptor enhancement and T3 repression of nTRE1 but not nTRE2. Both nTREs bound nuclear corepressor and binding increased in response to T3. Nuclear corepressor knockdown resulted in loss of T3 repression of both nTRE1 and nTRE2. COUP-TF1, which usually represses T3 induction of positive thyroid hormone response elements, reverses T3 repression mediated by nTRE1 in the mouse KBP gene. Endogenous KBP expression is repressed by T3 and two functional nTREs, both of which are required, have been characterized in the KBP gene. COUP-TF1 may be an important factor to modulate expression of genes that are repressed by T3.
Rodent kallikrein-binding protein (KBP) and its human counterpart, kallistatin, are heparin-binding serine peptidase inhibitors (1, 2). KBP inhibits kallikrein peptidase activity by covalently binding to kallikrein, forming a heat-stable serpin-proteinase complex (3, 4). KBP mediates a range of processes, including vasodilatation (5, 6), angiogenesis (7, 8), and the inflammatory response (9, 10). Recent studies indicate that KBP is a potent inhibitor of tumor growth by inhibiting vascular endothelial growth factor- or basic fibroblast growth factor-induced angiogenesis (11, 12). We previously identified the KBP gene as one modulated by T3, based on DNA microarray analysis of mouse embryonic stem cells (13).
The rodent KBP gene is composed of five exons and four introns. The amino acid sequences of KBP proteins are highly homologous among species, the rat 423 amino acids (10), the mouse 417 amino acids (14), and the human 427 amino acids (15). KBP is expressed in the kidney, heart, testis, uterus, lung, salivary gland, liver, and blood vessels. The rat KBP gene contains two promoters, one in the 5′ flanking region and the other in the first intron (16). Two activator protein-1 (AP-1) sites, GH and glucocorticoid response elements, have been identified in the 5′flanking region. A thyroid hormone response element (TRE) was identified by sequence inspection in the first intron of rat KBP (16); however, it was not characterized functionally.
In most genes positively regulated by T3, TR/retinoid X receptor (RXR) heterodimer binds to a TRE with hexamers arranged as a direct repeat with a 4-bp gap, an inverted repeat, or palindrome TRE (17, 18). The unliganded thyroid hormone receptor (TR) binds to a TRE and recruits corepressors, nuclear receptor corepressor (NCoR), and silencing mediator of retinoid and thyroid hormone receptors (SMRT) and reduces expression (19). Ligand binding disrupts corepressor bound to TR and promotes coactivator binding, such as members of steroid receptor coactivator family (20) and cAMP response element-binding protein (CREB) binding protein (CBP)/p300 (21). This leads to activation of histone acetyltransferase (22, 23) and initiation of transcription.
Negative regulation by T3 is less well understood, although at least three different molecular mechanisms have been proposed. In the first model, T3 binds to TR and triggers a switch from recruiting coactivator to corepressor (24–26). This switch may be induced by a negative thyroid hormone response element (nTRE) capable of allosterically altering receptor conformation upon TR-T3 binding (26), as has been shown for the TSHβ nTRE (27–29). The unliganded TR binds to TSHβ nTRE, recruits a coactivator, and enhances gene transcription. Liganded TR recruits corepressors, such as SMRT, NCoR (30), and histone deacetylase and exerts T3-dependent repression (25, 31–34). Some genes, however, negatively regulated by T3 show increased histone acetylation (35). Both corepressor and coactivator play an important role in T3-dependent negative regulation of TSHβ (36). In the second model, TR does not bind directly to DNA, but unliganded TR/RXR heterodimer interacts with c-fos/c-jun on an AP-1 site and recruits coactivator. T3 binds to TR and triggers the switch from recruitment of coactivator to corepressor, resulting in transcription inhibition through AP-1 (37, 38). The third mechanism is referred as squelching. Unliganded TR/RXR heterodimer recruits corepressor away from the AP-1 site but does not directly interact with AP-1 protein, allowing AP-1 to recruit coactivator for transactivation. Conversely, in the presence of T3, liganded TR/RXR recruits a coactivator (CBP/p300). Because there is a limiting amount of coactivator, AP-1 protein predominantly recruits a corepressor, which leads to transcription inhibition (21, 39, 40). Unlike the well-characterized sequences of positive TREs, the DNA sequences of nTREs are more variable and a consensus sequence has not been established.
Chicken ovalbumin upstream promoter transcription factor 1 (COUP-TF1) is known to inhibit T3-, retinoic acid-, and other nuclear receptor-mediated transcriptional activation, and the best characterized is the inhibition of T3 action in neural development (41). One of the mechanisms of transcriptional inhibition by COUP-TF1 is direct competition with TR, retinoic acid receptor, and steroid receptors for binding to the response element (42), which has been demonstrated in gel shift assays (43–45). The silencing activity of COUP-TFI is enhanced by the corepressors, NCoR and SMRT (46). COUP-TF1 can also function as a coactivator and stimulate gene expression (41). In T3-mediated negative regulation, COUP-TF1 action may depend on the promoter sequence and context.
We demonstrate that mouse KBP gene expression is negatively regulated by T3 in vivo. We identified two functional nTREs: nTRE1 located in the 5′ flanking region and nTRE2 located in the first intron. COUP-TF1 binds to nTRE1, stimulates KBP gene expression, and antagonizes T3-dependent repression. We demonstrate that nTRE1 and nTRE2 bind NCoR in response to T3, and NCoR is required for T3-dependent repression from both. KBP nTRE1 and nTRE2 both bind TR, but binding increases to nTRE2 after treatment with T3. nTREs are more variable in sequence and position compared with positive TREs, and characterization of cofactor binding shows similarities and differences among the nTRE elements.
Materials and Methods
Cell culture, transient transfection, and luciferase assays
HepG2 cells were maintained in Eagle's MEM supplemented with 10% fetal bovine serum. Mouse testes cells, GC-1spg, were maintained in DMEM with 10% serum. The transient transfection and luciferase assays were performed in cells that were seeded in a 24-well plate and grown in serum-free medium with 10% serum replacement (Invitrogen Inc., Carlsbad, CA) for 24 h before transfection. Unless otherwise stated, 0.1 μg of reporter construct, 0.1 μg TRβ, and/or 0.1 μg COUP-TF1 expression vectors were used in transient transfection assays using the Effectene system (QIAGEN Inc., Valencia, CA). Empty vector (pcDNA 3.1) was used to keep a constant concentration of DNA in all transfections. Transfected cells were treated with T3 (50 nm final concentration) in serum-free medium for 24 h before the luciferase assay. The dual-luciferase reporter system (Promega, Madison, WI) was used and results normalized to Renilla luciferase expression. All transfections were performed in triplicate.
Reporter constructs, mutant reporter constructs, and expression vectors
The mouse KBP gene (Gene Bank accession no. X61597) sequence, from −59 to 1778, including a portion of the promoter region, first exon (noncoding exon, 93 bp) and first intron, was cloned by PCR into the pGL-3 promoter vector (Promega). The deletion constructs were also cloned by PCR into the pGL-3 promoter vector. Smaller reporter constructs −59/+66-luc, 93/135-luc, 323/342-luc, 381/431-luc, and mutant reporters were cloned using DNA oligonucleotides and inserted into the pGL-3 promoter vector at BglII-MluI site. Mutations of nTRE1 and nTRE2, constructs M1 and M2, were generated by site-directed mutagenesis using reporter −59/+723-luc. In M1, nTRE1 was mutated and nTRE2 was intact, and in M2 reporter, nTRE2 was mutated and nTRE1 was intact. All constructs and mutations were checked for accuracy by direct DNA sequencing. COUP-TF1, in pBlueScript, was a gift from Dr. Ming J. Tsai (Baylor College of Medicine, Houston, TX) and was subcloned into vector pcDNA3.0 for eukaryotic expression. The TRβ1 expression vector was PCR cloned into pCMVTNT expression vector (Promega).
RNA isolation, reverse transcription, and quantitative PCR analysis
Total RNA was isolated from cells using RNeasy Plus kit (QIAGEN) and then reverse transcribed using Superscript III (Invitrogen). cDNA (1 μl) was used in PCR amplification of specific genes. Quantification by real-time PCR was described previously (13). The relative mRNA expression levels were expressed as a ratio of the mRNA being tested to the levels of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA. Primers used for detection of mRNA expression of KBP were 5′-cagcctggagaacagaca3′ (sense) and 5′-gtgagactgtcgagttgtgt3′ (antisense).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed according to the protocol provided by the manufacturer (Millipore, Bedford, MA). In brief, mouse testis cells (GC-1spg) were plated in a 10-cm dish (∼2 × 106 cells /dish) in complete medium (DMEM supplemented with 10% serum) overnight. Cells were plated at a relatively low density to optimize the response to T3 treatment. On the first day, the medium was replaced with serum-free medium and cells were transfected with TRβ and COUP-TF1 expression vectors and incubated overnight. On the second day, cells were fed with fresh serum-free medium and treated with 50 nm T3 for time periods of 0, 15, 30, 45, and 60 min. At the end of each time point, cells (three dishes) were incubated with formaldehyde (final concentration 1%) for 10 min to cross-link the chromatin and then washed twice with cold PBS. The rest of procedure was the same as the manufacturer protocol. To reach the desired resolution for detecting binding to the response elements, we optimized the sonication procedure to obtain 150- to 200-bp DNA fragments. The antibodies used for each time point of the ChIP assay were anti-TR antibody (Thermofisher Scientific-Pierce Antibody, Rockville, IL), anti-NCoR (Millipore, Bedford, MA), anti-COUP-TF1, anti-SMRT, and anti-CBP antibodies (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Upon completion of immunoprecipitation, cross-linked chromatin DNA was recovered. DNA was purified using MiniElute column (QIAGEN). DNA (1 μl) was used for PCR for 42 cycles with specific primers. For nTRE1 −59 to +66, the primers were 5′-CCAGCATTTCCT AAGAGGAG-3′ (sense) and 5′-TTACTCCAGCCAGCTT CAG-3′ (antisense). The PCR product was 125 bp. For nTRE2 93-135, the primers were 5′-TGGAAGCTGGCTGGAGTAAG-3′ (sense) and reverse, 5′-TGGTTTCTCTTGC TTGTGGT-3′ (antisense). The PCR probe was 104 bp. For the ChIP detection of receptor and cofactor binding to necdin nTRE (−30 to +246), the primers used were as previously described (47). Input material was one tenth of the diluted total ChIP material. A negative control was performed using mouse IgG without the primary antibody. Transfection efficiency was assessed by the mRNA level of TRβ and COUP-TF1.
Gene silencing
Unless stated otherwise, On-Target small interfering RNA (siRNA; Dharmacon RNAi Technologies, Lafayette, CO) was used in gene-silencing experiments. Control siRNA (supplied by the manufacturer) was used in control conditions (non-gene silencing). Cells were seeded on six-well plates with a density 0.2–0.5 × 106 cells/well. For each well, 100 nm siRNA (final concentration) was transfected using DharmaFact. On the next day, medium was removed and replaced with fresh serum-free medium with 10% serum replacement and grown for an additional 36 h before T3 treatment. For T3 treatment, cells were incubated with 50 nm T3 (final concentration) for an additional 16 h before RNA isolation. After reverse transcription, cDNA was used for PCR detection or quantitative PCR quantification. For the gene silencing combined with luciferase assay experiments, cells were seeded on a 24-well plate in serum-free medium with 10% serum replacement, siRNA. Control (scrambled RNA), siRNA COUP-TF, or siRNA NCoR (final 100 nm) and 0.1 μg of each plasmid (reporter and expression vector) were cotransfected using DharmaDuo reagent for each well. The next day, cells were fed with fresh serum-free medium and grown for an additional 24 h. Cells were then treated with T3 (50 nm) for an additional 16 h before the luciferase assay. All transfections were performed in triplicates. The results were expressed as the mean value with se (±se).
Animals.
All animal experiments were approved by the Institutional Animal Care Use Committee. Two groups of mice were used in the study, euthyroid and hypothyroid (n = 4 in both groups). Hypothyroidism was induced by feeding an iodine-deficient diet supplemented with 0.15% propylthiouracil for a period of 6 wk starting at age 4 wk. The mice were then euthanized at age 10 wk. Total RNA was isolated from testis and KBP mRNA levels were detected by quantitative PCR.
Statistical analysis
Reporter assays and quantitative PCR experiments were performed in triplicate. Statistical analysis was done using t test and one-way ANOVA. Statistical significance was defined as P < 0.05.
Results
Thyroid hormone inhibits KBP gene expression
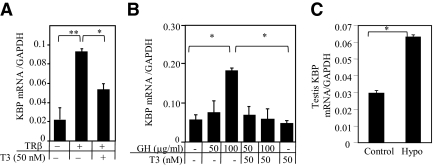
We investigated thyroid hormone regulation of the endogenous KBP gene expression in HepG2 cells. HepG2 cells were transfected with a TRβ expression vector and treated with or without T3 (50 nm) for 24 h. Unliganded TRβ enhanced KBP mRNA expression 2.6-fold (P < 0.01). Enhanced basal expression in the presence of unliganded TR is characteristic of genes negatively regulated by T3 (47, 48). Addition of T3 (50 nm) repressed KBP gene expression (Fig. 1A).
Fig. 1.
Effect of T3 on endogenous KBP mRNA expression. A, HepG2 cells were transfected with TRβ-expressing vector and treated with or without T3 (50 nm) for 24 h. B, Testis spermatogonia cells (GC-1spg) were grown in serum-free medium for 24 h before treatment with GH (0, 50, and 100 μg/ml) with or without T3 (50 nm) for 24 h. After completion of hormone treatment, KBP and GAPDH mRNA expression was determined by quantitative PCR. C, KBP mRNA expression in testis of euthyroid (control) and hypothyroid (Hypo) mice (n = 4 in each group). Values shown are mean ± se (error bars) of triplicates (for transfections). *, P < 0.05, **, P < 0.01, when compared with conditions without TRβ or added T3.
Previous studies have reported that GH stimulates expression of the rat KBP promoter (9, 49). We studied the influence of T3 on GH-induced mouse KBP gene expression. Mouse testis cells (GC-1spg), which are more responsive to hormonal stimulation compared with HepG2, were used for these studies. Testis cells were treated with human GH (50 and 100 μg/ml) either in the presence or absence of T3 (50 nm). Low concentrations of GH had minimal effect on KBP mRNA, but high concentrations of GH (100 μg/ml) stimulated KBP mRNA 3.2-fold (P < 0.05). In the presence of T3, GH did not stimulate KBP mRNA expression (Fig. 1B).
The influence of thyroid status on KBP gene expression in mice was studied. Mice made hypothyroid for 6 wk were compared with euthyroid mice. The hypothyroid mice had increased (2.1-fold, P < 0.05) KBP mRNA expression in the testis compared with the euthyroid control (Fig. 1C). These findings are consistent with negative regulation of the endogenous KBP gene by T3.
Localization of nTREs in the KBP gene
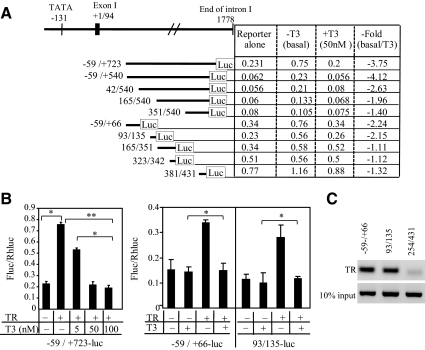
We examined the sequence of the mouse KBP gene and identified an AGGTCA-like octamer half-sites in the 5′ flanking region and the first intron. The reporter construct, KBP −59/+723-luc, including both regions, was used to test these putative elements (Fig. 2A). Cotransfection of the unliganded TRβ expression vector with KBP −59/+723-luc induced luciferase activity 3.3-fold (P < 0.05) (Fig. 2B). Addition of T3 at increasing concentrations (5, 50, and 100 nm) diminished TRβ-induced gene expression in a dose-dependent fashion, 3.75-fold inhibition at 50 nm, consistent with a nTRE located within the sequence −59/+723 (P < 0.05) (Fig. 2B).
Fig. 2.
KBP gene is negatively regulated by T3. A, Diagram of mouse KBP gene and reporter construct basal expression, response to T3, and fold T3 repression (basal/T3 treated). B, Transfection assays. HepG2 cells grown in serum-free medium were cotransfected with reporter construct and TRβ expression vector. The fragment −59/+723 includes nTRE1 (contained in −59/+66) and nTRE2 (contained in 93/135). Cells were treated with T3 to a final concentration of 0, 5, 50, or 100 nm for 24 h before luciferase assay. The dual-luciferase assay system was used to determine relative Firefly luciferase activity (FLuc)/Rhenilla luciferase (RhLuc) of the reporters. Values shown are mean ± se (error bars) of triplicates. *, P < 0.05, **, P < 0.01, when compared with conditions without TRβ or added T3. C, ChIP analysis of TR binding to nTRE1 −59/+66 and nTRE2 93/135. The segment 254/431 is a control fragment without a known TRE or nTRE. ChIP was performed in GC-1spg cells transfected with TRβ.
To localize the nTRE, several deletion constructs of this region (−59-540-luc, 42-540-luc, 165-540-luc, and 351-540-luc) were analyzed for T3 responsiveness. Analysis of the −59/+540-luc reporter demonstrated that luciferase activity was reduced 4.12-fold in response to T3 (Fig. 2A). T3-mediated inhibition was still present with the 42/540-luc construct, 2.63-fold. Further deletions showed significant loss of T3 inhibition, 165/540-luc, 1.96-fold, and 351/540-luc, 1.40-fold. These data indicate by deletion analysis that the nTRE activity is located primarily in the sequence −59/+165.
We further narrowed the location of the nTRE with reporter constructs −59/+66-luc, 93/135-luc, 165/351-luc, 323/342-luc, and 381/431-luc (Fig. 2A). In transient transfection assays, T3 repressed reporter activity of the construct −59/+66-luc 2.24-fold and the 93/135-luc construct 2.15-fold. Reporter constructs 165/351-luc, 323/342-luc, and 381/431-luc had no significant inhibition in response to T3. T3-inhibitory activity therefore was primarily contained in the two regions, −59/+66 and 93/135. We used a ChIP assay to confirm that TR bound to the regions −59/+66 and 93/135 but not to a control region 254/431 (Fig. 2C). Based on the sequence inspection and deletion analysis, we defined the region −59/+3 as nTRE1 and 93/135 as nTRE2 (Fig. 3A).
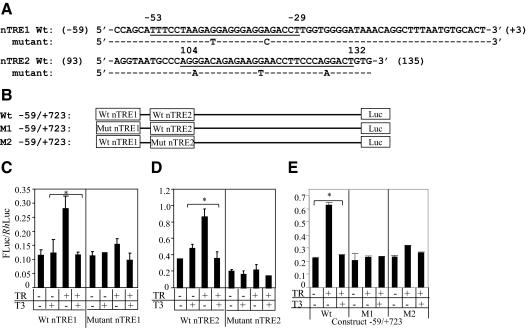
Fig. 3.
Mutational analysis of KBP nTREs. A, Alignment of KBP nTRE1 (−53/−29) and nTRE2 (104/132) sequences, wild-type (wt) sequence, and location of the point mutations. Sequences containing half-sites are underlined. B, Diagram of wild-type reporter −59/+723 and mutant constructs M1 and M2. In M1 and M2 reporters, the locations of the point mutations are exactly the same as the mutations in nTRE1 (−53/−29) and nTRE2 (104/102), respectively. C–E, T3 repression of wild-type and mutant nTRE1 and nTRE2 elements. HepG2 cells were transfected with reporter alone or cotransfected with TRβ expression vector and treated with or without T3 (50 nm) for 24 h. The dual-luciferase assay system was used to determine relative Firefly luciferase activity (FLuc)/Rhenilla luciferase (RhLuc) of the reporters. Values shown are mean ± se (error bars) of triplicates. *, P < 0.05, when compared with conditions without TRβ or added T3.
Mutational analysis of nTREs
We examined TR repression of the nTREs using mutational analysis (Fig. 3A). The nTRE 1 (Fig. 3B) and nTRE2 (Fig. 3C) were significantly enhanced by cotransfection of unliganded TRβ and significantly repressed by the addition of T3 (P < 0.05). The nTRE1 and nTRE2 were mutated and analyzed for T3-mediated response (Fig. 3A). T3 inhibition was lost in both mutant nTRE-luc constructs compared with the wild-type nTREs (Fig. 3, B and C). These data demonstrate that the regions −59/+3 and 93/135 contain regulatory elements that confer T3-mediated repression of gene expression.
Role of nTRE1 and nTRE2
To investigate the relative role of nTRE1 and nTRE2 in T3-dependent KBP gene repression, we mutated each nTRE in the context of the −59/+723-luc. M1 contains a mutation in nTRE1 and M2 a mutation in nTRE2 (Fig. 3B). There was no significant T3 repression with mutation of either nTRE1 (M1 reporter) or nTRE2 (M2 reporter) (Fig. 3E). The data indicate that in the context of the −59/+723 element, both nTREs were required for T3 repression.
Regulation of KBP gene by TR/T3 and COUP-TF1
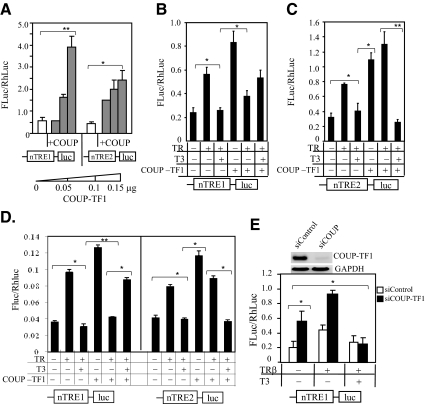
COUP-TF1 is known to compete with TR for binding to a TRE and inhibits T3 induction (42, 50–52). In addition to competing for a binding site, COUP-TF1 is also capable of heterodimerizing with TR on a palindromic TRE to suppress T3 stimulation (53). To assess whether COUP-TF1 was involved in the regulation of KBP gene expression, HepG2 cells were cotransfected with reporter constructs (nTRE1-luc or nTRE2-luc) and COUP-TF1 expression vector at increasing concentrations (0.05, 0.10, and 0.15 μg). COUP-TF1 stimulated basal luciferase activity of both reporters, nTRE1-luc, 8.9-fold (P < 0.01), and nTRE2, 5.4-fold (P < 0.05) (Fig. 4A). Interestingly, the effect of cotransfection of TRβ on COUP-TF1-stimulated expression differed between the two nTREs (Fig. 4, B and C). In the absence of T3, COUP-TF1 inhibited unliganded TR-mediated enhancement of nTRE1-luc but increased expression of nTRE2-luc. In the presence of T3, COUP-TF1 blunted nTRE1-luc-mediated repression (Fig. 4B) but did not modify nTRE2-luc (Fig. 4C). In the presence of TR, COUP-TF1 inhibited the nTRE1 but not the nTRE2. To determine whether COUP-TF1 antagonism of TR was cell type specific, we evaluated the COUP-TF1 effects on the KBP nTREs in testis cells (GC-1spg) (Fig. 4D). Similar results were obtained as those in HepG2 cells (Fig. 4, B and C), supporting the findings that COUP-TF1 antagonizes T3 repression mediated by nTRE1.
Fig. 4.
Influence of COUP-TF1 on KBP nTREs and gene expression. A, HepG2 cells were transfected with reporter constructs and COUP-TF1 expression vector at increasing concentrations (0, 0.05, 0.1, and 0.15 μg). Differential effects of COUP-TF1 on TR/T3-mediated gene expression of nTRE1 (B) and nTRE2 (C). Cells were cotransfected with reporter construct and expression vectors expressing TRβ (0.1 μg) and/or COUP-TF1 (0.1 μg). Cells were then treated with or without T3 (50 nm) for 16 h before a luciferase assay. D, Reporter assay using testis GC-1spg cells. Conditions are the same as described in B and C. E, COUP-TF1 knockdown. Cells were cotransfected with siRNA COUP-TF1 or siRNA control (scrambled RNA), expression vector were transfected with TRβ and nTRE1-luc, and the cells were grown in serum-free medium. Forty-eight hours after transfection, cells were incubated with T3 (50 nm) for 16 h before luciferase assay or RNA isolation. The effectiveness of gene silencing of COUP-TF1 was assessed by PCR detection of COUP-TF1 mRNA expression in the cells. PCR was performed using 1 μl of cDNA for 30 cycles for COUP-TF1 and 1 μl of 5-fold diluted cDNA for 25 cycles for GAPDH. The dual-luciferase assay system was used to determine relative Firefly luciferase activity (FLuc)/Rhenilla luciferase (RhLuc) of the reporters. Values shown are mean ± se (error bars) of triplicates. *, P < 0.05, **, P < 0.01, when compared with conditions without TRβ, COUP-TF1, or added T3.
The influence of COUP-TF1 on TR-mediated repression by nTRE1 should be reversed when the COUP-TF1 gene is silenced. We performed COUP-TF1 gene knockdown by transfection of cells with siRNA COUP-TF1. Expression of nTRE1 in the presence of TRβ was repressed with addition of COUP-TF1 (Fig. 4B) but was significantly enhanced after COUP-TF1 knockdown (P < 0.05) (Fig. 4E). In the presence of T3, COUP-TF1 knockdown enhanced T3-dependent repression, 3.7-fold (P < 0.05), compared with repression without COUP-TF1 knockdown (2-fold) (Fig. 4B). COUP-TF1 has been shown to have different effects based on the response element sequence. It strongly inhibits T3-mediated transcription of a direct repeat with a 4-bp gap TRE but not TREs in the malic enzyme gene or myosin heavy-chain gene (53). A similar phenomenon has been demonstrated in estrogen response elements (EREs). COUP-TF1 enhanced estradiol-estrogen receptor (ER) binding to consensus ERE but did not affect tamoxifen aziridine-ER binding to an ERE (54).
Influence of TR, T3, and COUP-TF1 on endogenous KBP gene expression
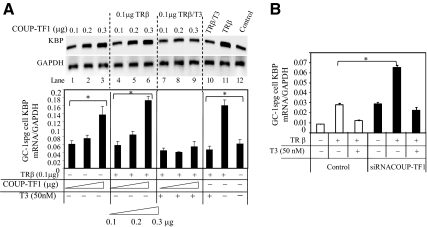
To evaluate the effects of TR, T3, and COUP-TF1 on endogenous KBP expression, KBP mRNA levels were determined in mouse testicular cells (GC-1spg) transfected with constant TRβ (0.1 μg) and COUP-TF1 at increasing concentrations (0.1, 0.2, 0.3 μg). COUP-TF1 alone increased endogenous KBP mRNA levels (Fig. 5A, lanes 1–3), consistent with the results from the promoter analysis of nTRE1 and nTRE2 (Fig. 4A). In the absence of T3, cotransfection of COUP-TF1 with TRβ (Fig. 5A, lanes 4–6) had no effect compared with COUP-TF1 alone (Fig. 5A). In the presence of COUP-TF1 (Fig. 5A, lanes 7–9), T3 repression was seen only with the highest level of COUP-TF1 (Fig. 5A, lane 9). In the presence of unliganded TR, KBP mRNA expression was increased and then repressed by the addition of T3 (Fig. 5A, lanes 10 and 11). We performed siRNA gene silencing of COUP-TF1 to determine the influence on endogenous KBP mRNA expression with transfected TRβ (Fig. 5B). In the absence of T3, KBP mRNA expression was enhanced compared with control (P < 0.05), and in the presence of T3, KBP mRNA was reduced 3.1-fold in COUP-TF1 knockdown cells compared with 2.2-fold in control cells expressing COUP-TF1. The pattern of endogenous KBP expression after COUP-TF1 knockdown (Fig. 5B) showed enhanced response to unliganded TR and enhanced T3 repression compared with control. This is consistent with endogenous COUP-TF1 blunting TR-induced expression and T3-mediated repression.
Fig. 5.
Effects of T3 and COUP-TF1 on endogenous KBP mRNA expression. A, KBP mRNA levels in transfected GC-1spg cells. Cells were transfected with COUP-TF1 alone or cotransfected with TR with or without T3 (50 nm). After 24 h of T3 treatment, RNA was isolated. The mRNA level from each sample examined using cDNA (1 μl) and PCR for KBP mRNA (amplified 40 cycles) and GAPDH mRNA (amplified 22 cycles). B, KBP mRNA expression in GC-1spg control cells and cells with knockdown of COUP-TF1. Cells in six-well dish were cotransfected with 1 μg of TRβ expression vector and either siRNA control (100 μm) or siRNA COUP-TF1 (100 μm). Cotransfections were carried out using DharmaDuo reagent (Dharmacon). After transfection (48 h), RNA was isolated and reverse transcribed. KBP mRNA expression was examined by quantitative PCR. Values shown are mean ± se (error bars) of triplicates. *, P < 0.05, when compared with conditions without TRβ, COUP-TF1, or added T3.
TR, NCoR, and COUP-TF1 binding to nTRE1 and nTRE2
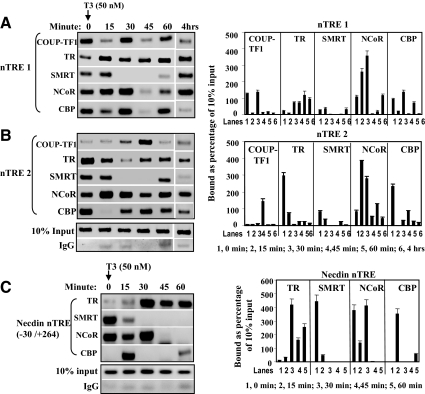
We next tested receptor binding to the KBP nTREs and the recruitment of coactivators and corepressors. We performed ChIP assay in GC1-spg mouse testis cells transfected with TRβ1 and COUP-TF1 and treated with T3 (50 nm) for 0, 15, 30, 45, and 60 min and 4 h. In the absence of T3 (0 min), the corepressor NCoR was bound to nTRE1 (−53/−29) and nTRE2 (104/132), and binding increased to both elements after addition of T3, reaching a peak at 30–60 min (Fig. 6, A and B). nTRE1 and nTRE2 also bound CBP. TR bound to nTRE2 in the absence of ligand, and TR binding was reduced after the addition of ligand. nTRE1 bound only a small amount of TR in the absence of ligand, and binding was increased after addition of T3. COUP-TF1 bound most strongly to nTRE1, and binding was reduced after the addition of ligand as TR binding increased. nTRE2 bound COUP-TF1 only at the 45-min time point after the addition of T3.
Fig. 6.
TR and COUP-TF1 DNA binding and recruitment of corepressor and coactivator in GC-1spg mouse testis cells. A, TR and COUP-TF1 binding to nTRE1 and recruitment of cofactors. PCR amplified region from −59 to +62 (121 bp). B, TR and COUP-TF1 binding to nTRE2 and recruitment of cofactors. PCR amplified 99 bp from 72 to 171. C, TR and cofactor binding pattern of the necdin nTRE. PCR amplification was carried out for 42 cycles using primers for necdin nTRE region −30 to +264. Quantitation of density of bands (mean ± se of three repeated measurements of image) shown in ChIP assay, arranged by each binding factor across the time course. PCR was performed 40 cycles for all samples including input and negative control (IgG only).
Pattern of TR and coactivator binding to the necdin nTRE
The necdin nTRE is positioned downstream of the transcription start site at +88 in both the human and mouse gene. The expression of the nTRE was enhanced by unliganded TR and repressed after the addition of T3 (47). We performed the same ChIP assay as for the KBP nTREs to track the interaction with TR, SMRT, NCoR, and CBP (Fig. 6C). A pattern of factor binding with similarities to that seen for KBP nTRE1 and nTRE2 was observed. The nTRE strongly bound the corepressors SMRT and NCoR, which diminished after the addition of ligand and recruitment of TR.
NCoR is required for T3-mediated repression of gene expression
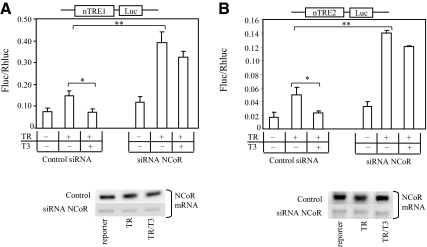
To confirm the role of NcoR, we performed siRNA gene silencing of NCoR and then examined the T3-dependent repression using both nTRE reporter constructs. NCoR knockdown significantly increased reporter expression in the presence of TR compared with control (P < 0.01) (Fig. 7, A and B). Significant T3-mediated repression was demonstrated in the control (P < 0.05), but not after NCoR knockdown (Fig. 7, A and B), indicating that NCoR is required for T3 repression by nTRE1 and nTRE2.
Fig. 7.
The role of NCoR in T3-dependent inhibition of KBP gene expression. Cells were cotransfected with siRNA NCoR or control siRNA (scrambled RNA), TRβ expression vector, and reporter nTRE1-luc (A) or nTRE2-luc (B). After transfection, cells were grown in serum-free medium supplied with serum replacement for 48 h and then treated with 50 nm T3 for 16 h before the luciferase assay. The effectiveness of gene silencing of NCoR was assessed by NCoR mRNA expression. Values shown are mean ± se (error bars) of triplicates. *, P < 0.05, **, P < 0.01, when compared with conditions without TRβ or added T3.
Discussion
We have identified two nTREs in the mouse KBP gene, nTRE1 located in the 5′ flanking region −53 to −29, and nTRE2, located in the first intron 104–132. These elements confer T3-mediated negative regulation in the KBP gene. T3-dependent repression was demonstrated for both nTRE1 and nTRE2 and involves TR interaction with DNA and requires recruitment of NCoR (Table 1). This is consistent with the primary role of NCoR in T3-mediated repression. We demonstrated that COUP-TF1 enhances basal expression of both nTRE1 and nTRE2. COUP-TF1 binds predominantly to nTRE1 and blunts unliganded TR enhancement of expression and reduces T3-mediated repression. Although either element alone binds TR and cofactors and confers T3 repression, both nTRE elements were required for T3 repression in the context of the −59 to +723 fragment. This suggests that elements contained in this additional sequence likely influence the interaction and function of the two sites.
Table 1.
Summary of Kallikrein-Binding Protein (KBP) gene expression and nTRE properties based on binding and functional studies
| Transcription factors | KBP endogenous mRNA | KBP nTRE1 (5′ flank −53/−29) | KBP nTRE2 (first intron 104/132) |
|---|---|---|---|
| Addition of TR (functional) | Enhances expression | Enhances expression | Enhances expression |
| TR + ligand (T3) (functional) | Repress | Repress | Repress |
| COUP-TF1 (functional and binding to nTRE) | Enhances basal expression, blunts TR enhancement, blunts T3 repression | Enhances basal expression, blunts TR enhancement, blunts/reverses T3 repression | Enhances basal expression, no effect on TR enhancement or T3 repression |
| NCoR (functional and binding to nTRE) | Not tested | Represses basal and unliganded TR expression, required for repression | Represses basal and unliganded TR expression, required for repression |
| TR (binding to nTRE) | Not tested | Increased binding in response to T3 | Reduced binding in response to T3 |
Interestingly, the two nTREs have different patterns of T3-dependent repression. TR binds minimally to nTRE1 in the absence of T3. In the presence of T3, TR binds strongly to DNA, recruits NCoR, and releases CBP. This process is the typical reported pattern of TR repression and has been previously shown with the TSHβ nTRE (26, 31, 34). The position of the nTRE, in close proximity to the transcription start site, is also characteristic of other nTREs (55). NCoR, however, bound with a similar pattern to both nTRE1 and nTRE2.
In contrast to the pattern of TR binding to nTRE1, unliganded TR binds to nTRE2 in the absence of T3 and recruits CBP. In the presence of T3, liganded-TR was dissociated from DNA, CBP was released, and NCoR was recruited. This process was similar to the previously proposed non-DNA-binding model. In this model, ligand-induced suppression is via TR interaction with AP-1 (Jun/Jun or Jun/Fos) rather than nTRE (26, 37). Presumably, unliganded TR/RXR interacts with AP-1 and recruits coactivator for transactivation, and liganded-TR recruits corepressor to AP-1/TR/RXR complex for inhibition. This model has been used to explain T3-induced inhibition of gene expression without receptor binding to DNA (37, 56). For the non-DNA-binding model, it is essential that an AP-1 binding site is present, allowing TR to interact with AP-1 on the AP-1 site. However, in the case of the AP-1 retinoic acid-mediated transcription, AP-1 can directly interact with the retinoic acid receptor to antagonize retinoic acid-mediated transcription, without binding to an AP-1 site (57).
In the KBP gene, we found an AP-1-like site located in the first intron at 226–232 (TGAGTTC), which is not far from the nTRE2 (93–136). In this study, however, the putative AP-1 site was not contained in the reporter construct for functional assays or the PCR primed region (from 66 to 172) used in the ChIP assay. We observed that TR binds to nTRE2, although it has not established a role for TR interaction with AP-1. Further investigation is needed to understand whether AP-1 is involved in T3-mediated repression via nTRE2 and whether the AP-1 site is functional, although our deletion studies suggest that it is not required.
We have demonstrated that TR and COUP-TF1 bind intermittently to the nTREs. The intervals of DNA binding differed with different DNA configuration. It has been reported that TR has distinct dynamic binding pattern to the positive TREs, cholesterol 7α-hydroxylase (Cyp7) TRE, and phosphoenol-pyruvate carboxykinase TRE and for the recruitment of coactivators, steroid receptor coactivator-1 p160, glucocorticoid-interacting protein-1, and thyroid hormone receptor-associated protein-220, to these positive TREs (58). In addition to DNA configuration, other factors also influence receptor DNA binding, such as assembling protein-protein complex, chromatin remodeling, and transcription initiation. The cycle of ER binding to DNA and the recruitment of coactivators is associated with phosphorylation of RNA Pol II (59). Phosphorylation of Pol II C-terminal domain initiates the transcription, which, in turn, disassembles the protein complex and releases ER from the DNA.
We observed that in the absence of T3, coactivator (CBP) and corepressor (NCoR and SMRT) were recruited to DNA. NCoR and SMRT have been shown to function as coactivator of unliganded TR as evidenced by the fact that cotransfection of NCoR or SMRT increases unliganded TR-stimulated transcription in transient transfection assays (40, 60). It is necessary for corepressors to interact with CBP to assemble a transcription complex. In fact, direct association of NCoR with CBP has been visualized in MCF-7 cells using immunofluorescence techniques and has also been demonstrated in COS cells by coimmunoprecipitation (61), indicating that NCoR may be involved in modulating histone acetyltransferase activity. To determine whether unliganded TR can recruit both corepressor and coactivator to an nTRE, we examined the well-characterized necdin nTRE (47). We demonstrated a pattern of TR and cofactor binding to the necdin nTRE, with similarities to those seen with nTRE1 and nTRE2 (Fig. 7). We conclude that the mode of recruiting coactivator or corepressor to nTRE has similarities and differences among the various nTRE configurations we studied and likely also due to the availability of corepressor and coactivator.
COUP-TF1 has a significant role in early neural development and is recognized as repressing genes stimulated by T3 (62, 63). It has been increasingly recognized in developmental models that thyroid hormone activation and inactivation are both required for a normal developmental program (19). A physiological role for COUP-TF1 in modulation T3-dependent gene expression in the adult has not been reported, but the KBP gene may be an example.
Thyroid hormone inhibition of gene expression is important in brain and sensory development and in a range of regulatory processes in the adult. Inhibition of TSH by thyroid hormone is the key thyroid hormone action used to evaluate overall thyroid status. Although there are greater variations of the mechanisms of T3-mediated repression, compared with T3-mediated stimulation, a number of consistent mechanistic patterns are emerging. The KBP gene is notable for two nTREs, nTRE1 in a typical nTRE position and nTRE2 downstream of the transcription start site, although both are required for T3 repression. The recruitment of NCoR to both nTRE1 and nTRE2 was strongly shown as was the absolute requirement of NCoR for functional T3-mediated repression. The COUP-TF1 effect on antagonizing T3 repression is complementary to COUP-TF1 repression of T3 induction and suggests a broader role for COUP-TF1 in modulating thyroid hormone action. The physiological relevance of these interactions will need to be tested in whole-animal models.
Acknowledgments
This work was supported by Merit Review funds from the Department of Veterans Affairs and Grant RO1 DK67233 from the National Institutes of Health.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AP-1
- activator protein-1
- CBP
- cAMP response element-binding protein (CREB) binding protein
- ChIP
- chromatin immunoprecipitation
- COUP-TF1
- chicken ovalbumin upstream promoter transcription factor 1
- CREB
- cAMP response element-binding protein
- ER
- estrogen receptor
- ERE
- estrogen response element
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- KBP
- kallikrein-binding protein
- NCoR
- nuclear receptor corepressor
- nTRE
- negative thyroid hormone response element
- RXR
- retinoid X receptor
- siRNA
- small interfering RNA
- SMRT
- silencing mediator of retinoid and thyroid hormone receptor
- TR
- thyroid hormone receptor
- TRE
- thyroid hormone response element.
References
- 1. Chao J, Chai KX, Chen LM, Xiong W, Chao S, Woodley-Miller C, Wang LX, Lu HS, Chao L. 1990. Tissue kallikrein-binding protein is a serpin. I. Purification, characterization, and distribution in normotensive and spontaneously hypertensive rats. J Biol Chem 265:16394–16401 [PubMed] [Google Scholar]
- 2. Chen VC, Chao L, Pimenta DC, Bledsoe G, Juliano L, Chao J. 2001. Identification of a major heparin-binding site in kallistatin. J Biol Chem 276:1276–1284 [DOI] [PubMed] [Google Scholar]
- 3. Zhou GX, Chao L, Chao J. 1992. Kallistatin: a novel human tissue kallikrein inhibitor. Purification, characterization, and reactive center sequence. J Biol Chem 267:25873–25880 [PubMed] [Google Scholar]
- 4. Chen VC, Chao L, Chao J. 2000. Roles of the P1, P2, and P3 residues in determining inhibitory specificity of kallistatin toward human tissue kallikrein. J Biol Chem 275:38457–38466 [DOI] [PubMed] [Google Scholar]
- 5. Chao J, Stallone JN, Liang YM, Chen LM, Wang DZ, Chao L. 1997. Kallistatin is a potent new vasodilator. J Clin Invest 100:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Madeddu P, Emanueli C, El-Dahr S. 2007. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nephrol 3:208–221 [DOI] [PubMed] [Google Scholar]
- 7. Miao RQ, Agata J, Chao L, Chao J. 2002. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood 100:3245–3252 [DOI] [PubMed] [Google Scholar]
- 8. Gao G, Shao C, Zhang SX, Dudley A, Fant J, Ma JX. 2003. Kallikrein-binding protein inhibits retinal neovascularization and decreases vascular leakage. Diabetologia 46:689–698 [DOI] [PubMed] [Google Scholar]
- 9. Chao J, Chai KX, Chao L. 1996. Tissue kallikrein inhibitors in mammals. Immunopharmacology 32:67–72 [DOI] [PubMed] [Google Scholar]
- 10. Chai KX, Chen VC, Ni A, Lindpaintner K, Rubattu S, Chao L, Chao J. 1997. Molecular cloning and expression of rat kallistatin gene. Biochim Biophys Acta 1353:277–286 [DOI] [PubMed] [Google Scholar]
- 11. Cody V, Davis PJ, Davis FB. 2007. Molecular modeling of the thyroid hormone interactions with αvβ3 integrin. Steroids 72:165–170 [DOI] [PubMed] [Google Scholar]
- 12. Lu L, Yang Z, Zhu B, Fang S, Yang X, Cai W, Li C, Ma JX, Gao G. 2007. Kallikrein-binding protein suppresses growth of hepatocellular carcinoma by anti-angiogenic activity. Cancer Lett 257:97–106 [DOI] [PubMed] [Google Scholar]
- 13. Liu YY, Brent GA. 2005. Thyroid hormone-dependent gene expression in differentiated embryonic stem cells and embryonal carcinoma cells: identification of novel thyroid hormone target genes by deoxyribonucleic acid microarray analysis. Endocrinology 146:776–783 [DOI] [PubMed] [Google Scholar]
- 14. Chai KX, Chao J, Chao L. 1991. Molecular cloning and sequence analysis of the mouse kallikrein-binding protein gene. Biochim Biophys Acta 1129:127–130 [DOI] [PubMed] [Google Scholar]
- 15. Chai KX, Chen LM, Chao J, Chao L. 1993. Kallistatin: a novel human serine proteinase inhibitor. Molecular cloning, tissue distribution, and expression in Escherichia coli. J Biol Chem 268:24498–24505 [PubMed] [Google Scholar]
- 16. Ma JX, Chao J, Chao L. 1996. Identification and characterization of two promoters of rat kallikrein-binding protein gene. Biochim Biophys Acta 1307:285–293 [DOI] [PubMed] [Google Scholar]
- 17. Brent GA. 1994. The molecular basis of thyroid hormone action. N Engl J Med 331:847–853 [DOI] [PubMed] [Google Scholar]
- 18. Brent GA, Moore DD, Larsen PR. 1991. Thyroid hormone regulation of gene expression. Annu Rev Physiol 53:17–35 [DOI] [PubMed] [Google Scholar]
- 19. Shi YB. 2009. Dual functions of thyroid hormone receptors in vertebrate development: the roles of histone-modifying cofactor complexes. Thyroid 19:987–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glass CK, Rose DW, Rosenfeld MG. 1997. Nuclear receptor coactivators. Curr Opin Cell Biol 9:222–232 [DOI] [PubMed] [Google Scholar]
- 21. Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403–414 [DOI] [PubMed] [Google Scholar]
- 22. Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. 1996. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell 87:953–959 [DOI] [PubMed] [Google Scholar]
- 23. Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, O'Malley BW. 1997. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature 389:194–198 [DOI] [PubMed] [Google Scholar]
- 24. Hörlein AJ, Näär AM, Heinzel T, Torchia J, Gloss B, Kurokawa R, Ryan A, Kamei Y, Söderström M, Glass CK. 1995. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature 377:397–404 [DOI] [PubMed] [Google Scholar]
- 25. Shibusawa N, Hollenberg AN, Wondisford FE. 2003. Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. J Biol Chem 278:732–738 [DOI] [PubMed] [Google Scholar]
- 26. Lazar MA. 2003. Thyroid hormone action: a binding contract. J Clin Invest 112:497–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carr FE, Kaseem LL, Wong NC. 1992. Thyroid hormone inhibits thyrotropin gene expression via a position-independent negative l-triiodothyronine-responsive element. J Biol Chem 267:18689–18694 [PubMed] [Google Scholar]
- 28. Chin WW, Carr FE, Burnside J, Darling DS. 1993. Thyroid hormone regulation of thyrotropin gene expression. Recent Prog Horm Res 48:393–414 [DOI] [PubMed] [Google Scholar]
- 29. Bodenner DL, Mroczynski MA, Weintraub BD, Radovick S, Wondisford FE. 1991. A detailed functional and structural analysis of a major thyroid hormone inhibitory element in the human thyrotropin β-subunit gene. J Biol Chem 266:21666–21673 [PubMed] [Google Scholar]
- 30. Chen JD, Evans RM. 1995. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377:454–457 [DOI] [PubMed] [Google Scholar]
- 31. Sasaki S, Lesoon-Wood LA, Dey A, Kuwata T, Weintraub BD, Humphrey G, Yang WM, Seto E, Yen PM, Howard BH, Ozato K. 1999. Ligand-induced recruitment of a histone deacetylase in the negative-feedback regulation of the thyrotropin β gene. EMBO J 18:5389–5398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. 1997. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature 387:49–55 [DOI] [PubMed] [Google Scholar]
- 33. Heinzel T, Lavinsky RM, Mullen TM, Söderstrom M, Laherty CD, Torchia J, Yang WM, Brard G, Ngo SD, Davie JR, Seto E, Eisenman RN, Rose DW, Glass CK, Rosenfeld MG. 1997. A complex containing N-CoR, mSin3 and histone deacetylase mediates transcriptional repression. Nature 387:43–48 [DOI] [PubMed] [Google Scholar]
- 34. Shibusawa N, Hashimoto K, Nikrodhanond AA, Liberman MC, Applebury ML, Liao XH, Robbins JT, Refetoff S, Cohen RN, Wondisford FE. 2003. Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J Clin Invest 112:588–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D, Xia X, Liu Y, Oetting A, Walker RL, Zhu Y, Meltzer P, Cole PA, Shi YB, Yen PM. 2009. Negative regulation of TSHα target gene by thyroid hormone involves histone acetylation and corepressor complex dissociation. Mol Endocrinol 23:600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ortiga-Carvalho TM, Shibusawa N, Nikrodhanond A, Oliveira KJ, Machado DS, Liao XH, Cohen RN, Refetoff S, Wondisford FE. 2005. Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface. J Clin Invest 115:2517–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pfahl M. 1993. Nuclear receptor/AP-1 interaction. Endocr Rev 14:651–658 [DOI] [PubMed] [Google Scholar]
- 38. Rogatsky I, Zarember KA, Yamamoto KR. 2001. Factor recruitment and TIF2/GRIP1 corepressor activity at a collagenase-3 response element that mediates regulation by phorbol esters and hormones. EMBO J 20:6071–6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tagami T, Madison LD, Nagaya T, Jameson JL. 1997. Nuclear receptor corepressors activate rather than suppress basal transcription of genes that are negatively regulated by thyroid hormone. Mol Cell Biol 17:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tagami T, Park Y, Jameson JL. 1999. Mechanisms that mediate negative regulation of the thyroid-stimulating hormone alpha gene by the thyroid hormone receptor. J Biol Chem 274:22345–22353 [DOI] [PubMed] [Google Scholar]
- 41. Park JI, Tsai SY, Tsai MJ. 2003. Molecular mechanism of chicken ovalbumin upstream promoter-transcription factor (COUP-TF) actions. Keio J Med 52:174–181 [DOI] [PubMed] [Google Scholar]
- 42. Tsai SY, Tsai MJ. 1997. Chick ovalbumin upstream promoter-transcription factors (COUP-TFs): coming of age. Endocr Rev 18:229–240 [DOI] [PubMed] [Google Scholar]
- 43. Umesono K, Murakami KK, Thompson CC, Evans RM. 1991. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell 65:1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klinge CM, Silver BF, Driscoll MD, Sathya G, Bambara RA, Hilf R. 1997. Chicken ovalbumin upstream promoter-transcription factor interacts with estrogen receptor, binds to estrogen response elements and half-sites, and inhibits estrogen-induced gene expression. J Biol Chem 272:31465–31474 [DOI] [PubMed] [Google Scholar]
- 45. Kliewer SA, Umesono K, Heyman RA, Mangelsdorf DJ, Dyck JA, Evans RM. 1992. Retinoid X receptor-COUP-TF interactions modulate retinoic acid signaling. Proc Natl Acad Sci USA 89:1448–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shibata H, Nawaz Z, Tsai SY, O'Malley BW, Tsai MJ. 1997. Gene silencing by chicken ovalbumin upstream promoter-transcription factor I (COUP-TFI) is mediated by transcriptional corepressors, nuclear receptor-corepressor (N-CoR) and silencing mediator for retinoic acid receptor and thyroid hormone receptor (SMRT). Mol Endocrinol 11:714–724 [DOI] [PubMed] [Google Scholar]
- 47. Nygård M, Becker N, Demeneix B, Pettersson K, Bondesson M. 2006. Thyroid hormone-mediated negative transcriptional regulation of necdin expression. J Mol Endocrinol 36:517–530 [DOI] [PubMed] [Google Scholar]
- 48. Brent GA, Larsen PR, Harney JW, Koenig RJ, Moore DD. 1989. Functional characterization of the rat growth hormone promoter elements required for induction by thyroid hormone with and without a co-transfected beta type thyroid hormone receptor. J Biol Chem 264:178–182 [PubMed] [Google Scholar]
- 49. Hatcher HC, Wright NM, Chao J, Chao L, Ma JX. 1999. Kallikrein-binding protein is induced by growth hormone in the dwarf rat. FASEB J 13:1839–1844 [DOI] [PubMed] [Google Scholar]
- 50. Anderson GW, Larson RJ, Oas DR, Sandhofer CR, Schwartz HL, Mariash CN, Oppenheimer JH. 1998. Chicken ovalbumin upstream promoter-transcription factor (COUP-TF) modulates expression of the Purkinje cell protein-2 gene. A potential role for COUP-TF in repressing premature thyroid hormone action in the developing brain. J Biol Chem 273:16391–16399 [DOI] [PubMed] [Google Scholar]
- 51. Liu YY, Brent GA. 2002. A complex deoxyribonucleic acid response element in the rat Ca(2+)/calmodulin-dependent protein kinase IV gene 5′-flanking region mediates thyroid hormone induction and chicken ovalbumin upstream promoter transcription factor 1 repression. Mol Endocrinol 16:2439–2451 [DOI] [PubMed] [Google Scholar]
- 52. Qiu Y, Krishnan V, Pereira FA, Tsai SY, Tsai MJ. 1996. Chicken ovalbumin upstream promoter-transcription factors and their regulation. J Steroid Biochem Mol Biol 56:81–85 [DOI] [PubMed] [Google Scholar]
- 53. Tran P, Zhang XK, Salbert G, Hermann T, Lehmann JM, Pfahl M. 1992. COUP orphan receptors are negative regulators of retinoic acid response pathways. Mol Cell Biol 12:4666–4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Klinge CM. 1999. Role of estrogen receptor ligand and estrogen response element sequence on interaction with chicken ovalbumin upstream promoter transcription factor (COUP-TF). J Steroid Biochem Mol Biol 71:1–19 [DOI] [PubMed] [Google Scholar]
- 55. Brent GA, Williams GR, Harney JW, Forman BM, Samuels HH, Moore DD, Larsen PR. 1991. Effects of varying the position of thyroid hormone response elements within the rat growth hormone promoter: implications for positive and negative regulation by 3,5,3′-triiodothyronine. Mol Endocrinol 5:542–548 [DOI] [PubMed] [Google Scholar]
- 56. Zhang XK, Wills KN, Husmann M, Hermann T, Pfahl M. 1991. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol 11:6016–6025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang-Yen HF, Zhang XK, Graupner G, Tzukerman M, Sakamoto B, Karin M, Pfahl M. 1991. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol 3:1206–1219 [PubMed] [Google Scholar]
- 58. Liu Y, Xia X, Fondell JD, Yen PM. 2006. Thyroid hormone-regulated target genes have distinct patterns of coactivator recruitment and histone acetylation. Mol Endocrinol 20:483–490 [DOI] [PubMed] [Google Scholar]
- 59. Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. 2000. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103:843–852 [DOI] [PubMed] [Google Scholar]
- 60. Kim SW, Ho SC, Hong SJ, Kim KM, So EC, Christoffolete M, Harney JW. 2005. A novel mechanism of thyroid hormone-dependent negative regulation by thyroid hormone receptor, nuclear receptor corepressor (NCoR), and GAGA-binding factor on the rat cD44 promoter. J Biol Chem 280:14545–14555 [DOI] [PubMed] [Google Scholar]
- 61. Cowger JJ, Torchia J. 2006. Direct association between the CREB-binding protein (CBP) and nuclear receptor corepressor (N-CoR). Biochemistry 45:13150–13162 [DOI] [PubMed] [Google Scholar]
- 62. Francoise A, Sourisseau T, Metivier R, Page YL, Desbois C, Michel D, Salbert G. 2000. COUP-TFI (Chicken Ovalbumin Upstream Promoter-Transcription Factor I) regulates cell migration and axogenesis in differentiating P19 embryonal carcinoma cells. Mol Endocrinol 14:1918–1933 [DOI] [PubMed] [Google Scholar]
- 63. Yamaguchi H, Zhou C, Lin SC, Durand B, Tsai SY, Ming-Jer Tsai M-J. 2004. The nuclear orphan receptor COUP-TFI is important for differentiation of oligodendrocytes. Dev Biol 266:238–251 [DOI] [PubMed] [Google Scholar]