Leptin directly modulates the activity of midbrain LepRb expressing urocortin 1 neurons by recruiting JAK-STAT signaling mediated by STAT3 phosphorylation.
Abstract
A recent study systematically characterized the distribution of the long form of the leptin receptor (LepRb) in the mouse brain and showed substantial LepRb mRNA expression in the nonpreganglionic Edinger-Westphal nucleus (npEW) in the rostroventral part of the midbrain. This nucleus hosts the majority of urocortin 1 (Ucn1) neurons in the rodent brain, and because Ucn1 is a potent satiety hormone and electrical lesioning of the npEW strongly decreases food intake, we have hypothesized a role of npEW-Ucn1 neurons in leptin-controlled food intake. Here, we show by immunohistochemistry that npEW-Ucn1 neurons in the mouse contain LepRb and respond to leptin administration with induction of the Janus kinase 2-signal transducer and activator of transcription 3 pathway, both in vivo and in vitro. Furthermore, systemic leptin administration increases the Ucn1 content of the npEW significantly, whereas in mice that lack LepRb (db/db mice), the npEW contains considerably reduced amount of Ucn1. Finally, we reveal by patch clamping of midbrain Ucn1 neurons that leptin administration reduces the electrical firing activity of the Ucn1 neurons. In conclusion, we provide ample evidence for leptin actions that go beyond leptin's well-known targets in the hypothalamus and propose that leptin can directly influence the activity of the midbrain Ucn1 neurons.
Energy homeostasis requires tight regulation of food intake and fat storage (e.g. see Refs. 1–3). A very critical hormone is leptin, the product of ob gene and produced by adipocytes. It conveys information to the brain about the amount of peripherally stored fat (4). Leptin acts through the leptin receptor long form (LepRb), the product of db gene (5, 6), to activate the Janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3) pathway (7, 8). It has been primarily shown to act in the hypothalamus, especially on the arcuate nucleus, and it plays a key role in the regulation of food intake and energy expenditure (9, 10). However, some evidence is appearing that leptin would also have direct effects on brain centers outside the hypothalamus. For example, LepRb expression has been shown in the cerebral cortex, hippocampus, midbrain, and brain stem (11). In a recent study, using a novel LepRb-internal ribosomal entry site (IRES)-Cre-enhanced yellow fluorescent protein-reporter mouse and in situ hybridization, substantial amounts of LepRb expressions were found in extrahypothalamic brain areas in adult mice (12, 13), such as in the nonpreganglionic Edinger-Westphal nucleus (npEW) in the rostroventral part of the midbrain. This nucleus is of particular interest, because its electrical lesioning substantially decreased general food intake (14) and this nucleus is the major source of urocortin 1 (Ucn1), a member of the corticotropin releasing factor family that has strong anorexigenic actions (15–17).
Recently, we showed that the rat npEW reveals substantial LepRb mRNA expression and responds to fasting (18). Moreover, the npEW of rats fed a high-fat diet showed a decrease in Ucn1 mRNA expression (19). In addition, peripheral injection of low doses of Ucn1 produces strong and prolonged inhibition of food intake (20, 21), an effect that can also be seen in leptin-deficient (ob/ob) mice (22). Intracerebroventricular (icv) administration of Ucn1 also potently reduces food intake in food-deprived rats (23), an action most probably mediated by the ventromedial hypothalamic nucleus (24).
The colocalization of LepRb and Ucn1 in npEW neurons is interesting, because a number of experimental studies have shown the interactions of leptin and Ucn1 on food intake: 1) the satiety effect of Ucn1 is enhanced by its own ability to induce plasma leptin (25), 2) cotreatment with doses of leptin and Ucn1 that are ineffective while given alone effectively suppresses appetite (26), 3) leptin facilitates Ucn1 transport across the blood-brain barrier (27), and 4) Ucn1 seems to potentiate leptin signaling by leptin receptor-mediated STAT3 phosphorylation (27).
Although these data taken together indicate that the collaboration of peripheral leptin signaling and Ucn1 in the npEW may play an important role in regulating feeding behavior, the mechanism by which leptin would influence the activity of the npEW-Ucn1 neurons is unclear. Here, we hypothesize that in mouse, leptin-mediated signaling occurs in Ucn1 neurons in the npEW and that modulation of leptin signaling can modify Ucn1 neuron activity.
Materials and Methods
Animals
Male C57BL/6J, B6.Cg-m+/+Leprdb/J (db/db), and LepRbEGFP mice were used. In vivo experiments were performed with young-adult mice (10–12 wk old; obtained from The Jackson Laboratory, Bar Harbor, ME) and housed in the University of Texas Southwestern Medical Center at Dallas (in situ hybridization) or in the Unit for Laboratory Animal Medicine at the University of Michigan (immunohistochemistry) in groups of two to four unless stated otherwise. In vitro studies were performed with wild-type (WT) C57BL/6J pups (16–21 d old), housed with their mother, who was obtained from Janvier (Le-Genest-St-Isle, France), in the Central Animal Laboratory at Radboud University Nijmegen. They were all housed at a 12-h light, 12-h dark cycle (lights on at 0600 h) in a humidity- and temperature (22 C)-controlled environment and had access to food and water ad libitum. All animal procedures had the approval of the respective University care and use committees.
Production of LepRbEGFP mice
To identify LepRb-expressing neurons, Leprcre mice were crossed with RosaEGFP reporter mice to produce LepRbEGFP offspring with enhanced green fluorescent protein (EGFP) expressed in LepRb neurons. Briefly, in Leprcre mice, an IRES-driven second cistron encoding cre recombinase is “knocked in” to the 3′-untranslated region of the LepRb-specific exon of Lepr, rendering the cre-coding sequence part of the LepRb-specific mRNA such that its expression is restricted to LepRb-expressing neurons (28). The Gt(ROSA)26Sortm2Sho (ROSAEGFP) line from The Jackson Laboratory has been engineered such that cre-mediated deletion of a floxed transcription-blocking cassette results in the expression of EGFP from the ubiquitously expressed ROSA26 locus (29). Because IRES-mediated cre expression is modest, mice were bred to homozygosity (Leprcre/cre, RosaEGFP/EGFP) to enhance the detection of LepRb neurons by EGFP expression. All matings were carried out in the University of Michigan Unit for Laboratory Animal Medicine.
Peptide and antisera
Recombinant mouse leptin was obtained from the National Hormone and Peptide Program (A. F. Parlow, Los Angeles, CA), rabbit anti-Ucn1 was a generous gift from W. W. Vale (no. 5779; The Salk Institute, La Jolla, CA), chicken anti-EGFP (no. 13970) was from Abcam (Cambridge, MA), rabbit antiphosphorylation of STAT3 (pSTAT3) (no. 9131) from Cell Signaling Technology (Danvers, MA), mouse antiglial fibrillary acidic protein (GFAP) (no. GA-5) from ICN Biomedicals (Irvine, CA), goat anti-Ucn1 (no. sc-1825) from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and Alexa 488-conjugated goat antichicken and Alexa 594-conjugated donkey antirabbit from Invitrogen (Carlsbad, CA). Normal donkey serum and the Cy2-conjugated donkey-antichicken, Cy2-conjugated donkey-antigoat, Cy3-conjugated donkey-antirabbit, and Cy5-conjugated donkey-antimouse sera were from Jackson ImmunoResearch (West Grove, PA). All other immunolabeling supplies were purchased from Sigma Chemical (St. Louis, MO).
Tissue preparations
In vivo study with in situ hybridization (LepRb mRNA)
Four C57BL/6J male mice were deeply anesthetized with an ip injection of chloral hydrate (350 mg/kg) and perfused transcardially with diethylpyrocarbonate (DEPC)-treated 0.9% saline followed by 10% neutral buffered formalin. After decapitation, brains were removed, postfixed in 10% neutral buffered formalin for 4 h at 4 C, cryoprotected in 20% sucrose in DEPC-treated PBS (pH 7.0) overnight at 4 C, and cut coronally into five equal series of 25-μm sections on a freezing microtome, which were stored at −20 C in antifreeze solution (30) until processing for radioactive in situ hybridization.
In vivo studies with immunohistochemistry
The untreated LepRbEGFP mice were used to demonstrate LepRb protein. To study the effect of leptin on Ucn1 in the npEW, 16 LepRbEGFP mice were single housed and injected ip with either leptin (5 mg/kg) or equal volume vehicle (sterile PBS, pH 7.4) and killed 2 or 4 h later. To assess the effect of disrupted leptin signaling on the npEW, five db/db and five WT mice were studied. All mice were deeply anesthetized with ip sodium pentobarbital (150 mg/kg), transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde (PFA), for 30 min, decapitated, and brains removed and postfixed in 4% PFA (31), for 16 h. Four representative series of coronal sections (30 μm) were cut with a sliding microtome, into a cryoprotective solution (30% ethylene glycol, 30% glycerol; in PBS), and stored at −20 C until use for immunohistochemistry.
In vitro brain-slice study with immunohistochemistry
Twelve C57BL/6J pups were decapitated, their brains were quickly removed and placed into ice-cold slicing medium containing 83.5 mm NaCl, 30 mm KCl, 1.3 mm KH2PO4, 1.2 mm MgCl2, 2.4 mm CaCl2, 2.6 mm NaHCO3, 2.5 mm glucose, and 1.0 mm HEPES (pH 7.4) (Merck Chemical, Darmstadt, Germany). The olfactory bulb and cerebellum were removed, and the remaining tissue was glued onto the holding block of a slicing chamber with the cerebellum side pointing upward, flooded with the slicing medium, and 300-μm coronal slices containing the npEW (between Bregma −3.2 and −3.6 mm) (32) cut with a VT1000S vibratome (Leica Microsystems, Wetzlar, Germany) and kept submerged on a grid in room temperature artificial cerebral spinal fluid (ACSF) containing 120 mm NaCl, 2.5 mm NaHCO3, 3.5 mm KCl, 1.25 mm NaH2PO4, 1.3 mm MgSO4, 2.5 mm glucose, and 2.5 mm CaCl2 (pH 7.4). Slices were incubated with ACSF (controls) or with ACSF + 100 nm leptin, for 30 min at 35 C. Then they were fixed in 4% PFA, for 2 d at 4 C, and 10 25-μm coronal sections at the midlevel of the npEW were cut on the freezing microtome (Microm GmbH, Walldorf, Germany) for immunohistochemistry.
In situ hybridization
The in situ hybridization procedure was a modification of that previously reported (11, 12). In short, sections were rinsed with DEPC-PBS, for 1 h, and treated with 1% sodium borohydride (Sigma Chemical) in DEPC-PBS, for 15 min, briefly rinsed in 0.1 m tetrachlorammonium (pH 8.0), treated with 0.25% acetic anhydride in 0.1 m tetrachlorammonium, for 10 min, and rinsed in DEPC-treated 2× sodium chloride/sodium citrate (SSC). Sections were incubated for 16 h at 57 C, with LepR riboprobes, generated by in vitro transcription using 35S-labeled uridine triphosphate as previously described (12) and diluted to 106 cpm/ml in a solution containing 50% formamide, 10 mm Tris-HCl (pH 8.0) (Life Technologies, Inc.-BRL, Bethesda, MD), 5 mg tRNA (Invitrogen), 10 mm dithiothreitol, 10% dextran sulfate, 0.3 m NaCl, 1 mm EDTA (pH 8.0), and 1× Denhardt's solution (Sigma Chemical). Then they were rinsed four times in 4× SSC, and incubated in 0.002% ribonuclease (RNase) A (Roche Applied Bioscience, Indianapolis, IN) diluted in a mixture of 0.5 m NaCl, 10 mm Tris-HCl (pH 8.0) and 1 mm EDTA (RNase buffer), for 30 min at 37 C. After another 30 min in RNase buffer and two rinses at room temperature in 2× SSC, the sections were rinsed three times in 50% formamide in 0.2× SSC, for 10 min at 50 C, and rinsed 2× SSC at 50 C, 0.2× SSC at 55 C, and 0.2× SSC at 60 C, each rinse for 1 h. After rinsing twice in 2× SSC at room temperature, sections were mounted on SuperFrost Plus slides (Fisher, Pittsburgh, PA), dehydrated in a graded ethanol series, and delipidated in chloroform. After rinses in 100 and 95% ethanol, slides were air-dried and placed in x-ray film cassettes with BMR-2 film (Kodak, Rochester, NY), for 2 d. Next, they were dipped in NTB2 emulsion (Kodak), air-dried, and stored in light-tight boxes at 4 C for 4 wk. Slides were developed with Kodak Dd-19 developer, counterstained with thionin, dehydrated in ethanol, cleared in xylene, and mounted with Permaslip. The high specificity of the probes has been shown before (12).
Immunohistochemistry
Single immunolabeling of EGFP was performed on the nontreated LepRbEGFP mice. Single immunolabeling of Ucn1 was performed on the PBS/leptin injected LepRbEGFP mice (4 h) and WT and db/db mice. Sections were treated with 0.5% Triton X-100 in PBS, for 30 min, blocked in 2% normal donkey serum, for 1 h, and incubated in primary chicken anti-EGFP (1:1000), goat anti-Ucn1 (1:100; PBS/leptin injected mice, 4 h), or rabbit anti-Ucn1 sera (1:30,000; WT and db/db mice) overnight. This was followed by a 2-h incubation with the secondary antibodies Alexa 488-conjugated goat antichicken (1:200), Cy2-conjugated antigoat IgG (1:100), or Cy3-conjugated antirabbit IgG (1:100).
Double immunolabeling of EGFP with Ucn1 or with pSTAT3 was performed on control or PBS/leptin-injected LepRbEGFP mice (2 h), respectively. For double immunolabeling of EGFP and Ucn1, sections were processed as described for single immunolabeling but with incubation in a mixture of the chicken anti-EGFP (1:2000) and rabbit anti-Ucn1 sera (1:30,000), for 16 h, and then in a mixture of Cy2-conjugated antichicken IgG and Cy3-conjugated antirabbit IgG (1:100), for 2 h. For double immunolabeling of EGFP and pSTAT3, sections were pretreated sequentially in 3% H2O2 and 1% NaOH, for 20 min, 0.3% glycine, for 10 min, and 0.03% sodium dodecyl sulfate, for 10 min, and processed as described above, with chicken anti-EGFP (1:1000) and rabbit anti-pSTAT3 (1:500) sera for 16 h, and Alexa 488-conjugated goat antichicken (1:200) and Alexa 594-conjugated donkey antirabbit sera, respectively, for 2 h (1:200).
Triple immunolabeling of Ucn1, pSTAT3, and GFAP was performed on in vitro brain slices. The same protocol for pSTAT3 immunohistochemistry was applied, with incubation in goat anti-Ucn1 (1:100), rabbit anti-pSTAT3 (1:400), and monoclonal mouse anti-GFAP (1:100) sera, for 16 h, and then in a mixture of Cy2-conjugated antigoat IgG, Cy3-conjugated antirabbit IgG, and Cy5-conjugated antimouse IgG (1:100) sera, respectively, for 2 h.
Antibody characterization
The high specificities of chicken anti-EGFP (8, 19, 28), mouse anti-GFAP (33), rabbit anti-pSTAT3 (12, 34), goat anti-Ucn1 (35, 36), and rabbit anti-Ucn1 (19, 36, 37) have been previously reported. In addition, preabsorption of antibodies with the respective synthetic peptides abolished immunostaining in the mouse npEW, and omission of primary antisera completely prevented immunoreaction in all cases.
Image analysis
Immunostainings were studied with a BX-51 bright field microscope with DP30BW camera (Olympus, Tokyo, Japan) or a confocal laser scanning microscopy (Leica Microsystems TCS SP2 AOBS system). For quantitative determinations of the amounts of immunoreactive peptide in the npEW, two parameters were determined: 1) the number of neuronal perikarya was counted in four medial sections of the npEW, and 2) per perikaryon, the specific immunoreactive signal density (SSD) was measured in each of all perikarya present in these sections, using ImageJ software (version 1.37; NIH, Bethesda, MD). Data were corrected for background density outside the npEW, yielding the SSD expressed in arbitrary units per perikaryon.
Electrophysiology
Brain slices of WT C57BL/6J pups (thickness, 300 μm) made as described above were incubated in ACSF, at 37 C for 30 min, and then stored 2–4 h at room temperature until use. Neurons in the npEW, 100–300 μm ventral to the periaqueductal gray, were studied in the cell-attached patch-clamp mode, using an EPC-9 patch-clamp amplifier with patchMaster software version 2.20 (HEKA, Lambrecht/Pfaltz, Germany). Data were recorded at 10 kHz and filtered using a 12.9-kHz Bessel filter. Patch pipettes with a resistance between 4 and 6 mΩ were pulled from Wiretrol II glass capillaries (Drummond Scientific Co., Broomall, PA) with a PP-83 pipette puller (Narishige Scientific Instrument Laboratories, Tokyo, Japan). All solutions were continuously carbogenized (5% CO2-95% O2), and the temperature of the superfusate was controlled with an SH-27B in-line temperature heater coupled to a TC-324B single channel heater controller (Harvard Apparatus, Holliston, MA). Electrical activity was recorded in the cell-attached patch voltage-clamp mode at 0 mV pipette potential (38). Performing measurements in cell-attached mode preserved the cytoplasm, and in this configuration, spontaneous firing activity was observed. Cell firing activity was recorded in the form of action potential currents, which are the first derivatives of action potentials and are mainly due to the capacitive current (39). During recording, cells were incubated in ACSF, followed by ACSF containing 100 nm leptin, and finally in ACSF to washout leptin. During each incubation, which lasted 3 min, the interspike interval (ISI), i.e. the time between two successive action currents, was averaged over all currents measured. The coefficient of variation (CV) was calculated as sd/mean of ISI, according to Yang et al. (38).
Statistical analyses
Per experimental group, data were expressed as the mean ± sem and entered in Student's t test after tests for normality (Shapiro-Wilk test; see Ref. 40) and homogeneity of variance (Bartlett's χ2 test; 41) (α = 5%).
Results
Expression of LepRb in npEW-Ucn1 neurons
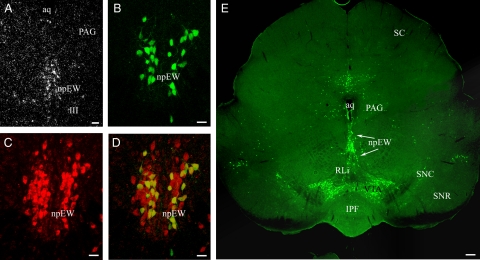
First, we used in situ hybridization to study the presence of LepRb mRNA in C57BL/6J mice. Sections of the midbrain were hybridized with a long-form specific antisense LepRb probe. In the npEW, neuronal perikarya revealed substantial labeling (Fig. 1A). No staining was seen in the npEW when the sense LepRb probe was used (data not shown). Next, we applied single-labeling immunofluorescence histochemistry to show the presence of LepRb protein in LepRbEGFP mice (Fig. 1E). They revealed an almost continuous, boomerang-shaped population of EGFP-immunoreactive (EGFP-ir) neurons in the midbrain, starting dorsomedially in the npEW, continuing through the rostral linear raphe and ventral tegmental area (VTA), and terminating with a few scattered neurons in the substantia nigra pars compacta. Using double-labeling immunofluorescence, we showed in npEW neurons the coexistence of EGFP-ir with Ucn1-ir (Fig. 1, B–D). Nearly all (>90%) EGFP-ir neurons revealed Ucn1-ir, whereas about 45% of the Ucn1-ir neurons were EGFP-ir (Fig. 1D). None of the EGFP-positive neurons in the linear raphe, VTA, and substantia nigra pars compacta showed Ucn1-ir (data not shown). One limitation of the genetic model used in this study is that not all of the LepRb neurons will exhibit EGFP-ir, because only a subpopulation of LepRb neurons expresses EGFP-ir (19, 42).
Fig. 1.
Visualization of midbrain LepRb neurons. A, LepRb mRNA is present in the npEW by radioactive in situ hybridization. E, LepRb-expressing neurons are revealed by EGFP-ir (green) in the midbrain of LepRbEGFP mice. B–D, EGFP-ir (green) (B) neurons are colocalized (yellow) (D) with Ucn1-ir (red) (C) in the npEW of LepRbEGFP mice. Scale bar, 20 μm (A–D) and 100 μm (E). Aq, Central aqueduct; III, nucleus of oculomotor nerve; IPF, fasciculus interpeduncularis; PAG, periaqueductal gray; RLi, rostral linear nucleus; SC, colliculus superior; SNC, substantia nigra pars compacta; SNR, substantia nigra reticular part.
LepRb signaling increases STAT3 phosphorylation
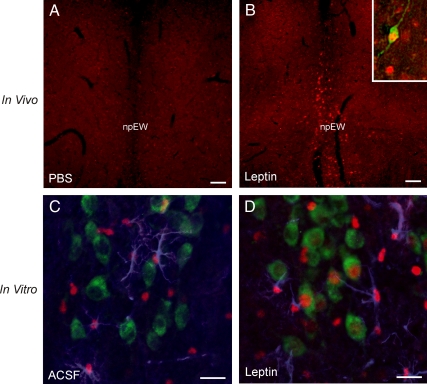
To assess whether LepRb in the npEW is functional, the JAK2-STAT3 pathway was evaluated. This signaling pathway is induced when leptin binds to LepRb, resulting in pSTAT3. Subsequently, the pSTAT3 is translocated into the nucleus to mediate gene transcription (43, 44). To determine whether leptin stimulates pSTAT3 in the npEW in vivo, LepRbEGFP mice were ip injected with PBS or leptin. Immunohistochemistry revealed no staining of pSTAT3-ir anywhere in the midbrain 2 h after PBS injection (Fig. 2A). However, 2 h after leptin administration, clear pSTAT3-ir was seen to be colocalized with the EGFP-ir neurons in the npEW (Fig. 2B). To assess whether this stimulatory effect would be due to a direct action of leptin on the npEW, brain slices containing the npEW were incubated in vitro with leptin. In fresh, control slices, incubated in ACSF, pSTAT3-ir was seen throughout the tissue in GFAP-ir astrocytes. This was not surprising, because the mere preparation of brain slices is sufficient to induce the JAK2-STAT3 pathway in astrocytes (42). Such fresh slices never revealed any pSTAT3-ir in neurons, including the Ucn1-ir neurons in the npEW (Fig. 2C). However, in slices incubated for 30 min with ACSF + 100 nm leptin, pSTAT3-ir was not only observed in GFAP-ir astrocytes but also in about 40% of the Ucn1-ir neurons, which revealed strong labeling of nucleus (triple labeling; Fig. 2D).
Fig. 2.
Increased STAT3 phosphorylation (pSTAT3) by leptin both in vivo and in vitro. A and B, In vivo, systemic leptin administration (5 mg/kg ip, 2 h) of LepRbEGFP mice induces pSTAT3-ir (red) in the npEW compared with PBS injection. The inset shows a high magnification of colocalization of EGFP-ir (green) and nuclear pSTAT3-ir (red). C and D, In vitro, incubation with ACSF on midbrain slices, pSTAT3-ir (red) is observed in GFAP-ir (blue) astrocytes (C); incubation with leptin induces pSTAT3-ir (red) not only in GFAP-ir (blue) astrocytes but also in the Ucn1-ir (green) neurons (D). Scale bar, 20 μm.
Leptin changes Ucn1 peptide content in npEW neurons
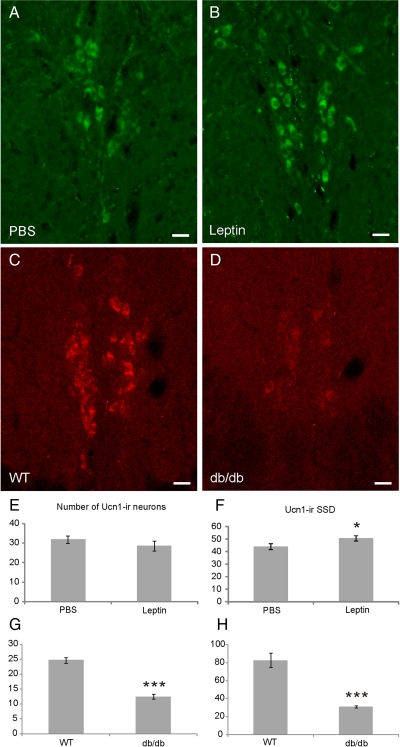
Studying the effect of leptin on the degree of Ucn1-ir of the npEW of C57BL/6J mice by semiquantitative immunohistochemistry revealed that, in leptin-injected mice, the number of Ucn1-ir cells was not different from that in PBS-injected controls (P > 0.05; n = 5; PBS, 31.9 ± 1.8; Leptin, 28.6 ± 2.4). However, the abundance of Ucn1-ir per individual perikaryon, as assessed by measuring the SSD, was significantly higher in leptin-injected mice than in the controls (P < 0.05; n = 5) (Fig. 3, A, B, E, and F). Next, we performed the same experiment with db/db mice and their control littermates (WT). In the db/db mice, the number of npEW-Ucn1-ir neurons and the SSD of Ucn1-ir were significantly lower (P < 0.0005 and P < 0.005; n = 5, respectively) than in WT (Fig. 3, C, D, G, and H).
Fig. 3.
Regulation of Ucn1 content in the npEW by increased and disrupted leptin signaling. A and B, Fluorescent immunohistochemistry reveals Ucn1-ir (green) in the npEW in PBS and leptin injected mice (n = 5). E and F, Systemic leptin administration (5 mg/kg ip, 4 h) of LepRbEGFP mice increases SSD of Ucn1-ir significantly (F) but not the number of Ucn1-ir neurons (E). C and D, Fluorescent immunohistochemistry reveals Ucn1-ir (red) in the npEW in WT and db/db mice (n = 5). G and H, Both the number and SSD of Ucn1-ir neurons are decreased significantly in db/db mice compared with WT. Scale bar, 20 μm. *, P < 0.05; ***, P < 0.005.
Leptin inhibits electrical activity of Ucn1 neurons
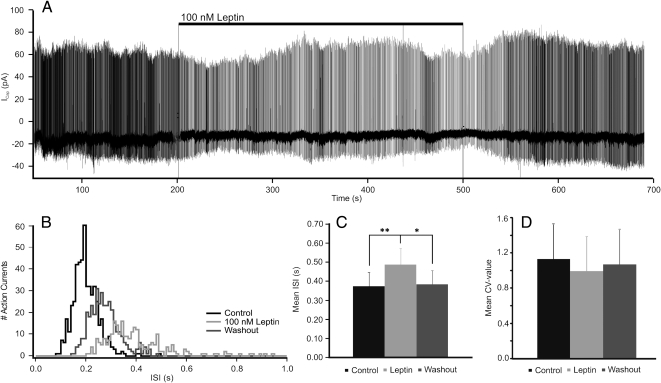
To determine the effect of leptin on electrical activity of Ucn1-neurons in the npEW, cell-attached voltage-clamp patch-clamp measurements were performed on brain slices of C57BL/6J pups. All recorded neurons (n = 11) spontaneously displayed action current firing, with an ISI of 0.37 ± 0.08 sec (Fig. 4, A–C). When ACSF + 100 nm leptin was used as superfusate, cells showed a reduction in action current frequency (Fig. 4, A–C), reflected by an increase in the ISI by 32.9% (P < 0.01; n = 11) (Fig. 4, A–C). In the majority of cells (n = 9), this effect was reversible upon washing out leptin, returning the ISI to control value (0.39 ± 0.07 sec; P < 0.05) (Fig. 4, A–C). To test the possibility that leptin regulates the electrical activity of npEW-LepRb neurons (see Discussion), we performed patch-clamp electrophysiology in acute brain slices with direct leptin administration. Our data demonstrates that leptin inhibits the neurons' electrical activity in approximately 80% of patched neurons. Of two cells only, the leptin-effect could not be reversed by leptin washout. In contrast to its effect on the ISI, leptin did never affect the CV (P > 0.05; n = 11) (Fig. 4D). The recorded cells likely represent Ucn1 producing neurons, because the immunohistochemistry data show that approximately 90% of EGFP neurons are Ucn1 neurons.
Fig. 4.
Cell-attached patch-clamp recordings from the npEW neurons in voltage-clamp mode. A, Example of one neuron responding to leptin and washout. B, Number of action current firing plotted with ISI of the neuron shown in A. C, Mean ISI increased by leptin application and reversed by washout. D, The CV-value is not affected by leptin treatment. *, P < 0.05; **, P < 0.01.
Discussion
Based on the expression of LepRb in the npEW (12, 13, 18), we hypothesized that the peripheral metabolic status would directly modulate the activity of the npEW via an action of leptin on LepRb of npEW-Ucn1 neurons by recruiting JAK-STAT signaling. Our findings on the mouse npEW support this hypothesis, for the following reasons: 1) LepRb is present in the npEW, and nearly all LepRb-containing neurons produce Ucn1; 2) administration of leptin either peripherally in vivo or directly onto the npEW in vitro causes pSTAT3 in npEW-Ucn1 neurons; 3) leptin administration significantly increases the abundance of Ucn1 in npEW-neurons; 4) LepRb-deficient (db/db) mice contain considerably less Ucn1 than WT mice; and 5) leptin acutely reduces the electrical activity of npEW-Ucn1 neurons. Together, the present results reveal that leptin directly targets npEW-Ucn1 neurons to modulate the activity of these neurons, at both the short-term (electrical response of Ucn1 neurons) and long-term (abundance of Ucn1 peptide in npEW neurons) level. Below, we will discuss these conclusions into detail.
LepRb occurs in npEW-Ucn1 neurons
First, we demonstrated the production of LepRb by npEW neurons by showing the expression of LepRb mRNA with in situ hybridization, which is in agreement with two recent, independent studies (12, 13). Next, we detected the LepRb by applying immunohistochemistry to a reporter mouse strain (LepRbEGFP mice) (19, 28, 45). Our finding that EGFP-labeled LepRb neurons occur in a characteristic, homogeneous, boomerang-shaped midbrain population of neurons, encompassing not only the npEW but also the rostral linear raphe, the VTA, and the substantia nigra pars compacta, is interesting, because these anatomically distinct neuronal subgroups all originate from the midbrain floor plate (46). This shared phenotype and common origin of these midbrain nuclei suggest an important coordinating role for leptin in the midbrain. Although leptin's action on VTA (47, 48) is well documented, little is known about the hormone's action on other midbrain LepRb expressing neurons and more in particular on LepRb expressing neurons in the npEW. Therefore, and in view of the dominant presence of Ucn1 in the npEW, we tested whether leptin would act on Ucn1 dynamics in this nucleus and found that almost all npEW-neurons that contain the LepRb also contain Ucn1 and that, conversely, 45% of Ucn1 neurons in the npEW produce LepRb. This situation is similar to that for other brain centers that are under physiological control by leptin. For example, 47% of the NPY-neurons in the arcuate nucleus and 60% of the dopaminergic neurons in the VTA express LepRb mRNA (47, 49). This analogy supports the general notion that even though brain centers may be anatomically distinctly structured, they may have many different functions, some of which are associated with similar functions of other brain centers, in this way establishing networks of collaborating neurons that serve the coordination of the various central regulations necessary to maintain physiological homeostasis.
Leptin recruits pSTAT3 in npEW neurons
In view of the capability of leptin to cross the blood-brain barrier in most parts of the brain (including the midbrain) (50) and the presence of leptin receptors throughout the brain (11–13), we tested whether leptin, by binding to LepRb, would be able to induce direct response on the npEW-Ucn1 neurons or whether additional input to these neurons from remote brain centers would be necessary for such leptin-mediated activation. For this purpose, we deprived the npEW from peripheral nervous input, by dissecting it out as a thin brain slice that was kept in vitro and treated with leptin. STAT3-tyrosine phosphorylation was used as a read-out parameter for npEW-Ucn1 neuron signal, because it represents response of the common leptin-signaling JAK-STAT pathway (7, 8, 31). Because we showed that acute leptin administration to such brain slices induced within 30 min STAT3 phosphorylation in many npEW-Ucn1-ir neurons, we conclude that leptin is indeed able to activate these neurons directly, without the support of neuronal input from remote brain centers. This finding is likely relevant for the in vivo situation as well, because we also found that systemic administration of leptin induces pSTAT3 in npEW-LepRb neurons. Our observation of leptin-induced recruitment of Ucn1 neurons in the npEW extends the recent report on leptin-induction of STAT3 phosphorylation in mouse npEW neurons (13). Because the JAK-STAT signaling pathway is a major signaling alternative to the second messenger system and transduces external neurochemical information to gene promoters on nuclear DNA (8), our data indicate that leptin may be involved in the control of (possibly Ucn1) gene expression and/or Ucn1 biosynthesis.
Leptin regulates npEW Ucn1 production
Although the administration of exogenous leptin often evokes only modest effects in normal animals, deficiency in leptin signaling (e.g. lack of leptin receptor) promotes marked alterations in neuronal function and mammalian physiology. We show that peripheral injection of leptin increases the abundance of Ucn1 within the npEW-Ucn1 neurons, whereas in db/db mice, the abundance was strongly decreased. These results clearly show that leptin regulates the Ucn1 content in npEW neurons and suggest that although short-term action of leptin (as mimicked by our injection study) evokes a moderate increase in cellular Ucn1 content, long-term absence of leptin signaling (as occurs in db/db mice) leads to a profound decrease in Ucn1 in the npEW.
Leptin inhibits electrical activity of npEW neurons
To test the possibility that leptin regulates the electrical activity of npEW-LepRb neurons, we performed patch-clamp electrophysiology in acute brain slices with direct leptin administration. Our data demonstrates that leptin inhibits the neurons' electrical activity in approximately 80% of patched neurons. This finding is consistent with the described action of leptin on the electrical activity of the VTA (47), the dorsal vagal nucleus (51), the premammillary nucleus of the hypothalamus (45), the lateral hypothalamic area (19), and the dorsal and ventral raphe nucleus (52). Based on the fact that approximately 90% of EGFP and pSTAT3 positive neurons are Ucn1 positive and the responses in the electrophysiological study, we argue that the observed effects of leptin are through a direct interaction of leptin via LepRb on the Ucn1 neurons, although we cannot exclude a (although not very likely) possible involvement of interneurons. The electrophysiological response of the mouse npEW after leptin administration indicates that rapid signaling downstream of LepRb is an important facet of leptin regulating the activity of npEW neurons. Meanwhile, leptin's ability to induce STAT3 activation supports the idea that the messenger may control long-term (e.g. transcriptional) events, such as (Ucn1) gene expression and Ucn1 biosynthesis as well.
Based on the anorexigenic action of centrally administered Ucn1, one would expect an increased release of Ucn1 from the npEW after leptin injection. Our results do not support this expectation but are well in line with the facts that lesioning the EW results in inhibition of food intake (14) and that decreased leptin by starvation increases Ucn1 mRNA in the rat npEW (18). In addition, high-fat diet decreases Ucn1 mRNA in the npEW too, concomitant with increased plasma leptin level (53). Furthermore, the physiological role(s) of a centrally administered neuropeptide has always been difficult to interpret. Ucn1 administered via an icv route has access to both corticotropin-releasing factor receptor subtypes, which could lead to differential (even opposing) behavioral, physiological, and neuroendocrine responses. Central injection of Ucn1 not only reduces food intake (23) but also increases energy expenditure (54), increases anxiety-like behavior (55–57), and even improves learning and memory (58), suggesting that the physiological role(s) of icv-injected Ucn1 will highly depend on its concentration, its site of action, and its binding with its cognate receptor(s). Taken together, we argue that a negative correlation exists between plasma leptin and Ucn1 specifically in the npEW.
Conclusion and possible functional consideration
The relationship between leptin and the regulation of short and long-term aspects of the activity of Ucn1 neurons in the npEW, as emanate from the present studies, support a notion that these neurons play an important role in the processing of information about the metabolic status of the animal. Interestingly, developmental studies of midbrain (dopaminergic) neurons strongly suggest a common origin for midbrain LepRb neurons, including the substantia nigra, VTA, rostral linear raphe nucleus, and the npEW (19, 46). Regulation of the mesolimbic dopamine system plays an important role in feeding behavior, as well as in reward processing (59–61), and metabolic status is an important modulator of such motivational behaviors. It has been suggested that leptin, a major signal of long-term energy balance, can modulate the response to food and nonfood rewards via the mesolimbic dopaminergic system (62). Although the VTA is a well-known center modulating reward processing (59), as well as expresses LepRb, in a recent study, Leshan et al. (63) have found that LepRb VTA neurons represent a subclass of VTA dopaminergic neurons that specifically innervate and control the central amygdala and send only scarce projections to the nucleus accumbens (63). This clearly indicates that yet another population(s) of midbrain LepRb neurons may play roles in mediating/controlling reward processing. In support of such action, particularly by the midbrain npEW-Ucn1/LepRb expressing neurons, are the facts that these neurons are directly regulated by leptin (this study) and that Ucn1's ability to reduce food intake was interestingly accompanied by a reduced motivation to eat (64). This intriguing (inter)action of npEW-Ucn1/LepRb is currently under investigation in our group.
Acknowledgments
We thank Dr. Christian Bjorbaeck (Beth Israel Deaconess Medical Center, Harvard University, Boston, MA) for the LepR cDNA.
This work was supported by The Netherlands Organization for Scientific Research Grant 864.05.008 (to T.K.) and the National Institutes of Health Grants DK078056 and DK057768 (to M.G.M.) and HD061539 (to C.F.E.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- Artificial cerebral spinal fluid
- CV
- coefficient of variation
- DEPC
- diethylpyrocarbonate
- EGFP
- enhanced green fluorescent protein
- EGFP-ir
- EGFP-immunoreactive
- GFAP
- glial fibrillary acidic protein
- icv
- intracerebroventricular
- IRES
- internal ribosomal entry site
- ISI
- interspike interval
- JAK2
- Janus kinase 2
- LepRb
- leptin receptor long form
- npEW
- nonpreganglionic Edinger-Westphal nucleus
- PFA
- paraformaldehyde
- pSTAT3
- phosphorylation of STAT3
- RNase
- ribonuclease
- SSC
- sodium chloride/sodium citrate
- SSD
- specific immunoreactive signal density
- STAT3
- signal transducer and activator of transcription 3
- Ucn1
- urocortin 1
- VTA
- ventral tegmental area
- WT
- wild type.
References
- 1. Arora S, Anubhuti 2006. Role of neuropeptides in appetite regulation and obesity—a review. Neuropeptides 40:375–401 [DOI] [PubMed] [Google Scholar]
- 2. Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. 2006. Central nervous system control of food intake and body weight. Nature 443:289–295 [DOI] [PubMed] [Google Scholar]
- 3. Dhillo WS. 2007. Appetite regulation: an overview. Thyroid 17:433–445 [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- 5. Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP. 1996. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495 [DOI] [PubMed] [Google Scholar]
- 6. Hegyi K, Fülöp K, Kovács K, Tóth S, Falus A. 2004. Leptin-induced signal transduction pathways. Cell Biol Int 28:159–169 [DOI] [PubMed] [Google Scholar]
- 7. Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC. 1996. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 93:6231–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Villanueva EC, Myers MG., Jr 2008. Leptin receptor signaling and the regulation of mammalian physiology. Int J Obes 32(Suppl 7):S8–S12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sahu A. 2004. Minireview: a hypothalamic role in energy balance with special emphasis on leptin. Endocrinology 145:2613–2620 [DOI] [PubMed] [Google Scholar]
- 10. Simerly RB. 2008. Hypothalamic substrates of metabolic imprinting. Physiol Behav 94:79–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. 1998. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- 12. Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. 2009. Leptin targets in the mouse brain. J Comp Neurol 514:518–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Caron E, Sachot C, Prevot V, Bouret SG. 2010. Distribution of leptin-sensitive cells in the postnatal and adult mouse brain. J Comp Neurol 518:459–476 [DOI] [PubMed] [Google Scholar]
- 14. Weitemier AZ, Ryabinin AE. 2005. Lesions of the Edinger-Westphal nucleus alter food and water consumption. Behav Neurosci 119:1235–1243 [DOI] [PubMed] [Google Scholar]
- 15. Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C. 1995. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotrophin-releasing factor. Nature 378:287–292 [DOI] [PubMed] [Google Scholar]
- 16. Kozicz T, Yanaihara H, Arimura A. 1998. Distribution of urocortin-like immunoreactivity in the central nervous system of the rat. J Comp Neurol 391:1–10 [DOI] [PubMed] [Google Scholar]
- 17. Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. 1999. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J Comp Neurol 415:285–312 [PubMed] [Google Scholar]
- 18. Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz T. 2009. Sex-specific effects of fasting on urocortin 1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience 162:1141–1149 [DOI] [PubMed] [Google Scholar]
- 19. Leinninger GM, Jo YH, Leshan RL, Louis GW, Yang H, Barrera JG, Wilson H, Opland DM, Faouzi MA, Gong Y, Jones JC, Rhodes CJ, Chua S, Jr, Diano S, Horvath TL, Seeley RJ, Becker JB, Münzberg H, Myers MG., Jr 2009. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab 10:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asakawa A, Inui A, Ueno N, Makino S, Fujino MA, Kasuga M. 1999. Urocortin reduces food intake and gastric emptying in lean and ob/ob obese mice. Gastroenterology 116:1287–1292 [DOI] [PubMed] [Google Scholar]
- 21. Wang L, Martínez V, Rivier JE, Taché Y. 2001. Peripheral urocortin inhibits gastric emptying and food intake in mice: differential role of CRF receptor 2. Am J Physiol Regul Integr Comp Physiol 281:R1401–R1410 [DOI] [PubMed] [Google Scholar]
- 22. Asakawa A, Inui A, Ueno N, Makino S, Fujimiya M, Fujino MA, Kasuga M. 2001. Urocortin reduces oxygen consumption in lean and ob/ob mice. Int J Mol Med 7:539–541 [DOI] [PubMed] [Google Scholar]
- 23. Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. 1996. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science 273:1561–1564 [DOI] [PubMed] [Google Scholar]
- 24. Ohata H, Suzuki K, Oki Y, Shibasaki T. 2000. Urocortin in the ventromedial hypothalamic nucleus acts as an inhibitor of feeding behavior in rats. Brain Res 861:1–7 [DOI] [PubMed] [Google Scholar]
- 25. Kotz CM, Wang C, Levine AS, Billington CJ. 2002. Urocortin in the hypothalamic PVN increases leptin and affects uncoupling proteins-1 and -3 in rats. Am J Physiol Regul Integr Comp Physiol 282:R546–R551 [DOI] [PubMed] [Google Scholar]
- 26. Pan W, Kastin AJ. 2008. Urocortin and the brain. Prog Neurobiol 84:148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pan W, Tu H, Hsuchou H, Daniel J, Kastin AJ. 2007. Unexpected amplification of leptin-induced Stat3 signaling by urocortin: implications for obesity. J Mol Neurosci 33:232–238 [DOI] [PubMed] [Google Scholar]
- 28. Myers MG, Jr, Münzberg H, Leinninger GM, Leshan RL. 2009. The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mao X, Fujiwara Y, Orkin SH. 1999. Improved reporter strain for monitoring Cre recombinase-mediated DNA excisions in mice. Proc Natl Acad Sci USA 96:5037–5042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Simmons DM, Arriza JL, Swanson LW. 1989. A complete protocol for in situ hybridization of messenger RNAs in brain and other tissues with radiolabelled single stranded RNA probes. J Histotechnol 12:169–181 [Google Scholar]
- 31. Münzberg H, Huo L, Nillni EA, Hollenberg AN, Bjørbaek C. 2003. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic proopiomelanocortin gene expression by leptin. Endocrinology 144:2121–2131 [DOI] [PubMed] [Google Scholar]
- 32. Paxinos G, Franklin KBJ. 2001. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press [Google Scholar]
- 33. Kimura N, Nishikawa S, Tamai M. 2000. Müller cells in developing rats with inherited retinal dystrophy. Tohoku J Exp Med 191:157–166 [DOI] [PubMed] [Google Scholar]
- 34. Ladyman SR, Grattan DR. 2004. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology 145:3704–3711 [DOI] [PubMed] [Google Scholar]
- 35. Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. 2003. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J Neurosci 23:2477–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaszner B, Jensen KO, Farkas J, Reglodi D, Csernus V, Roubos EW, Kozicz T. 2009. Effects of maternal separation on dynamics of urocortin 1 and brain-derived neurotrophic factor in the rat non-preganglionic Edinger-Westphal nucleus. Int J Dev Neurosci 27:439–451 [DOI] [PubMed] [Google Scholar]
- 37. Turnbull AV, Vaughan J, Rivier JE, Vale WW, Rivier C. 1999. Urocortin is not a significant regulator of intermittent electrofootshock-induced adrenocorticotropin secretion in the intact male rat. Endocrinology 140:71–78 [DOI] [PubMed] [Google Scholar]
- 38. Yang JH, Li LH, Lee S, Jo IH, Lee SY, Ryu PD. 2007. Effects of adrenalectomy on the excitability of neurosecretory parvocellular neurones in the hypothalamic paraventricular nucleus. J Neuroendocrinol 19:293–301 [DOI] [PubMed] [Google Scholar]
- 39. Cornelisse LN, Deumens R, Coenen JJ, Roubos EW, Gielen CC, Ypey DL, Jenks BG, Scheenen WJ. 2002. Sauvagine regulates Ca2+ oscillations and electrical membrane activity of melanotrope cells of Xenopus laevis. J Neuroendocrinol 14:778–787 [DOI] [PubMed] [Google Scholar]
- 40. Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality. Biometrika 52:591–599 [Google Scholar]
- 41. Snedecor GW, Cochran WG. 1989. Statistical Methods, Eighth Edition, Iowa State University Press, Ames, IA [Google Scholar]
- 42. Damiani CL, O'Callaghan JP. 2007. Recapitulation of cell signaling events associated with astrogliosis using the brain slice preparation. J Neurochem 100:720–726 [DOI] [PubMed] [Google Scholar]
- 43. Banks AS, Davis SM, Bates SH, Myers MG., Jr 2000. Activation of downstream signals by the long form of the leptin receptor. J Biol Chem 275:14563–14572 [DOI] [PubMed] [Google Scholar]
- 44. Robertson SA, Leinninger GM, Myers MG., Jr 2008. Molecular and neural mediators of leptin action. Physiol Behav 94:637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leshan RL, Louis GW, Jo YH, Rhodes CJ, Münzberg H, Myers MG., Jr 2009. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci 29:3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Joksimovic M, Anderegg A, Roy A, Campochiaro L, Yun B, Kittappa R, McKay R, Awatramani R. 2009. Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci USA 106:19185–19190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. 2006. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51:801–810 [DOI] [PubMed] [Google Scholar]
- 48. Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. 2006. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51:811–822 [DOI] [PubMed] [Google Scholar]
- 49. Baskin DG, Breininger JF, Schwartz MW. 1999. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48:828–833 [DOI] [PubMed] [Google Scholar]
- 50. Banks WA, Clever CM, Farrell CL. 2000. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab 278:E1158–E1165 [DOI] [PubMed] [Google Scholar]
- 51. Williams KW, Zsombok A, Smith BN. 2007. Rapid inhibition of neurons in the dorsal motor nucleus of the vagus by leptin. Endocrinology 148:1868–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yadav VK, Oury F, Suda N, Liu ZW, Gao XB, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. 2009. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138:976–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Legendre A, Papakonstantinou E, Roy MC, Richard D, Harris RB. 2007. Differences in response to corticotropin-releasing factor after short- and long-term consumption of a high-fat diet. Am J Physiol Regul Integr Comp Physiol 293:R1076–R1085 [DOI] [PubMed] [Google Scholar]
- 54. De Fanti BA, Martínez JA. 2002. Central urocortin activation of sympathetic-regulated energy metabolism in Wistar rats. Brain Res 930:37–41 [DOI] [PubMed] [Google Scholar]
- 55. Spiga F, Lightman SL, Shekhar A, Lowry CA. 2006. Injections of urocortin 1 into the basolateral amygdala induce anxiety-like behavior and c-Fos expression in brainstem serotonergic neurons. Neuroscience 138:1265–1276 [DOI] [PubMed] [Google Scholar]
- 56. Gehlert DR, Shekhar A, Morin SM, Hipskind PA, Zink C, Gackenheimer SL, Shaw J, Fitz SD, Sajdyk TJ. 2005. Stress and central Urocortin increase anxiety-like behavior in the social interaction test via the CRF1 receptor. Eur J Pharmacol 509:145–153 [DOI] [PubMed] [Google Scholar]
- 57. D'Anna KL, Stevenson SA, Gammie SC. 2005. Urocortin 1 and 3 impair maternal defense behavior in mice. Behav Neurosci 119:1061–1071 [DOI] [PubMed] [Google Scholar]
- 58. Telegdy G, Tiricz H, Adamik A. 2005. Involvement of neurotransmitters in urocortin-induced passive avoidance learning in mice. Brain Res Bull 67:242–247 [DOI] [PubMed] [Google Scholar]
- 59. Saper CB, Chou TC, Elmquist JK. 2002. The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211 [DOI] [PubMed] [Google Scholar]
- 60. Kelley AE. 2004. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron 44:161–179 [DOI] [PubMed] [Google Scholar]
- 61. Volkow ND, Wise RA. 2005. How can drug addiction help us understand obesity? Nat Neurosci 8:555–560 [DOI] [PubMed] [Google Scholar]
- 62. Cota D, Barrera JG, Seeley RJ. 2006. Leptin in energy balance and reward: two faces of the same coin? Neuron 51:678–680 [DOI] [PubMed] [Google Scholar]
- 63. Leshan RL, Opland DM, Louis GW, Leinninger GM, Patterson CM, Rhodes CJ, Münzberg H, Myers MG., Jr 2010. Ventral tegmental area leptin receptor neurons specifically project to and regulate cocaine- and amphetamine-regulated transcript neurons of the extended central amygdala. J Neurosci 30:5713–5723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kinney JW, Scruggs B, Avery DD. 2001. Peripheral administration of urocortin suppresses operant responding for food reward. Peptides 22:583–587 [DOI] [PubMed] [Google Scholar]