Mouse model of premature ovarian failure generated by oocyte-specific ablation of complex N- and O-glycans reveals new roles for the oocyte in ovarian function.
Abstract
Premature ovarian failure (POF) affects up to 1.4% of women under the age of 40 yr and less than 30% of cases have a known cause. Here we describe a new mouse model of POF resulting from oocyte-specific ablation of core 1-derived (mucin) O-glycans and complex and hybrid N-glycans. Females carrying floxed alleles of both the C1galt1 (T-syn) and Mgat1 glycosyltransferase genes and a ZP3Cre transgene, generate oocytes lacking complex O- and N-glycans following oocyte-specific deletion at the primary follicle stage. We previously showed that few double-mutant females are fertile, and those produce only a single small litter. Here we show that ovarian function declined rapidly in double-mutant females with less than 1% ovulating at 11 wk of age after superovulation with exogenous gonadotropins. Ovary weight was significantly decreased in double-mutant females by 3 months of age, consistent with a decrease in the number of developing follicles. FSH levels in double-mutant females were elevated at 3 months of age, and testosterone and inhibin A were decreased, showing that the loss of complex N- and O-glycans from oocyte glycoproteins affected hypothalamic-pituitary-gonadal feedback loops. The absence of developing follicles, ovary dysfunction, reduced testosterone and inhibin A, and elevated FSH in double-mutant females lacking C1galt1 and Mgat1 in oocytes represents a new mouse model for the study of follicular POF.
Premature ovarian failure (POF) affects 1% of women younger than 40 yr of age (1), increasing to 1.4% in African-American and Hispanic women (2). There are no reliable markers for the onset of POF, and it is currently irreversible, leading to considerable heartache for couples with elevated anxiety, depression, and sleep disturbance in women (3–5). POF is characterized by ovary dysfunction, a decrease in the number of growing ovarian follicles, increased concentrations of gonadotropins, and reduced concentrations of ovarian steroids in serum (6). POF can be classified as either afollicular or follicular (6). Ovaries of women with afollicular POF are devoid of follicles and thus lack the potential for future follicle development and ovulation. Afollicular POF results primarily from either a reduction in the number of primordial follicles generated, leading to premature exhaustion of the primordial pool, or from an increase in the number of primordial follicles that resume development, thereby depleting the pool prematurely (6). By contrast, follicular POF ovaries contain follicles and retain the potential to return to a functional state (reviewed in Ref. 7). Indeed, women with POF can occasionally become pregnant (6, 8). Follicular POF, which includes resistant ovary syndrome, represents about 45% of POF cases (9, 10). Despite the relatively high prevalence of POF, the number of POF cases with a known cause is less than 30%, and thus, further investigation into ovarian function and the onset of POF is of considerable importance (6, 11). We describe here a mouse model with oocyte-specific deletion of two glycosyltransferase genes, C1galt1 and Mgat1, that develops follicular POF by 3 months of age.
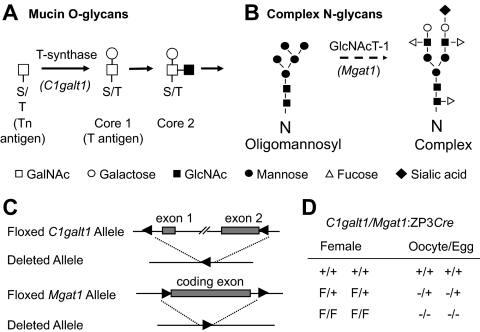
T-synthase encoded by the C1galt1 gene (also known as T-syn) is required for the generation of core 1-derived O-glycans (12, 13), whereas Mgat1 (also known as N-acetylglucosaminyltransferase I; GlcNAc-TI) encoded by the Mgat1 gene is required for the generation of complex and hybrid N-glycans (14) (Fig. 1). Glycosylation is the most abundant posttranslational protein modification, and has various effects on protein structure and function (15). Embryos lacking complex and hybrid N-glycans die at approximately embryonic d 9.5 (16, 17) and embryos lacking core 1-derived O-glycans die at approximately embryonic d 12.5 (18, 19). Therefore, to study roles for these glycans in oogenesis, female mice were created with oocyte-targeted deletion of both Mgat1 and C1galt1 (T-syn) (19), herein termed double-mutant females. We previously showed that female mice generating oocytes in which all glycoproteins lack complex and hybrid N-glycans ovulate fewer eggs due to aberrant preovulatory follicle development (20). By contrast, female mice generating oocytes lacking core 1-derived O-glycans have increased fertility due to an increase in follicle numbers, resulting in the ovulation of more eggs (21). However, female mice generating oocytes lacking both core 1-derived O-glycans and complex N-glycans have decreased fertility (19).
Fig. 1.
Pathways for the generation of complex O-glycans (A) and N-glycans (B) are shown. C, Diagram of the floxed and deleted C1galt1 and Mgat1 alleles. D, Genotypes of female mice and the oocytes they generate after the action of the zona pellucida protein 3 (ZP3) Cre transgene. F, Floxed; +, wild type; −, deleted gene.
In the ovary, each oocyte is surrounded by granulosa cells and later theca cells, which together constitute a follicle. Although the granulosa and theca cells are essential for oocyte development, the oocyte in turn plays an active role in facilitating the development of the follicle (22). Therefore, the follicle is the functional unit within the ovary. A variety of mouse models that exhibit POF has been described (reviewed in Refs. 23 and 24). Although those with premature depletion of primordial follicles may be used to shed light on the resumption of primordial follicle growth, they have not been appropriate for studies into the onset of follicular POF. We demonstrate here that the C1galt1/Mgat1 oocyte-conditional double mutant is a new model for follicular POF that exhibits all the recognized markers for this condition. A proportion of double-mutant females produce a single, small litter (19). The lack of subsequent litters in this mouse model is particularly intriguing because follicle development was successful in 3- to 6-wk-old double-mutant females but not in those at 12 wk, even though oocyte-specific deletion occurred at the same time in the development of each oocyte. We show here that double-mutant females (C1galt1F/FMgat1F/F:ZP3Cre) undergo POF despite the presence of primordial and primary follicles.
Materials and Methods
Mice
Females of a mixed 129/B6 genetic background carrying floxed Mgat1 and floxed C1galt1 alleles and a ZP3Cre transgene that generates oocytes deficient in complex and hybrid N-glycans and core 1-derived O-glycans have been described previously (19). The ZP3Cre transgene is expressed at the primary stage of development (25, 26), after primordial follicle activation. Floxed C1galt1 and Mgat1 alleles function as wild-type genes and therefore both C1galt1+/+Mgat1+/+:ZP3Cre and C1galt1F/FMgat1F/F were used as controls. The ZP3Cre transgene does not affect fertility (19–21). Mouse experiments were approved by the Animal Institute Committee of the Albert Einstein College of Medicine (New York, NY).
Superovulation
Mutant and control females aged 3–11 wk were treated with 5 IU pregnant mare serum gonadotropin (Calbiochem, EMD Chemicals Inc., San Diego, CA) followed 46–48 h later by 5 IU human chorionic gonadotropin (Sigma-Aldrich, St. Louis, MO). Fourteen to 16 h later, females were killed and oviducts dissected into warmed M2 medium (Specialty Media, Phillipsburg, NJ). Hyaluronidase (Sigma-Aldrich) was added to the M2 medium at a concentration of 0.3 mg/ml, and oviducts were ripped to release the cumulus mass. The cumulus mass was incubated in the M2 containing hyaluronidase for about 5 min at room temperature until eggs were freed for counting.
Ovarian histology
Ovaries were collected and weighed from females aged 3 wk, 6 wk, or 3 months. Ovaries were fixed in 10% buffered formalin (Sigma-Aldrich) or Bouins fixative (Sigma-Aldrich) and paraffin embedded, after which 5 μm sections were cut and stained with hematoxylin and eosin.
Determination of follicle numbers
Follicle numbers at each stage of follicle development were determined in 3-month females (control, n = 3; mutant, n = 3). Body and uterine weights were noted and ovaries were collected and prepared as described above. One ovary was serially sectioned and every 10th serial section of 5 μm was used to count follicles in which the nucleus was visible. Morphologically healthy follicles were staged according to the classification by Pedersen and Peters (27) as follows: primordial, less than 1 complete layer of cuboidal granulosa cells; stage 3a, one complete layer of 20 or fewer cuboidal granulosa cells per section; stage 3b, one complete layer of 21–60 cuboidal granulosa cells per section; stage 4, two complete layers of 61–100 cuboidal granulosa cells per section; stage 5, multiple layers of 100–400 granulosa cells per section; antral, large follicles with an antral space. All counts were performed blinded.
3β-Hydroxysteroid dehydrogenase (3β-HSD) immunohistochemistry
Formalin-fixed paraffin sections were dewaxed and rehydrated. Endogenous peroxidase was quenched by incubation in 3% hydrogen peroxide in PBS for 5 min at room temperature. Sections were washed in double distilled H2O and Tris-buffered saline (TBS) with Tween 20 (TBST) before blocking with normal goat serum (Vectastain ABC Elite kit; Vector Laboratories, Peterborough, UK) in TBS for 30 min or longer at room temperature. The polyclonal rabbit 3β-HSD antibody against recombinant type 2 human 3β-HSD, which recognizes mouse type 1 3β-HSD [generously provided by Professor Ian Mason (28)] was used at a 1:2000 dilution in normal goat serum/TBS and incubated at 4 C overnight. The following day, slides were washed in TBST three times for 3 min each. Sections were incubated with biotinylated goat antirabbit antibody (Vectastain ABC Elite kit; Vector Laboratories) in TBS for 30 min at room temperature. Slides were washed again in TBST three times for 3 min each before incubation with Vectastain ABC Elite to enhance avidin/biotin signal for 30 min at room temperature. Slides were washed in PBS and 0.05% Tween 20 three times for 5 min each before sections were exposed to diaminobenzidine stain (peroxidase substrate kit; Vector Laboratories) for the same amount of time. Slides were rinsed with double-distilled H2O before counterstaining with hematoxylin, dehydrating, and mounting. Images were taken with the same exposure and lighting.
Gonadotropin and steroid analysis
Blood was collected by cardiac puncture from avertin-anesthetized, 3-month females. Blood was left at room temperature for 1 h to clot before being centrifuged and serum collected. Serum concentrations of FSH, LH, inhibin A, inhibin B, estradiol, progesterone, and testosterone were determined by the University of Virginia Center for Research in Reproduction, Ligand Assay, and Analysis Core Laboratory (Charlottesville, VA). Assay information is available (http://www.healthsystem.virginia.edu/internet/crr/ligand.cfm). The mouse FSH RIA has a readable range of 3.60–25.44 ng/ml and an intraassay coefficient of variation (CV) of 13.2 and 2.0% for low and high control samples, respectively. The mouse LH sandwich immunoradiometric assay has a readable range of 0.04–37.4 ng/ml and an intraassay CV of 0.6 and 3.9% for low and high control samples, respectively. The progesterone RIA has a readable range of 0.14–11.5 ng/ml and an intraassay CV of 2.6, 7.0, and 0.4% for low, medium, and high controls, respectively. The estradiol RIA has a readable range of 11.3–679.7 pg/ml and an intraassay CV of 10.2 and 16.4% for low and high control samples, respectively. The testosterone RIA has a readable range of 15.2–1002.8 ng/dl and an intraassay CV of 3.3, 4.5, and 0.5% for low, medium, and high controls, respectively. The inhibin A ELISA has a readable range of 10–1000 pg/ml and an intraassay CV of 4.1%. The inhibin B ELISA has a readable range of 20–1000 pg/ml and an intraassay CV of 7.7%. Samples reading outside the readable range were given the value of the lowest readable value or the highest readable value. When necessary, serum samples were diluted in PBS to fall within the readable range.
Statistical analysis
All values for n ≥ 3 are mean ± sd. Significant heterogeneity of variances was detected for the endocrine data, and therefore, these values were log transformed before two-tailed t test analysis using the Microsoft Excel data analysis program (Richmond, CA). A P < 0.05 was considered to be statistically significant. The two-tailed t test was performed by the Microsoft Excel data analysis program. Statistical analysis was not performed for samples of n < 3 for which average and range of values are presented.
Results
Premature loss of fertility in C1galt1/Mgat1 double-mutant females
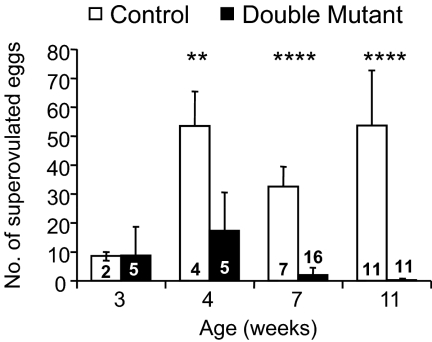
The ZP3Cre transgene is expressed at the primary stage of follicle development (25, 26), after primordial follicles resume development. Targeted deletion of both the C1galt1 and Mgat1 genes results in reduced fertility of C1galt1/Mgat1 double mutants, with only a few females producing a single small litter (19). The number of eggs obtained after superovulation of 4-wk females was reduced and 6-month double-mutant females failed to ovulate any eggs (19). To determine at what age double-mutant ovaries become refractory to exogenous gonadotropins, females from 3 to 11 wk were superovulated (Fig. 2). Double-mutant females responded to superovulation similarly to controls at 3 wk of age, but the ability of mutant females to respond declined to approximately 50% of controls 1 wk later (Fig. 2). At 7 wk, the number of eggs ovulated by double-mutant females in response to the superovulatory stimulus was less than 10% of controls, and by 11 wk the ovulation rate fell to less than 1% (Fig. 2). Therefore, the ovulation of eggs in C1galt1/Mgat1 mutant females was restricted to the period before 3 months of age, which is consistent with the inability of double mutants to produce a second litter (19).
Fig. 2.
Number of eggs superovulated by C1galt1/Mgat1 double-mutant females. The number in each bar is the number of mice analyzed. The average number of eggs collected at 3 wk is given with range for controls. Statistical analysis was not performed for the number of eggs collected at 3 wk due to small sample size for controls. **, P < 0.01; ****, P < 0.001.
Ovary histology deteriorates as C1galt1/Mgat1 mutant females age
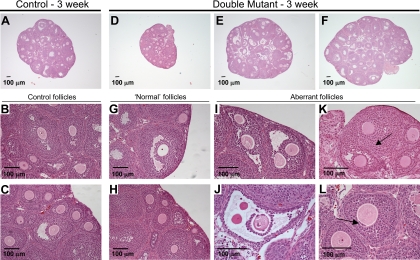
To determine whether follicle development and/or ovulation was involved in the demise of fertility, ovary sections were analyzed in prepubertal 3-wk females (when double mutants respond to exogenous gonadotropins similarly to controls), at 6 wk when double mutants have decreased fertility, and at 3 months when double mutants are infertile. Ovaries from 3-wk double mutants were grossly normal, containing many follicles at different stages of development, although ovary size was more variable in double mutants than controls [controls, 22.2 ± 3.3 mg (n = 3); mutants, 26.5 ± 20.0 mg (n = 3); Fig. 3A–H]. Closer examination revealed some abnormalities (Fig. 3, I–L). Ovaries carrying double-mutant oocytes contained multiple-oocyte follicles (MOFs; Fig. 3, I and J), which was not surprising, considering that these are found in C1galt1 mutant ovaries (21). In addition, not all oocytes within MOFs of double-mutant ovaries had normal morphology, with some oocytes lacking a zona pellucida and associated granulosa cells (Fig. 3J). Joined follicles were also present in ovaries with double-mutant oocytes, as indicated by the fold of basal lamina and theca cells that partially separates two connected follicles (Fig. 3K). In addition, granulosa cells were visible beneath the oocyte zona pellucida in double-mutant follicles (Fig. 3L), representing either abnormal follicle development or granulosa cell invasion that may be involved in oocyte elimination (29).
Fig. 3.
Ovary histology in 3-wk C1galt1/Mgat1 double-mutant females. Representative ovary sections from 3-wk control (A–C) and double-mutant (D–L) females contain follicles at all stages of development. Numerous follicles exist in control (A) and ovaries with double-mutant oocytes (D–F), but the size of the double-mutant ovary varied more than controls. The sections shown had the largest diameter observed in each ovary. Normal follicles in control (B and C) and ovaries with double-mutant oocytes (G and H) are shown. Immature ovaries with double-mutant oocytes contained many aberrant follicles including MOFs (I), large antral follicles with a thin granulosa layer and zona-free oocytes (J), mutant follicles fusing (K) as indicated by the visible basal lamina and theca cells between the oocytes (arrow), and follicles with granulosa cells beneath the zona pellucida (arrow) (L).
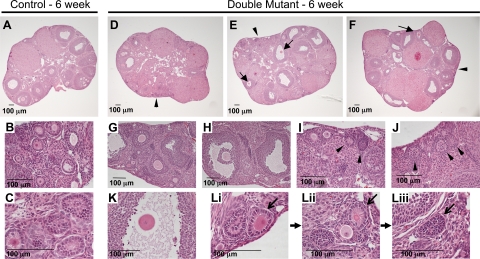
By 6 wk, ovaries from double mutants had more follicular abnormalities and appeared to contain fewer follicles (Fig. 4). Although developing follicles were evident in double-mutant ovaries, few of these follicles were histologically normal compared with controls (Fig. 4, D–F and H–J). Numerous oocytes lacking cumulus cells were visible in follicles with double-mutant oocytes (Fig. 4, E and F). Large antral follicles in double mutants had an irregular follicle boundary and contained granulosa cells with an asymmetric distribution (Fig. 4H), indicative of aberrant signaling between the oocyte lacking complex N- and O-glycans and follicular cells (30). Oocytes that lacked associated granulosa cells but had a zona pellucida were also observed (Fig. 4K). Toward the perimeter of the double-mutant ovary, small circular structures containing densely packed cells with little cytoplasm were observed and were termed empty follicles (Fig. 4, E, I, and J). It is possible that these structures develop after apoptosis of the double-mutant oocyte at an early stage, leading to empty follicles (Fig. 4, Li-Liii). Some corpora lutea contained red blood cells (Fig. 4F); however, these were found in both control and double-mutant ovaries.
Fig. 4.
Ovaries from 6-wk C1galt1/Mgat1 double mutants contain aberrant follicles and structures. A–C, Ovarian sections from 6-wk control females contained follicles at different stages of development and corpora lutea. D–F, Ovaries with double-mutant oocytes contained growing follicles with abnormalities visible at low magnification including oocytes lacking zona (E and F; solid arrows). Growing follicles were evident in ovaries with double-mutant oocytes (G and H), although granulosa cells of large antral follicles were distributed asymmetrically (H). Ovaries with double-mutant oocytes also contained empty follicles, which were located predominantly at the periphery of the ovary (arrowheads, D–F, I, and J). K, Oocyte lacking cumulus cells. Proposal for empty follicle development from a small primary follicle (arrow; Li) to a follicle with a diminishing or declining oocyte (arrow; Lii) to an empty follicle (arrow; Liii) are shown.
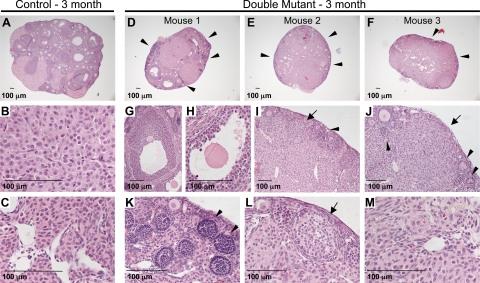
By 3 months of age, ovaries with double-mutant oocytes contained considerably fewer growing follicles compared with controls (Fig. 5, A and D–F). Two of the three ovaries examined from double-mutant females contained only small follicles, whereas the third ovary contained a few larger follicles (Fig. 5D and G). However, these larger double-mutant follicles were nevertheless abnormal with irregular borders (Fig. 5G) and zona-free double-mutant oocytes (Fig. 5H), as also observed in younger ovaries with double-mutant oocytes (Figs. 3 and 4). The majority of ovaries with double-mutant oocytes lacked developing follicles and normal corpora lutea at 3 months. However, primary follicles, numerous empty follicles, and many larger circular structures were present at the periphery of ovaries with double-mutant oocytes (Fig. 5, I–L). In addition, these ovaries contained considerable amounts of interstitial tissue (Fig. 5, E and F). Higher magnification revealed that stromal cells of 3-month double-mutant ovaries were luteinized (Fig. 5M) and looked more like the cells of control corpus lutea (Fig. 5B) than control stroma (Fig. 5C).
Fig. 5.
Ovaries from 3-month, double-mutant females contain few developing follicles. A, Control ovaries from 3-month-old females contained all stages of developing follicles and corpora lutea. High magnification of control corpora lutea (B) and stroma (C). D–F, Ovaries with double-mutant oocytes contained few preantral and antral follicles, and the majority of the tissue was interstitial tissue with empty follicles present at the periphery (arrowheads). The few follicles present in one double-mutant ovary (D) were abnormal with irregular follicle boundaries (G) and zona-free oocytes (H). This ovary also contained corpora lutea. I and J, The majority of the double-mutant ovary tissue was indistinct, although empty follicles (arrowheads) and larger circular structures (arrows) were visible. K, Higher magnification of empty follicles (arrowheads). L, Higher magnification of larger circular structures (arrow). M, Higher magnification of interstitial tissue in ovaries with double-mutant oocytes reveals luteinization.
Ovaries with double-mutant oocytes have decreased follicle development at 3 months
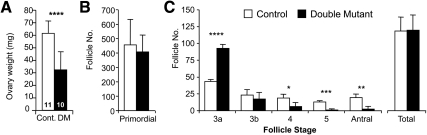
At 3 months of age, the weight of ovaries with double-mutant oocytes was less than controls (Fig. 6A), consistent with the observed lack of developing follicles (Fig. 5). To ascertain at which stage follicle development is modified in C1galt1/Mgat1 conditional oocyte mutants, all follicles with a visible oocyte nucleus were counted in every 10th section of 3-month ovaries. The number of primordial follicles in ovaries with double-mutant oocytes was equivalent to controls, as expected, because deletion by ZP3Cre does not occur until the primary stage of follicle development (Fig. 6B). However, there were significantly fewer secondary (stage 4), preantral (stage 5), and antral (stages 6–8) follicles in ovaries with double-mutant oocytes (Fig. 6C). On the other hand, the total number of follicles was the same in ovaries with double-mutant oocytes compared with control ovaries, apparently due to the dramatically increased numbers of primary follicles at the 3a stage. Body weight was unaltered in females with double-mutant oocytes (controls: 27.7 ± 2.9 g, n = 11; mutants: 26.8 ± 3.9 g, n = 10), indicating that the decrease in ovary weights and developing follicle numbers were not due to a change in body condition. Interestingly, uterine weight was not decreased in females with double-mutant oocytes (controls: 943 ± 450 mg, n = 11; mutants 940 ± 391 mg, n = 10) indicating that, despite ovarian dysfunction, estrogen production was not affected.
Fig. 6.
C1galt1/Mgat1 double-mutant, 3-month ovary weights and follicle counts. A, The average weight of ovaries with double-mutant oocytes was decreased at 3 months (the number in the bar is the n for each analysis). Cont, Control. ****, P < 0.00005. B and C, Ovaries with double-mutant oocytes have a decreased number of developing follicles at 3 months (control, n = 3; mutant, n = 3; one ovary per mouse). B, Primordial follicle numbers in 3-month ovaries. C, Number of follicles at each developmental stage and total follicle numbers. *, P = 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0005.
Females with double-mutant oocytes have a modified endocrine profile
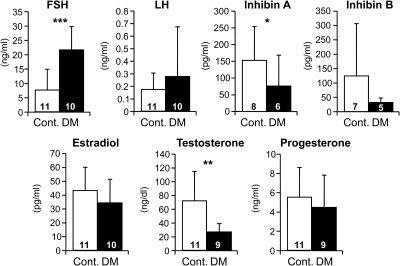
To determine what effect the decrease in follicle numbers in females with double-mutant oocytes (Fig. 6) had on circulating concentrations of gonadotropins, inhibins, and steroids, sera were analyzed from 3-month females. Serum concentrations of FSH in double-mutant females (Fig. 7) were significantly elevated compared with controls. This was consistent with a decrease in inhibin A levels and thus a reduction in negative feedback. The variation in inhibin B concentrations may reflect interanimal variation in the number and stage of ovarian follicles with control oocytes (Figs. 5 and 6). Testosterone was decreased in sera of double-mutant females, although estradiol levels were unaltered. Testosterone is a precursor for estradiol, although it does not undergo aromatization in granulosa cells as readily as androstenedione (31). However, the lack of a decrease in circulating estradiol concentrations was unexpected, considering the absence of growing follicles in females with double-mutant oocytes (Fig. 6). In addition, progesterone was unaltered in 3-month double-mutant females, which was also surprising because their ovaries contained less corpora lutea (Fig. 5). However the lack of ovulation does not preclude premature luteinization of follicles from which progesterone could be produced. The concentration of LH was not significantly altered in sera from females with double-mutant oocytes, consistent with their normal levels of progesterone, the predominant inhibitor of LH.
Fig. 7.
Gonadotropin and ovarian steroid levels in serum of 3-month controls and C1galt1/Mgat1 double mutants. The number in the bar is the n for each analysis. Cont, Control. *, P < 0.05; **, P < 0.005; ***, P = 0.001.
Abnormal 3-month, double-mutant ovarian tissue secretes progesterone
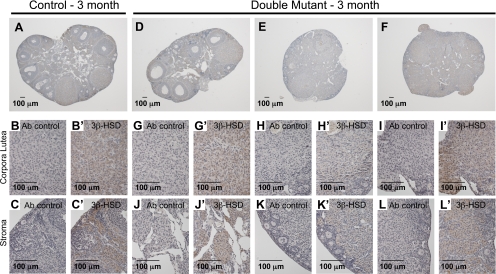
Three-month, double-mutant ovaries contain abnormal luteinized-like cells that express 3β-HSD, the enzyme required to convert pregnenolone to progesterone, indicating that these cells secrete progesterone (Fig. 8). 3β-HSD was detected in control and mutant corpora lutea (Fig. 8, B and G-I) but was not detected in follicles (Fig. 8, A and D). However, 3β-HSD was also detected in luteinized-like cells of the mutant ovary, which comprised the majority of the tissue of two of the ovaries (Fig. 8, E and F). 3β-HSD was also detected in nonluteinized stromal cells of the control ovary as previously described (32).
Fig. 8.
Double-mutant ovary luteinized-like cells express 3β-HSD. A, Control ovaries with 3β-HSD detected in corpora lutea and stroma but not follicles. B and B′, 3β-HSD detected in control corpora lutea. C and C′, 3β-HSD detected in control ovarian stroma. D–F, Mutant ovaries with detection of 3β-HSD in corpora lutea and abnormal ovary tissue. G–I′, Detection of 3β-HSD expression in mutant corpora lutea and corpora lutea-like tissue. J–L′, Detection of 3β-HSD expression in mutant stroma with a luteinized appearance. Ab control, Sections incubated without primary antibody.
Discussion
We show here that female mice generating mutant oocytes specifically lacking complex N- and O-glycans represent a new model of POF as characterized by ovary dysfunction and depletion of growing ovarian follicles as well as elevated FSH and decreased testosterone and inhibin A in sera (33). Females with double-mutant oocytes are infertile despite the presence of primordial and primary follicles, thus defining these mice as a model of follicular POF. Follicular POF represents approximately 45% of POF cases (9, 10) and models for this disease are scarce (reviewed in Refs. 23 and 24).
Follicle development in ovaries with oocytes lacking complex N- and O-glycans becomes increasingly aberrant with age. However, ovaries at all ages of double-mutant females contained some follicles. Interestingly, the suspension of follicle development is not universal in all follicles in all C1galt1/Mgat1 double-mutant ovaries. This could be because gene deletion did not occur in both glycosyltransferase genes in all oocytes at the same time, allowing some follicles to progress and mature further. Alternatively, this might be due to extrafollicular regulation of follicle development, such as changes in the density of the surrounding tissue (34). Nevertheless, oocyte-specific glycoproteins must play a key role in initiating the C1galt1/Mgat1 double-mutant phenotype, and future work is aimed at identifying these glycoproteins. However, at the present time, the number of females needed for collection of enough protein for analysis of oocytes is prohibitive (35). Therefore, the focus is on unraveling the basis of the altered physiology that gives rise to the double-mutant phenotype.
At 3 months of age, double-mutant ovaries contained decreased numbers of follicles at later stages of development. However, they had an increased number of follicles at the primary 3a stage. This could result from an increase in the number of primordial follicles resuming development, although the numbers of primordial follicles were not depleted at 3 months, and therefore, this explanation is unlikely. Furthermore, deletion of C1galt1 and Mgat1 occurs with ZP3Cre expression at the primary stage of development (25, 26), after primordial follicle activation. A second possibility for the increase in primary 3a stage follicles is that further follicle development is delayed or halted at this point. Another possibility is a decrease in atresia of follicles at the 3a stage. However, the levels of atresia are normally very low in follicles at this early stage of development. Therefore, the decrease in the number of late-stage follicles may be due to a decrease in the number of stage 3a follicles that go on to develop further. Alternatively this decrease in developing follicles may be due to increased atresia and is a possibility currently under investigation.
Numerous follicle abnormalities were observed in double-mutant ovaries of all ages. Zona-free oocytes most likely resulted from breakage of the thin fragile zona, which has been seen in oocytes lacking Mgat1 and C1galt1/Mgat1 double mutants (19, 36). MOFs were also observed in ovaries with double-mutant oocytes and are most likely generated by modified oocyte glycoproteins lacking core 1-derived O-glycans because these are a common feature of C1galt1 mutant ovaries (21). Empty follicles were also observed in ovaries of double-mutant females at each age examined. We hypothesize that empty follicles are the result of oocyte degeneration at an early stage of follicle development, when it is proposed that follicular cells have not yet acquired the capability for apoptosis (37). Structures similar to empty follicles, also termed nodules, have been observed in GDF-9−/− female mice (22) and also in streak ovaries from BMP-15−/− sheep (38–40). We propose that the granulosa cells of empty follicles continue to proliferate but lack oocyte-generated signals required for further differentiation and eventually undergo luteinization due to the lack of an oocyte-generated antiluteolytic signal (41–44), ultimately developing into the large circular luteinized structures that are visible at 3 months. The detection of 3β-HSD in the cells that comprise these structures, an enzyme that is required for progesterone synthesis and is thus also a marker of luteinized cells, supports this hypothesis.
C1galt1/Mgat1 conditional oocyte mutants at 3 months of age exhibited elevated serum FSH levels and decreased levels of serum inhibin A and testosterone, both characteristic of POF. There were also trends toward a decline in serum inhibin B concentrations and an elevation in serum LH concentrations in the double mutants (Fig. 7). The fact that these data did not reach significance may be due to sample size and/or variation due to collection of samples from controls and mutants on the same day and thus not at a specific stage of the estrous cycle, which would have reduced much of the variation in the controls. In addition, as observed at 3 months, some ovaries with double-mutant oocytes contain a few developing follicles, which may reflect variation in the endocrine status of double mutants. The relatively normal progesterone concentrations in sera were unexpected, considering females with double-mutant oocytes were no longer ovulating. However, mutant ovaries occasionally contained corpora lutea or, more commonly, considerable amounts of luteinized tissue, which expressed 3β-HSD, and therefore, this is likely to be the progesterone source in ovaries of double-mutant females.
The double-mutant POF phenotype described here is distinct from the reproductive phenotype of females lacking only complex N-glycans, which exhibit moderately decreased fertility (20, 36, 45), or lacking only core 1-derived O-glycans, which exhibit increased fertility (21). Therefore, the POF phenotype must arise from either modified functions of one or more glycoprotein(s) lacking both complex N- and O-glycans or glycoproteins lacking one or the other types of glycan. Ubiquitous loss of function of galactose-1-phosphate uridyl transferase (GALT) in women also leads to POF; however, this is due to premature ovarian insufficiency (reviewed in Ref. 46) and therefore arises via a different mechanism to POF observed due to loss of complex N- and O-glycans. In the double mutant, because the deletion of both C1galt1 and Mgat1 is oocyte specific, the molecule(s) involved in initiating POF must be of oocyte origin. Furthermore, because POF in the double mutant is follicular, mouse models of afollicular POF due to premature loss of primordial follicles, including oocyte-deletion of Pten (47) or 3-phosphoinositide-dependent protein kinase-1 (48), or deposition of too few primordial follicles, for example by suppression of Notch signaling (49), are of limited relevance. Nevertheless, although modified oocyte glycoprotein(s) must initiate the phenotype, due to the complex signaling that occurs between the oocyte and granulosa cells during normal oocyte and follicle development, mouse models of POF that are initiated by follicular defects are also pertinent (reviewed in Refs. 23 and 24). For example, deletion of the Smad signaling pathway in granulosa cells results in follicular POF (50), and therefore, modified oocyte-derived glycoproteins of the TGF-β superfamily could be involved in the double-mutant phenotype.
In conclusion, female mice with oocytes generating glycoproteins lacking core 1-derived O-glycans and complex N-glycans represent a new model of follicular POF. This model is particularly interesting, considering that, despite gene deletion occurring at the same time of follicle development (the primary stage) and irrespective of age, C1galt1/Mgat1 double mutants exhibit a progressive decline in ovarian function. A key question is why removal of oocyte-derived complex N- and O-glycans affects follicle development to a much greater extent in older compared with younger females? The defined time of POF onset in the C1galt1/Mgat1 double mutant provides a unique opportunity to investigate the etiology of follicular POF to enhance our understanding of factors that might affect the onset of this disease.
Acknowledgments
We thank Rani Sellers, Radma Mahmood, and Anthony Michael for technical advice; Wen Dong, Jason Aglipay, and Maya James for technical assistance; and Patricia Grasa and Nicole Maree Krzys for critical reading of the manuscript.
Current address for S.A.W.: Department of Physiology, Anatomy, and Genetics, Oxford University, Oxford OX1 3QX, United Kingdom.
This work was supported by National Institutes of Health Grant RO1 30645 from the National Cancer Institute (to P.S.), and partial support was provided by the Albert Einstein Cancer Center Grant PO1 13330 and a Medical Research Council New Investigator Research Grant (to S.A.W.). The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core was supported by National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction Research) Grant U54-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CV
- Coefficient of variation
- 3β-HSD
- 3β-hydroxysteroid dehydrogenase
- MOF
- multiple-oocyte follicle
- POF
- premature ovarian failure
- TBS
- Tris-buffered saline
- TBST
- TBS with Tween 20.
References
- 1. Coulam CB, Adamson SC, Annegers JF. 1986. Incidence of premature ovarian failure. Obstet Gynecol 67:604–606 [PubMed] [Google Scholar]
- 2. Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. 2003. Premature menopause in a multi-ethnic population study of the menopause transition. Hum Reprod 18:199–206 [DOI] [PubMed] [Google Scholar]
- 3. van der Stege JG, Groen H, van Zadelhoff SJ, Lambalk CB, Braat DD, van Kasteren YM, van Santbrink EJ, Apperloo MJ, Weijmar Schultz WC, Hoek A. 2008. Decreased androgen concentrations and diminished general and sexual well-being in women with premature ovarian failure. Menopause 15:23–31 [DOI] [PubMed] [Google Scholar]
- 4. de Taraciuk MB, Nolting M, Fernandez G, Colela D, Onetto C, Straminsky V. 2008. Psychological assessment of patients with premature ovarian failure. Gynecol Endocrinol 24:44–53 [DOI] [PubMed] [Google Scholar]
- 5. Laissue P, Lakhal B, Benayoun BA, Dipietromaria A, Braham R, Elghezal H, Philibert P, Saâd A, Sultan C, Fellous M, Veitia RA. 2009. Functional evidence implicating FOXL2 in non-syndromic premature ovarian failure and in the regulation of the transcription factor OSR2. J Med Genet 46:455–457 [DOI] [PubMed] [Google Scholar]
- 6. Sinha P, Kuruba N. 2007. Premature ovarian failure. J Obstet Gynaecol 27:16–19 [DOI] [PubMed] [Google Scholar]
- 7. Hoek A, Schoemaker J, Drexhage HA. 1997. Premature ovarian failure and ovarian autoimmunity. Endocr Rev 18:107–134 [DOI] [PubMed] [Google Scholar]
- 8. Beck-Peccoz P, Persani L. 2006. Premature ovarian failure. Orphanet J Rare Dis 1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta AE, Matwijiw I, Lyons EA, Faiman C. 1992. Noninvasive diagnosis of resistant ovary syndrome by ultrasonography. Fertil Steril 57:56–61 [PubMed] [Google Scholar]
- 10. Massin N, Gougeon A, Meduri G, Thibaud E, Laborde K, Matuchansky C, Constancis E, Vacher-Lavenu MC, Paniel B, Zorn JR, Misrahi M, Kuttenn F, Touraine P. 2004. Significance of ovarian histology in the management of patients presenting a premature ovarian failure. Hum Reprod 19:2555–2560 [DOI] [PubMed] [Google Scholar]
- 11. Vujovic S. 2009. Aetiology of premature ovarian failure. Menopause Int 15:72–75 [DOI] [PubMed] [Google Scholar]
- 12. Ju T, Brewer K, D'Souza A, Cummings RD, Canfield WM. 2002. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem 277:178–186 [DOI] [PubMed] [Google Scholar]
- 13. Ju T, Cummings RD, Canfield WM. 2002. Purification, characterization, and subunit structure of rat core 1 β1,3-galactosyltransferase. J Biol Chem 277:169–177 [DOI] [PubMed] [Google Scholar]
- 14. Chen W, Stanley P. 2003. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology 13:43–50 [DOI] [PubMed] [Google Scholar]
- 15. Varki A, Lowe J. 2009. Biological roles of glycans. In: Varki ACR, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. eds. Essentials of glycobiology. 2nd ed Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [PubMed] [Google Scholar]
- 16. Ioffe E, Stanley P. 1994. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci USA 91:728–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. 1994. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J 13:2056–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP. 2004. Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol 164:451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Williams SA, Xia L, Cummings RD, McEver RP, Stanley P. 2007. Fertilization in mouse does not require terminal galactose or N-acetylglucosamine on the zona pellucida glycans. J Cell Sci 120:1341–1349 [DOI] [PubMed] [Google Scholar]
- 20. Shi S, Williams SA, Seppo A, Kurniawan H, Chen W, Ye Z, Marth JD, Stanley P. 2004. Inactivation of the Mgat1 gene in oocytes impairs oogenesis, but embryos lacking complex and hybrid N-glycans develop and implant. Mol Cell Biol 24:9920–9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams SA, Stanley P. 2008. Mouse fertility is enhanced by oocyte-specific loss of core 1-derived O-glycans. FASEB J 22:2273–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. 1996. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531–535 [DOI] [PubMed] [Google Scholar]
- 23. Skillern A, Rajkovic A. 2008. Recent developments in identifying genetic determinants of premature ovarian failure. Sex Dev 2:228–243 [DOI] [PubMed] [Google Scholar]
- 24. Jagarlamudi K, Reddy P, Adhikari D, Liu K. 2010. Genetically modified mouse models for premature ovarian failure (POF). Mol Cell Endocrinol 315:1–10 [DOI] [PubMed] [Google Scholar]
- 25. Philpott CC, Ringuette MJ, Dean J. 1987. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev Biol 121:568–575 [DOI] [PubMed] [Google Scholar]
- 26. Lewandoski M, Wassarman KM, Martin GR. 1997. Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol 7:148–151 [DOI] [PubMed] [Google Scholar]
- 27. Pedersen T, Peters H. 1968. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil 17:555–557 [DOI] [PubMed] [Google Scholar]
- 28. Thomas JL, Mason JI, Brandt S, Spencer BR, Jr, Norris W. 2002. Structure/function relationships responsible for the kinetic differences between human type 1 and type 2 3β-hydroxysteroid dehydrogenase and for the catalysis of the type 1 activity. J Biol Chem 277:42795–42801 [DOI] [PubMed] [Google Scholar]
- 29. Shimaoka H, Dohi Y, Ohgushi H, Ikeuchi M, Okamoto M, Kudo A, Kirita T, Yonemasu K. 2004. Recombinant growth/differentiation factor-5 (GDF-5) stimulates osteogenic differentiation of marrow mesenchymal stem cells in porous hydroxyapatite ceramic. J Biomed Mater Res A 68:168–176 [DOI] [PubMed] [Google Scholar]
- 30. Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. 1999. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol 13:1018–1034 [DOI] [PubMed] [Google Scholar]
- 31. Corbin CJ, Trant JM, Walters KW, Conley AJ. 1999. Changes in testosterone metabolism associated with the evolution of placental and gonadal isozymes of porcine aromatase cytochrome P450. Endocrinology 140:5202–5210 [DOI] [PubMed] [Google Scholar]
- 32. McNeilly JR, Saunders PT, Taggart M, Cranfield M, Cooke HJ, McNeilly AS. 2000. Loss of oocytes in Dazl knockout mice results in maintained ovarian steroidogenic function but altered gonadotropin secretion in adult animals. Endocrinology 141:4284–4294 [DOI] [PubMed] [Google Scholar]
- 33. Meskhi A, Seif MW. 2006. Premature ovarian failure. Curr Opin Obstet Gynecol 18:418–426 [DOI] [PubMed] [Google Scholar]
- 34. West ER, Xu M, Woodruff TK, Shea LD. 2007. Physical properties of alginate hydrogels and their effects on in vitro follicle development. Biomaterials 28:4439–4448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Easton RL, Patankar MS, Lattanzio FA, Leaven TH, Morris HR, Clark GF, Dell A. 2000. Structural analysis of murine zona pellucida glycans. Evidence for the expression of core 2-type O-glycans and the Sd(a) antigen. J Biol Chem 275:7731–7742 [DOI] [PubMed] [Google Scholar]
- 36. Williams SA, Stanley P. 2009. Oocyte-specific deletion of complex and hybrid N-glycans leads to defects in preovulatory follicle and cumulus mass development. Reproduction 137:321–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Markström E, Svensson ECh, Shao R, Svanberg B, Billig H. 2002. Survival factors regulating ovarian apoptosis—dependence on follicle differentiation. Reproduction 123:23–30 [DOI] [PubMed] [Google Scholar]
- 38. Braw-Tal R, McNatty KP, Smith P, Heath DA, Hudson NL, Phillips DJ, McLeod BJ, Davis GH. 1993. Ovaries of ewes homozygous for the X-linked Inverdale gene (FecXI) are devoid of secondary and tertiary follicles but contain many abnormal structures. Biol Reprod 49:895–907 [DOI] [PubMed] [Google Scholar]
- 39. Juengel JL, Quirke LD, Tisdall DJ, Smith P, Hudson NL, McNatty KP. 2000. Gene expression in abnormal ovarian structures of ewes homozygous for the inverdale prolificacy gene. Biol Reprod 62:1467–1478 [DOI] [PubMed] [Google Scholar]
- 40. Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O. 2000. Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 [DOI] [PubMed] [Google Scholar]
- 41. el-Fouly MA, Cook B, Nekola M, Nalbandov AV. 1970. Role of the ovum in follicular luteinization. Endocrinology 87:286–293 [PubMed] [Google Scholar]
- 42. Nekola MV, Nalbandov AV. 1971. Morphological changes of rat follicular cells as influenced by oocytes. Biol Reprod 4:154–160 [DOI] [PubMed] [Google Scholar]
- 43. Vitt UA, Hayashi M, Klein C, Hsueh AJ. 2000. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod 62:370–377 [DOI] [PubMed] [Google Scholar]
- 44. Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. 2001. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 276:11387–11392 [DOI] [PubMed] [Google Scholar]
- 45. Shi S, Williams SA, Kurniawan H, Lu L, Stanley P. 2005. Roles of complex and hybrid N-glycans and O-fucose glycans in oocyte development and function. Adv Exp Med Biol 564:99–100 [DOI] [PubMed] [Google Scholar]
- 46. Rubio-Gozalbo ME, Gubbels CS, Bakker JA, Menheere PP, Wodzig WK, Land JA. 2010. Gonadal function in male and female patients with classic galactosemia. Hum Reprod Update 16:177–188 [DOI] [PubMed] [Google Scholar]
- 47. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. 2008. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613 [DOI] [PubMed] [Google Scholar]
- 48. Reddy P, Adhikari D, Zheng W, Liang S, Hämäläinen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. 2009. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet 18:2813–2824 [DOI] [PubMed] [Google Scholar]
- 49. Trombly DJ, Woodruff TK, Mayo KE. 2009. Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150:1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pangas SA, Li X, Robertson EJ, Matzuk MM. 2006. Premature luteinization and cumulus cell defects in ovarian-specific Smad4 knockout mice. Mol Endocrinol 20:1406–1422 [DOI] [PubMed] [Google Scholar]