Abstract
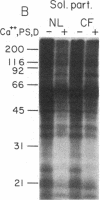
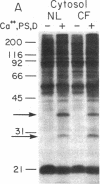
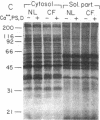
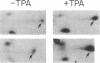
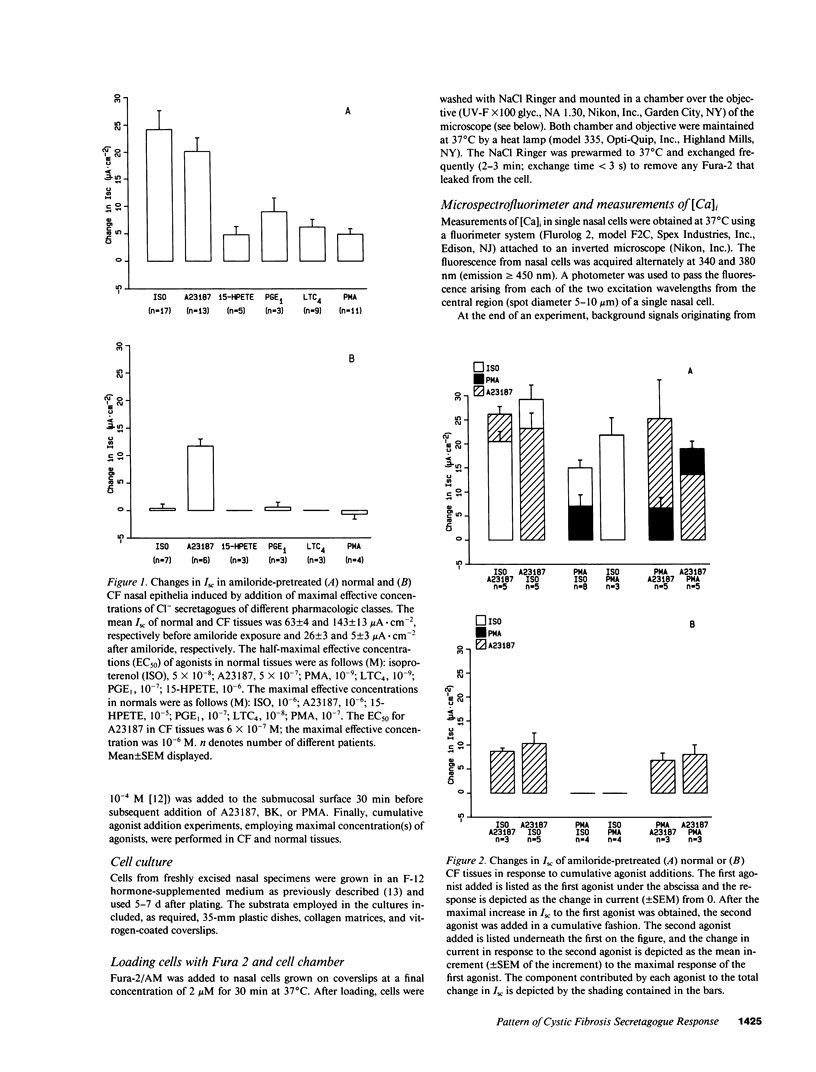
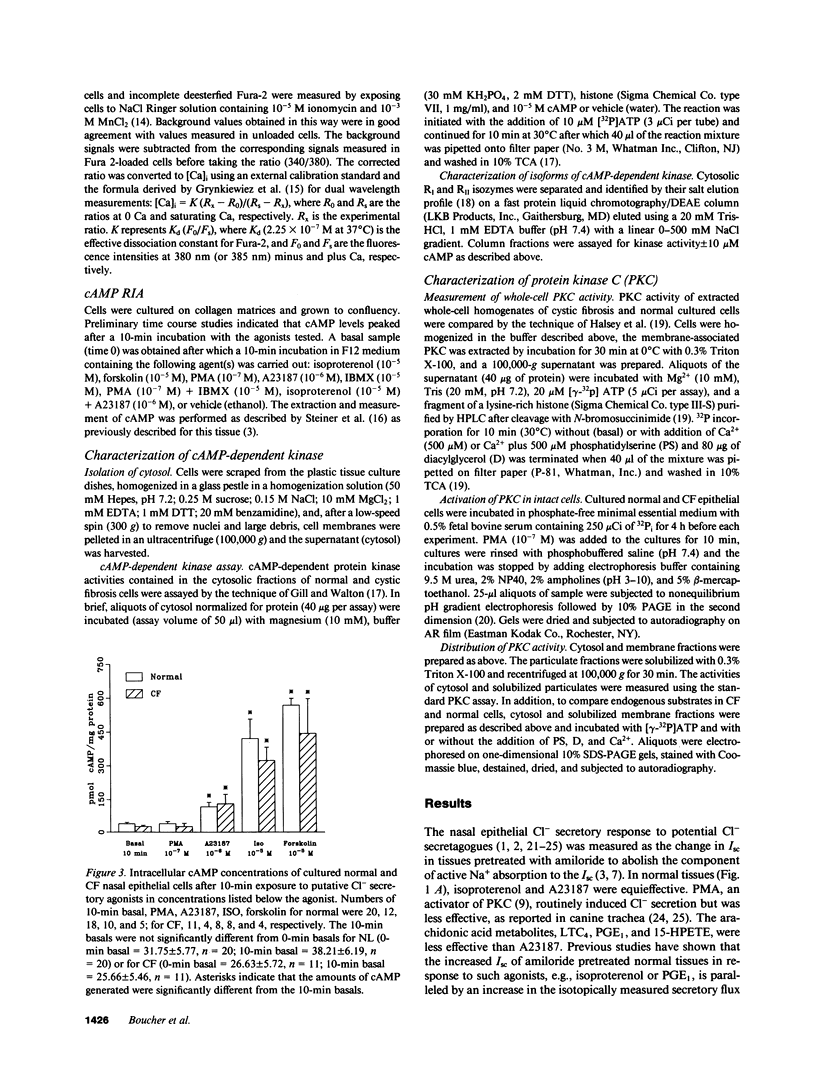
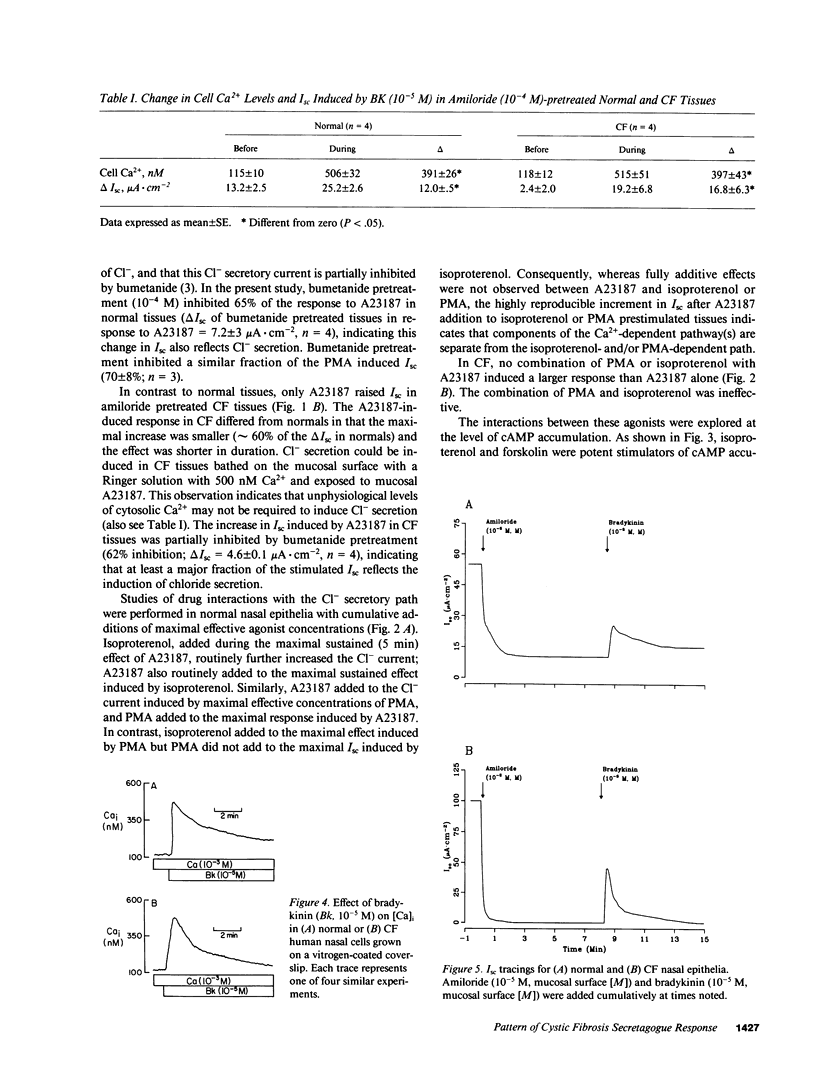
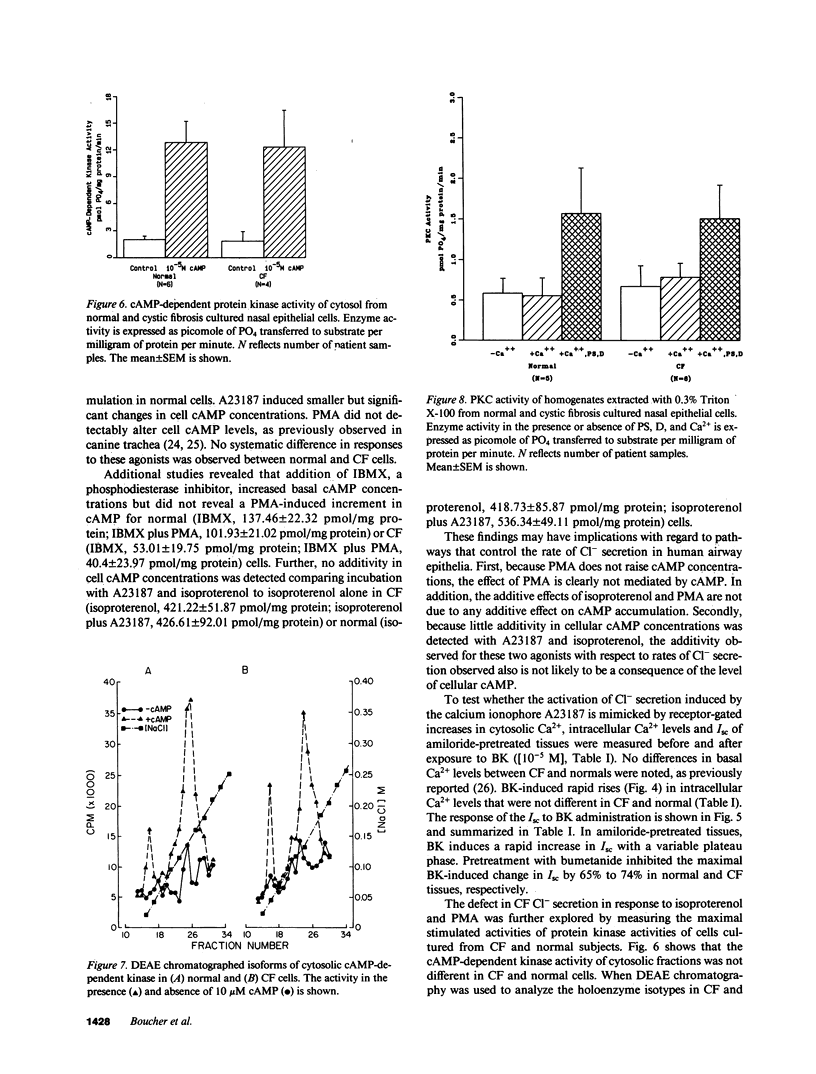
Because the defect in Cl- secretion exhibited by cystic fibrosis (CF) epithelia reflects regulatory rather than conductive abnormalities of an apical membrane Cl- channel, we investigated the role of different regulatory pathways in the activation of Cl- secretion in freshly excised normal and CF nasal epithelia mounted in Ussing chambers. A beta agonist (isoproterenol [ISO]), a Ca2+ ionophore (A23187), and a phorbol ester (PMA) were all effective Cl- secretagogues in normal human nasal epithelia. Agonist addition studies indicated that ISO and PMA but not A23187 may share a common regulatory pathway. In contrast, only A23187 induced Cl- secretion in CF epithelia. Bradykinin raised cytosolic Ca2+ and induced Cl- secretion in both normal and CF tissues, indicating that receptor gated Ca2+ dependent Cl- secretory mechanisms were preserved in CF. The defective Cl- secretory response in CF epithelia to ISO and PMA did not reflect abnormalities in cAMP-dependent (A) and phospholipid Ca2+-dependent (C) kinase activities. We conclude that (a) a Ca2+-sensitive mechanism for regulating Cl- secretion is maintained in CF airway epithelia, and (b) a regulatory pathway shared by two distinct protein kinases is defective in CF, indicating that the CF genetic lesion is not tightly coupled to a single (e.g., cAMP dependent) regulatory mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Bazzaz F. J., Cheng E. Effect of catecholamines on ion transport in dog tracheal epithelium. J Appl Physiol Respir Environ Exerc Physiol. 1979 Aug;47(2):397–403. doi: 10.1152/jappl.1979.47.2.397. [DOI] [PubMed] [Google Scholar]
- Al-Bazzaz F., Jayaram T. Ion transport by canine tracheal mucosa: effect of elevation of cellular calcium. Exp Lung Res. 1981 May;2(2):121–130. doi: 10.3109/01902148109052308. [DOI] [PubMed] [Google Scholar]
- Barthelson R. A., Jacoby D. B., Widdicombe J. H. Regulation of chloride secretion in dog tracheal epithelium by protein kinase C. Am J Physiol. 1987 Dec;253(6 Pt 1):C802–C808. doi: 10.1152/ajpcell.1987.253.6.C802. [DOI] [PubMed] [Google Scholar]
- Barthelson R., Widdicombe J. Cyclic adenosine monophosphate-dependent kinase in cystic fibrosis tracheal epithelium. J Clin Invest. 1987 Dec;80(6):1799–1802. doi: 10.1172/JCI113274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berschneider H. M., Knowles M. R., Azizkhan R. G., Boucher R. C., Tobey N. A., Orlando R. C., Powell D. W. Altered intestinal chloride transport in cystic fibrosis. FASEB J. 1988 Jul;2(10):2625–2629. doi: 10.1096/fasebj.2.10.2838365. [DOI] [PubMed] [Google Scholar]
- Boucher R. C., Cotton C. U., Gatzy J. T., Knowles M. R., Yankaskas J. R. Evidence for reduced Cl- and increased Na+ permeability in cystic fibrosis human primary cell cultures. J Physiol. 1988 Nov;405:77–103. doi: 10.1113/jphysiol.1988.sp017322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher R. C., Stutts M. J., Knowles M. R., Cantley L., Gatzy J. T. Na+ transport in cystic fibrosis respiratory epithelia. Abnormal basal rate and response to adenylate cyclase activation. J Clin Invest. 1986 Nov;78(5):1245–1252. doi: 10.1172/JCI112708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. Physiology. Chloride ions and cystic fibrosis. 1986 Jul 31-Aug 6Nature. 322(6078):407–407. doi: 10.1038/322407a0. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Frizzell R. A., Rechkemmer G., Shoemaker R. L. Altered regulation of airway epithelial cell chloride channels in cystic fibrosis. Science. 1986 Aug 1;233(4763):558–560. doi: 10.1126/science.2425436. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Walton G. M. Assay of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Res. 1979;10:93–106. [PubMed] [Google Scholar]
- Greengard P. Phosphorylated proteins as physiological effectors. Science. 1978 Jan 13;199(4325):146–152. doi: 10.1126/science.22932. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hunter J. A., Finkbeiner W. E., Nadel J. A., Goetzl E. J., Holtzman M. J. Predominant generation of 15-lipoxygenase metabolites of arachidonic acid by epithelial cells from human trachea. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4633–4637. doi: 10.1073/pnas.82.14.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. R., Stutts M. J., Spock A., Fischer N., Gatzy J. T., Boucher R. C. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science. 1983 Sep 9;221(4615):1067–1070. doi: 10.1126/science.6308769. [DOI] [PubMed] [Google Scholar]
- Leikauf G. D., Ueki I. F., Nadel J. A., Widdicombe J. H. Bradykinin stimulates Cl secretion and prostaglandin E2 release by canine tracheal epithelium. Am J Physiol. 1985 Jan;248(1 Pt 2):F48–F55. doi: 10.1152/ajprenal.1985.248.1.F48. [DOI] [PubMed] [Google Scholar]
- Li M., McCann J. D., Liedtke C. M., Nairn A. C., Greengard P., Welsh M. J. Cyclic AMP-dependent protein kinase opens chloride channels in normal but not cystic fibrosis airway epithelium. Nature. 1988 Jan 28;331(6154):358–360. doi: 10.1038/331358a0. [DOI] [PubMed] [Google Scholar]
- Murphy E., Cheng E., Yankaskas J., Stutts M. J., Boucher R. C. Cell calcium levels of normal and cystic fibrosis nasal epithelium. Pediatr Res. 1988 Jul;24(1):79–84. doi: 10.1203/00006450-198807000-00019. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Turnover of inositol phospholipids and signal transduction. Science. 1984 Sep 21;225(4668):1365–1370. doi: 10.1126/science.6147898. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Sato K., Sato F. Defective beta adrenergic response of cystic fibrosis sweat glands in vivo and in vitro. J Clin Invest. 1984 Jun;73(6):1763–1771. doi: 10.1172/JCI111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoumacher R. A., Shoemaker R. L., Halm D. R., Tallant E. A., Wallace R. W., Frizzell R. A. Phosphorylation fails to activate chloride channels from cystic fibrosis airway cells. Nature. 1987 Dec 24;330(6150):752–754. doi: 10.1038/330752a0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Welsh M. J. Effect of phorbol ester and calcium ionophore on chloride secretion in canine tracheal epithelium. Am J Physiol. 1987 Dec;253(6 Pt 1):C828–C834. doi: 10.1152/ajpcell.1987.253.6.C828. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Liedtke C. M. Chloride and potassium channels in cystic fibrosis airway epithelia. 1986 Jul 31-Aug 6Nature. 322(6078):467–470. doi: 10.1038/322467a0. [DOI] [PubMed] [Google Scholar]
- Widdicombe J. H. Cystic fibrosis and beta-adrenergic response of airway epithelial cell cultures. Am J Physiol. 1986 Oct;251(4 Pt 2):R818–R822. doi: 10.1152/ajpregu.1986.251.4.R818. [DOI] [PubMed] [Google Scholar]
- Willumsen N. J., Boucher R. C. Activation of an apical Cl- conductance by Ca2+ ionophores in cystic fibrosis airway epithelia. Am J Physiol. 1989 Feb;256(2 Pt 1):C226–C233. doi: 10.1152/ajpcell.1989.256.2.C226. [DOI] [PubMed] [Google Scholar]
- Wu R., Yankaskas J., Cheng E., Knowles M. R., Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985 Aug;132(2):311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]