Mice with combined gene deletion demonstrate that iodothyronine deiodinases (D1 and D2) are important in the manifestations and levels of impairment caused by Mct8 deficiency.
Abstract
Mice deficient in the thyroid hormone (TH) transporter Mct8 (Mct8KO) have increased 5′-deiodination and impaired TH secretion and excretion. These and other unknown mechanisms result in the low-serum T4, high T3, and low rT3 levels characteristic of Mct8 defects. We investigated to what extent each of the 5′-deiodinases (D1, D2) contributes to the serum TH abnormalities of the Mct8KO by generating mice with all combinations of Mct8 and D1 and/or D2 deficiencies and comparing the resulting eight genotypes. Adding D1 deficiency to that of Mct8 corrected the serum TH abnormalities of Mct8KO mice, normalized brain T3 content, and reduced the impaired expression of TH-responsive genes. In contrast, Mct8D2KO mice maintained the serum TH abnormalities of Mct8KO mice. However, the serum TSH level increased 27-fold, suggesting a severely impaired hypothalamo-pituitary-thyroid axis. The brain of Mct8D2KO manifested a pattern of more severe impairment of TH action than Mct8KO alone. In triple Mct8D1D2KO mice, the markedly increased serum TH levels produced milder brain defect than that of Mct8D2KO at the expense of more severe liver thyrotoxicosis. Additionally, we observed that mice deficient in D2 had an unexplained marked reduction in the thyroid growth response to TSH. Our studies on these eight genotypes provide a unique insight into the complex interplay of the deiodinases in the Mct8 defect and suggest that D1 contributes to the increased serum T3 in Mct8 deficiency, whereas D2 mainly functions locally, converting T4 to T3 to compensate for distinct cellular TH depletion in Mct8KO mice.
Thyroid hormones (TH) are essential for the development of the central nervous system and for peripheral tissue metabolism in all mammals. The predominant form, synthesized and secreted by the thyroid gland, is T4. Yet the biological effect depends mainly on its activation by removal of one outer ring iodine to form T3. Vertebrates have developed a series of specific mechanisms to maintain appropriate tissue levels of active TH. These mechanisms include regulation of TH synthesis and secretion (1), TH transport across the cell membrane (2), and intracellular TH metabolism, mainly through stepwise deiodination (3). Three deiodinases, D1, D2, and D3, play important roles in regulating local tissue T3 availability in a time- and cell-specific fashion (4). Both D1 and D2 catalyze T4 conversion to T3 through 5′ deiodination, whereas D3 inactivates TH through 5 deiodination, converting T4 to rT3 and T3 to 3,3′-diiodothyronine. D1 is predominantly expressed in liver, kidney, and thyroid and contributes to the plasma T3 in euthyroid and hyperthyroid states. D2, on the other hand, generates T3 mostly for local use at sites such as brain, pituitary, and brown adipose tissue. It is the key enzyme that maintains brain TH homeostasis during hypothyroidism and participates in the feedback system at the hypothalamo-pituitary-thyroid (HPT) axis (3, 4).
The recent identification of several classes of TH cell membrane transporters (2) indicated that the entry of TH into cells is an active process involving specific transporters. The monocarboxylate transporter 8 (MCT8), encoded by a gene located on the X-chromosome, was characterized to be a specific TH transporter (5). Patients deficient in MCT8 present a unique clinical phenotype: abnormal serum TH levels (increased serum T3, decreased T4 and rT3) associated with slightly elevated or normal TSH concentration and severe psychoneuromuscular deficits (6–8). Investigation of mice deficient in Mct8 (Mct8KO) revealed a similar thyroid phenotype but without any obvious neurological deficit (9, 10). Brains of these mice showed low T3 uptake, decreased T3 content, increased D2 activity, and decreased D3 activity, reflecting the impaired TH transport and TH depletion in this tissue. This impairment of TH entry into brain, including the hypothalamus, maintains elevated serum TSH concentrations despite high serum T3 level. Furthermore, Mct8KO mice exhibit more impairment of T3 than T4 feedback regulation of the HPT axis (9–11). In contrast, the liver has normal T3 uptake, increased T3 content, and high D1 activity, indicating that alternative transporters could compensate for the lack of Mct8, resulting in a hyperthyroid state (9, 10). Kidneys of Mct8KO mice have enhanced T4 and T3 uptake and increased D1 activity, indicating high T4 metabolism and loss of TH in the urinary tract (12). Recent studies also demonstrated that the impaired thyroidal TH secretion in these mice not only contributed to the low serum T4 but also produced thyroidal TH retention (13, 14). These findings set up the Mct8KO mouse as a unique model of coexisting tissue-specific TH excess and deficiency.
Nevertheless, findings in the Mct8KO mouse leave many questions unanswered. What roles do changes in deiodinases activity have on the phenotype of these mice? More specifically, how does TH transport interplay with modifications in deiodinase activity? Because the activities of both 5′ deiodinases are increased in these mice, one might ask are these changes beneficial compensatory responses or detrimental consequences resulting from impaired TH entry into cells? Finally, which of the two deiodinases contributes most to the high serum T3 level in Mct8 deficiency?
In this report we present the phenotypes of mice that we recently generated with combined deficiencies in D1 and Mct8, D2 and Mct8, and D1, D2, and Mct8. We found that D1 and D2 contribute in unique ways to the deficits in TH homeostasis of the Mct8KO mouse because their action or inaction appears to correct or exacerbate some of those defects. Our results provide novel insights into the physiological significance of 5′ deiodination and TH transport and underscore the important interplay between these factors to deliver appropriate levels of TH to the brain and peripheral tissues.
Materials and Methods
Generation and maintenance of knockout (KO) mice
Combined D1 and D2 KO (D1−/−/D2−/−) and Mct8 deficient (Mct8-/y) mice were generated as described (9, 15). All KO mice were backcrossed more than 10 times with the wild-type (Wt) C57BL/6 strain. The details of mice mating and genotyping are provided in supplemental data.
The strategy for maintaining the experimental genotypes is shown in Table 1. The combined KO male mice used for this study are: 1) wild type (Mct8+/y/D1+/+/D2+/+, abridged Wt); 2) Mct8KO (Mct8−/y, abridged M8KO); 3) D1KO (D1−/−); 4) Mct8 and D1 KOs (Mct8−/yD1−/−, abridged M8D1KO); 5) D2KO (D2−/−); 6) Mct8 and D2 KOs (Mct−/yD2−/−, abridged M8D2KO); 7) D1 and D2 KOs (D1−/−D2−/−, abridged D1D2KO); and 8) Mct8, D1, and D2 KOs (Mct8−/yD1−/−D2−/−, abridged M8D1D2KO).
Table 1.
Strategy for the generation of the eight genotypes used in this publication, litter size, and Mendelian genotype ratio
| Mating |
Litter size Pups/litter (n) | Mendelian ratio |
|||
|---|---|---|---|---|---|
| Mother | Father | Offspring | Expected (%) | Observed (%) | |
| Mct8+/− | Mct8+/y | 8.07 ± 0.45 | Male Mct8+/y (Wt)a | 2550 | 23.14 |
| Male Mct8−/y (M8KO)a | 25 | 23.14 | |||
| Female Mct8+/+ and Mct8+/− | 50 | 53.72 | |||
| Mct8+/−D1−/− | Mct8+/yD1−/− | 7.67 ± 0.35 | Male Mct8+/yD1−/− (D1KO)a | 25 | 29.56 |
| Male Mct8−/yD1−/− (M8D1KO)a | 25 | 25.22 | |||
| Female Mct8+/+D1−/− and Mct8+/−D1−/− | 50 | 45.22 | |||
| Mct8+/−D2−/− | Mct8+/yD2−/− | 8.67 ± 0.42 | Male Mct8+/yD2−/− (D2KO)a | 25 | 28.46 |
| Male Mct8−/yD2−/− (M8D2KO)a | 25 | 23.08 | |||
| Female Mct8+/+D2−/− and Mct8+/−D2−/− | 50 | 48.46 | |||
| Mct8+/−D1−/−D2−/− | Mct8+/yD1−/−D2−/− | 7.67 ± 0.57 | Male Mct8+/yD1−/−D2−/− (D1D2KO)a | 25 | 20.00 |
| Male Mct8−/yD1−/−D2−/− (M8D1D2KO)a | 25 | 25.22 | |||
| Female Mct8+/+D1−/−D2−/− and Mct8+/−D1−/−D2−/− | 50 | 54.78 | |||
Fifteen litters were tested in each mating. The litter size is expressed as mean ± se.
Genotypes used in these studies.
Experimental animals and treatment
Mice were housed in a controlled environment at 22 ± 2 C and 12-h alternating dark and 12-h light cycles. They were fed Purina Rodent Chow (0.8 ppm iodine; Purina Mills, St. Louis, MO) and water ad libitum. All studies were performed using male mice and a protocol approved by the Institutional Animal Care and Use Committee. Mice were weighed and their length (nose to base of the tail) measured weekly from 4 to 12 wk of age. Unless specifically indicated, experiments were performed on adult mice aged 90–100 d [postnatal day (P) 90–100]. All animals were humanely euthanized. Tissues were immediately dissected, placed on dry ice, and then stored at −80 C. Serum was stored at −20 C.
To assess the TH-responsive genes in mouse cerebrum under hypothyroid or hyperthyroid states, Wt mice were fed low iodine diet (LoI; Harlan Teklad Co., Madison, WI) and given 0.02% methimazole (MMI) and 0.5% perchlorate in the drinking water (LoI/MMI/ClO4) for 4 wk. During the last 7 d, while maintaining LoI/MMI/ClO4, animals were given daily ip injections of PBS (hypothyroid) or 10 μg l-T4 per 100 g body weight (hyperthyroid). The experiment was terminated 16 h after the last injection with blood samples and tissues collection.
Measurements in serum
Serum total T4, T3, rT3, and TSH concentrations were measured using RIAs as previously described (9, 16). Cholesterol and alkaline phosphatase (AP) were measured each on 10 μl serum using a clinical chemistry autoanalyzer. Isoenzymes of AP were measured by electrophoresis on agarose gels (hydragel ISO-PAL procedure; Sebia, Inc., Norcross, GA) on serum pooled from several animals per group.
Bioactivity of serum TSH was determined as previous reported (17, 18) by measurement of cAMP generation after the addition of the serum sample to the medium of cultured Chinese hamster ovary cells, stably transfected with a TSH receptor cDNA. The final TSH bioactivity is expressed as the ratio of cAMP generation to the amount of immunoreactive TSH.
Measurement of specific gene mRNA expression in tissues
Total RNA extraction from tissue, RT and the mRNA quantification by real-time quantitative PCR followed procedures described (9). Results are expressed relative to those in the Wt mice and normalized for RNA polymerase II mRNA (19).
Determination of deiodinase enzymatic activities
D2 and D1 enzymatic activities were measured as described (20, 21). These were expressed in femtomoles (D2) or picomoles (D1) of labeled iodide generated per hour per milligram of protein and were corrected for nonenzymatic deiodination observed in the tissue-free controls. Activities were also checked in tissues collected from the corresponding deiodinase deficient mice.
D3 enzymatic activity was measured as previously described (22). Deiodination was determined based on the amount of [125I]-3,3′-diiodothyronine produced after separation of reaction products by paper chromatography.
Tissue T4 and T3 content
To measure TH content in tissues, blood was removed by perfusion with heparinated PBS through a needle placed in the left cardiac ventricle. The extraction and determination of TH in brain and liver and thyroglobulin (Tg) TH and non-Tg TH in thyroid were performed as previously described (9, 13, 23, 24).
Histological analysis
Thyroid glands attached to the trachea were collected from adult mice, placed in formalin, and embedded in paraffin. Sections were stained with hematoxylin/eosin. Quantitative analysis of thyroid follicles was performed using NIH Image J software (http://rsb.info.nih.gov/ij/) on two randomly selected slides per sample and two thyroid samples per group. For each slide 50 thyroid follicles were analyzed as described in a previous report (25).
Statistics
Statistical analysis of multiple groups used ANOVA with Fisher's protected least significant differences. Statistical difference between two groups was determined using a Student's t test. Data are presented as mean ± se. P ≥ 0.05 was considered not significant (ns). Logarithmic transformation of data were used when means for different groups varied by more than 20-fold and sometimes by 1000-fold (see TSH).
Results
Litter size and growth (weight and length)
Mating female Mct8+/− with male Mct8+/y having the same corresponding deiodinase KO background generated the eight different genotypes in male mice. All mice were viable and fertile with no overt somatic defects and no obvious motor or behavioral abnormalities. As shown in Table 1, pups maintained correct Mendelian ratios. Litters were of similar sizes, with no significant difference compared with Wt mice mating of the same strain in our laboratory (26).
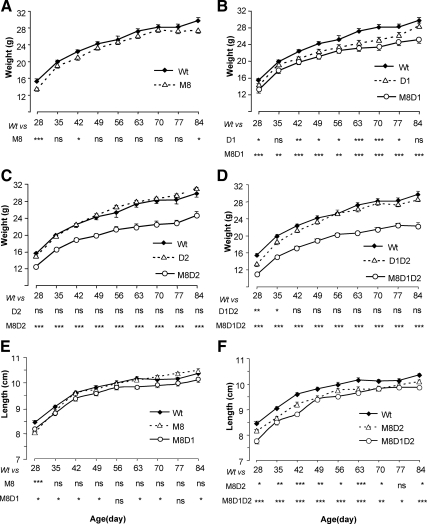
The body weight of M8KO mice from 28 to 84 d of age tended to be lower compared with Wt mice, although this was statistically significant at only few time points (Fig. 1A). When Mct8 deficiency was combined with that of deiodinases, weights were significantly lower at all data points: lower by 11–15, 16–20, and 19–25% in M8D1KO, M8D2KO, and M8D1D2KO mice, respectively, relative to Wt (Fig. 1, B–D). Of note is that D1KO (Fig. 1B), but not D2KO and D1D2KO, had significantly reduced body weight compared with Wt. The body length of M8KO mice was comparable with that of Wt mice. However, the combination of Mct8 deficiency with D1, D2, or D1D2 deficiencies produced significantly shorter mice. The decreases were minimal for M8D1KO (1.9–3.2%), slightly greater for M8D2KO (2.5–4.3%), and more than 8% for M8D1D2KO mice at early age (28–42 d), with a slight catch-up growth thereafter, resulting in only 3–4% lower length than Wt mice (Fig. 1, E and F). The lengths of D1KO, D2KO, and D1D2KO were comparable with Wt except that D1KO had a transient decrease around 60 d (data not shown).
Fig. 1.
Growth curves for mice of the eight genotypes under investigation. Panels A through D show weights and E and F show lengths. Weights in each panel compare Wt to M8KO mice and with those deficient in D1, D2, or D1 and D2 in the presence and absence of Mct8. Length curves are shown only for the genotypes with Mct8 deficiency in comparison with the Wt mice. Results are expressed as mean ± se for nine to 13 animals for each data point. Statistical differences from the Wt mice are shown for each data point and in each genotype below the x-axis: *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant. Genotype nomenclatures are abridged as follows: M8, M8KO; D1, D1KO; M8D1, M8D1KO; D2, D2KO; M8D2, M8D2KO; D1D2, D1D2KO; and M8D1D2, M8D1D2KO.
Serum thyroid hormones and TSH levels
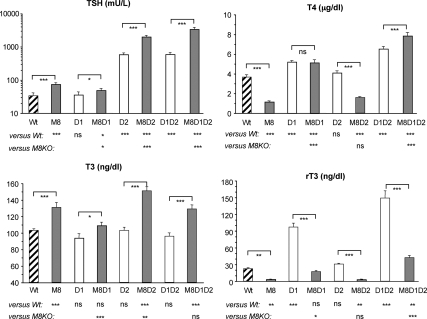
Serum TH and TSH concentrations were measured in mice from all eight genotypes at P90–100, and data are shown in Fig. 2. The M8KO mice maintained their typical thyroid phenotype including high T3, low T4, low rT3, and a slightly elevated TSH (9, 10). In contrast, removing D1 from M8KO mice resulted in increased T4 to the level similar to that of D1KO mice (5.1 ± 0.3 vs. 5.2 ± 0.2 μg/dl), the serum T3 concentration normalized to the level of the Wt animals (109 ± 4 vs. 103 ± 2 ng/dl) but was significantly higher than that of their D1KO littermates (94 ± 5 ng/dl). The rT3 also normalized. The T3 to T4 ratio declined (21.2 vs. 113.4 of M8KO) and became comparable with that of Wt mice (27.8). The T3 to rT3 ratio was also reduced (6.2 vs. 40.7 of M8KO) and became comparable with the Wt mice (4.6). We also observed reduced serum TSH in M8D1KO mice compared with M8KO mice (49 ± 7 vs. 75 ± 8 mU/liter, P < 0.05), although it did not normalize to the level of the Wt and D1KO mice (34 ± 7 and 36 ± 8 mU/liter, respectively), implying that some level of impairment at the HPT axis persisted.
Fig. 2.
TSH, T4, T3, and rT3 concentrations in serum of mice of the eight genotypes under investigation. Results are expressed as mean ± se for 12–16 mice per group. Statistical differences between Wt and all genotypes of mice and between M8KO and all other three genotypes with Mct8 deficiency are shown below the x-axis; differences between littermates, with and without Mct8 deficiency, are indicated above the bars. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant. Genotype nomenclatures are abridged as follows: M8, M8KO; D1, D1KO; M8D1, M8D1KO; D2, D2KO; M8D2, M8D2KO; D1D2, D1D2KO; and M8D1D2, M8D1D2KO.
The D2KO mice had a 17-fold elevation of serum TSH (584 ± 76 vs. 34 ± 7 mU/liter of Wt). The addition of Mct8 deficiency produced a further increase in TSH level (2005 ± 211 mU/liter) to be 59-fold that of the Wt. Serum T3 in M8D2KO mice was significantly higher than in M8KO mice (151 ± 5 vs. 131 ± 6 ng/dl, P < 0.01) and was the highest of all eight genotypes. Serum T4 and rT3 were maintained at the lower level similar to that of M8KO (T4, 1.6 ± 0.1 vs. 1.2 ± 0.1 μg/dl; rT3, 3.1 ± 0.4 vs. 3.2 ± 0.4 ng/dl).
The triple KO mice, M8D1D2KO, presented with dramatically high serum TSH level (3410 ± 440 mU/liter), 100-fold that of the Wt and 6-fold that of D1D2KO (598 ± 73 mU/liter) but not significantly different than M8D2KO mice (2005 ± 211 mU/liter). Both serum T4 and T3 concentrations in M8D1D2KO mice were elevated (7.9 ± 0.3 μg/dl and 129 ± 5 ng/dl, respectively), whereas rT3 (42 ± 4 ng/dl) was significantly lower than that of their littermate D1D2KO mice (149 ± 13 ng/dl). A similar pattern of rT3 reduction was observed in M8KO compared with Wt mice, M8D1KO vs. D1KO and M8D2KO vs. D2KO mice. This decrease in serum rT3 in Mct8 deficient mice could be due to impaired access of T4 to D3-containing cells such as those in brain.
Effect on brain (cerebrum)
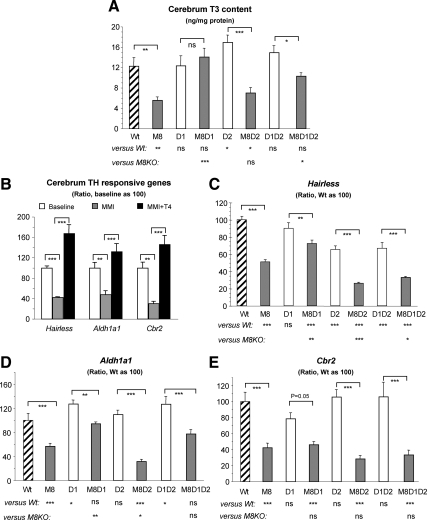
We measured the T3 content in cerebrum, and data are shown in Fig. 3A. M8KO mice had a 50% decrease in T3 content compared with Wt mice. Interestingly, this decrease was abolished in the M8D1KO mice. Relative to the M8KO mice, a comparable T3 level was found in the M8D2KO (7.0 ± 3.3 vs. 5.6 ± 2.0 pg/mg protein of M8KO), whereas it was significantly increased in the M8D1D2KO (10.3 ± 1.9 pg/mg protein). In addition, a slightly elevated and normal T3 content were presented in D2KO and D1D2KO mice, respectively.
Fig. 3.
Effect of genotype on brain (cerebrum) T3 content and TH action. A, T3 content for nine to 13 mice per group. B, Expression of TH-responsive genes relative to baseline in Wt mice deprived of TH [in LoI/MMI/ClO4 treatment (MMI)] and treated with 10 μg l-T4 per 100 g body weight (MMI+T4). There were five to six animals per group and statistical differences are indicated above the bar. Hairless (C), Aldh1a1 (D), and Cbr2 (E) mRNA levels are shown relative to Wt mice. Results are expressed as mean ± se for six to seven mice per group. Statistical differences between Wt and all genotypes of mice and between M8KO and all other three genotypes with Mct8 deficiency are shown below the x-axis; differences between littermates, with and without Mct8 deficiency, are indicated above the bars. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant. Genotype nomenclatures are abridged as follows: M8, M8KO; D1, D1KO; M8D1, M8D1KO; D2, D2KO; M8D2, M8D2KO; D1D2, D1D2KO; and M8D1D2, M8D1D2KO.
D2 and D3 enzymatic activities were measured, and results are presented in Table 2. In agreement with the tissue T3 content, a 12-fold increase of D2 activity was found in M8KO mice, and that in M8D1KO mice was intermediate to the Wt and M8KO, being 7-fold higher than Wt. All seven single and combined KO mice had slightly decreased D3 activity compared with the Wt, but it was lowest in the M8KO mice. D2 and D3 mRNA expression were also measured in the same mice (data not shown). The results followed the trend of activity, except the changes in D2 activity were of greater magnitude compared with the mRNAs, supporting the notion that the up-regulation of D2 in Mct8 deficiency is mainly posttranscriptional.
Table 2.
D1, D2, and D3 enzymatic activities in brain (cerebrum) and liver tissues
| Genotype | Cerebrum D2 (fmol/h·mg protein) | Cerebrum D3 (fmol/h·mg protein) | Liver D1 (pmol/h·mg protein) |
|---|---|---|---|
| Wt | 31.8 ± 3.4 | 3685 ± 139 | 59.2 ± 2.4 |
| M8KO | 392.4 ± 8.2a | 2257 ± 403a | 99.9 ± 2.6a |
| D1KO | 31.2 ± 3.0 | 2970 ± 209b | NA |
| M8D1KO | 227.0 ± 12.5a | 2733 ± 104c | NA |
| D2KO | NA | 2927 ± 56b | 55.9 ± 2.9 |
| M8D2KO | NA | 2815 ± 466b | 99.8 ± 7.0a |
| D1D2KO | NA | 2797 ± 134c | NA |
| M8D1D2KO | NA | 2584 ± 110c | NA |
Data are expressed as mean ± se for five to seven mice in each group. NA, Not applicable.
P < 0.001, significant differences in comparison with the Wt.
P < 0.05, significant differences in comparison with the Wt.
P < 0.01, significant differences in comparison with the Wt.
We next performed quantitative PCR to further investigate TH action in brain. Three gene markers were chosen, namely, hairless, aldehyde dehydrogenase 1a1 (Aldh1a1) and carbonyl reductase 2 (Cbr2). The latter two were recently identified to be TH responsive in hypothyroid pups at P21 (27). To verify whether these genes remain useful markers of TH action in older mice, we examined the expressions of these genes in adult Wt mice untreated (baseline), TH deprived (hypothyroid), and treated with 10 μg l-T4 per 100 g body weight (hyperthyroid). Results showed that all three genes had 30–48% decreases during TH depletion and 2.7- to 4.8-fold increases after l-T4 treatment (Fig. 3B), confirming that these were sensitive markers for TH effect in adult brain. We next measured their respective mRNAs in cerebra of all genotypes. Compared with expression in Wt mice, all Mct8-deficient mice showed a significant decrease in hairless gene expression, M8D1KO being the mildest, M8KO and M8D1D2KO being the intermediate, and M8D2KO being the most severe decline (28, 49, 67, and 74%, respectively; Fig. 3C). About a 34% decrease in hairless expression was also observed in D2KO and D1D2KO mice. A significant decrease of Aldh1a1 gene expression relative to that in Wt mice was found in M8KO and M8D2KO mice but not in M8D1KO and M8D1D2KO mice (Fig. 3D). A slight but significant (P < 0.05) elevation of Aldh1a1 mRNA levels was found in D1KO and D1D2KO mice. A significant decrease in Cbr2 gene expression (Fig. 3E) was noted in all Mct8-deficient mice (M8KO, M8D1KO, M8D2KO, and M8D1D2KO) but not in the mice lacking D1 and/or D2.
Effects on liver
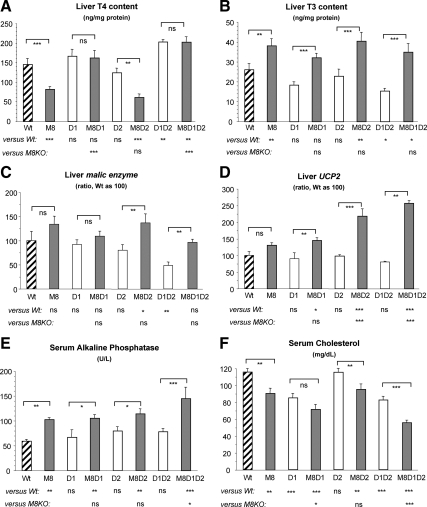
Data on liver T4 and T3 content are shown in Fig. 4, A and B. In general and for all genotypes, liver T4 and T3 content followed the concentrations observed in serum, indicating that TH uptake by liver is less dependent on Mct8.
Fig. 4.
Effect of genotype on liver TH content and action. T4 content (A) and T3 content (B) for nine to 13 animals per group are shown. Liver ME (C) and UCP2 (D) mRNA levels relative to values of Wt mice are shown. Serum AP (E) and cholesterol (F) concentration in five to six mice per group are shown. Results are expressed as mean ± se. Statistical differences between Wt and all genotypes of mice and between M8KO and all other three genotypes with Mct8 deficiency are shown below the x-axis; differences between littermates, with and without Mct8 deficiency, are indicated above the bars. *, P < 0.05; **, P < 0.01; ***, P < 0.001. ns, Not significant. Genotype nomenclatures are abridged as follows: M8, M8KO; D1, D1KO; M8D1, M8D1KO; D2, D2KO; M8D2, M8D2KO; D1D2, D1D2KO; and M8D1D2, M8D1D2KO.
We tested several well-documented markers to evaluate effect of TH on liver. These included hepatic D1 and malic enzyme (ME) and serum AP and cholesterol. The uncoupling protein-2 (UCP2) mRNA, known to be stimulated by TH in several tissues (28, 29), was also up-regulated by TH in liver (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). As expected on the basis of the tissue T3 content (Fig. 4B), compared with Wt mice, D1 enzymatic activity was increased by 68–69% in both M8KO and M8D2KO mice, whereas it was not different in the D2KO mice (Table 2). D1 mRNA expression shared the same pattern as the activity and is therefore not shown. When Mct8-deficient mice were compared with their Mct8-intact littermates (M8KO vs. Wt, M8D1KO vs. D1KO, M8D2KO vs. D2KO, and M8D1D2KO vs. D1D2KO), the absence of Mct8 produced a relative increase in liver ME and UCP2 expression (Fig. 4, C and D) and serum AP (Fig. 4E) concentration, whereas the serum cholesterol level was decreased (Fig. 4F). Most of the differences were statistically significant. These results are in agreement with the higher liver T3 content producing a hyperthyroid state in the liver of Mct8-deficient mice. It is of interest to note that in the absence of both deiodinases, M8D1D2KO, the effects of TH excess were more severe than deiodinases-intact M8KO mice (a 97% increase of UCP2 mRNA, 41% increase of serum AP, and 38% decrease of cholesterol). In contrast, and with the exception of the 67% increase in UCP2 expression, M8D2KO mice showed a similar degree of alteration as the M8KO mice. M8D1KO mice showed lesser increased ME expression compared with M8KO mice; however, there was no difference in the UCP2 expression and serum AP. Analysis of serum isoenzymes of AP in Wt, M8KO, and M8D1D2KO mice revealed that both liver and bone isoenzyme increases contributed to the total serum AP increase (Supplemental Table 1). Surprisingly, the decrease in serum cholesterol occurred not only in Mct8-deficient but also in D1-deficient mice, and its combination had an additive effect as seen in M8D1KO and M8D1D2KO.
Effects on thyroid: thyroid weight, TH content, histology, and serum TSH bioactivity
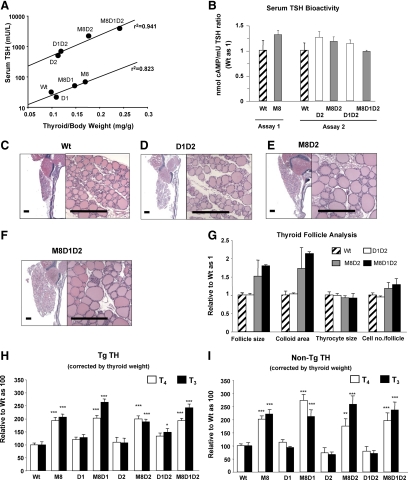
After correction for body weight, the thyroid weights of Wt, D1KO, D2KO, and D1D2KO mice were comparable without significant difference (0.099 ± 0.009, 0.109 ± 0.008, 0.115 ± 0.008, and 0.120 ± 0.010 mg/g, respectively, n = 7–9). All genotypes with Mct8 deficiency had significantly higher thyroid weights compared with Wt mice and all corresponding littermates with intact Mct8 (P < 0.001). M8D1KO mice showed the smallest increase, M8KO and M8D2KO mice intermediate, and M8D1D2KO mice the biggest increase (0.147 ± 0.005, 0.170 ± 0.003, 0.177 ± 0.005, and 0.241 ± 0.017 mg/g, respectively, n = 6–9). Comparison of serum TSH with thyroid gland size revealed two independent exponential correlation curves, those of mice with intact D2 locus (Wt, M8KO, D1KO, and M8D1KO) and those with D2 deficiency (D2KO, D1D2KO, M8D2KO, and M8D1D2KO) (Fig. 5A). Both curves showed tight correlations and had parallel slopes. The difference was the 19- to 25-fold higher TSH in D2 deficient mice for the same degree of increase in gland size. Because of this discrepancy, we measured the bioactivity of serum TSH in six genotypes, two without and four with D2 deficiency. No difference in bioactivity relative to immunoactivity was observed in any of these genotypes (Fig. 5B).
Fig. 5.
Effect of genotype on thyroid gland size, histology, and thyroidal TH content. A, Correlation of serum TSH concentration and thyroid gland weight corrected by body weight in the eight genotypes of mice under investigation. The mouse genotypes are indicated next to each data point. Data generated two exponential correlations are as follows: one involving mice with intact D2 (Wt, M8KO, D1KO, and M8D1KO), y = 6.1106 × 106.0151x (r2 = 0.823) and another involving mice with D2 deficiency (D2KO, M8D2KO, D1D2KO, and M8D1D2KO), y = 98.311 × 106.8113x (r2 = 0.941). There is a 19- to 25-fold difference in TSH for the same increase of thyroid gland size between mice with and without D2. B, Serum TSH bioactivity in different mouse genotypes (Wt, M8KO, D2KO, M8D2KO, D1D2KO, and M8D1D2KO). Activities were measured in six animals from each genotype and two independent assays. Data are expressed as the ratio of cAMP production relative to TSH immunoactivity, adjusted to unity for Wt mice. C–F, Low- (left panel) and high- (right panel) power views of hematoxylin/eosin-stained thyroid gland sections from Wt, D1D2KO, M8D2KO, and M8D1D2KO mice aged 90–100 d. Scale bar, 300 μm. G, Morphometric analysis of corresponding stained sections showing average thyroid follicle size, colloid area, thyrocyte size, and thyroid cell numbers per follicle. Tg TH (TH content within the Tg molecule) (H) and non-Tg TH (TH in thyroid gland not within the Tg molecule) (I) are shown after correction for thyroid weight and relative to those in Wt mice as 100. In Wt mice, Tg T4 and T3 were 232.2 ± 16.2 and 20.3 ± 2.5 ng/mg of thyroid weight, respectively; and non-Tg T4 and T3 were 3.35 ± 0.22 and 0.146 ± 0.017 ng/mg of thyroid weight, respectively. Results are expressed as mean ± se for six to nine mice per group. Significant differences for each genotype compared with Wt are presented above the bars. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Genotype nomenclatures are abridged as follows: M8, M8KO; D1, D1KO; M8D1, M8D1KO; D2, D2KO; M8D2, M8D2KO; D1D2, D1D2KO; and M8D1D2, M8D1D2KO.
We compared the thyroid gland histology of two mouse genotypes with increased thyroid gland size (M8D2KO and M8D1D2KO) (Fig. 5, E and F) with that of two mouse genotypes with thyroid glands of normal size (Wt and D1D2KO) (Fig. 5, C and D). Quantitative analysis showed that M8D2KO and M8D1D2KO mice had a 50–100% increase of follicle size and colloid area above measurements in the Wt mice, no change of thyrocyte size, and slight elevation in thyrocyte number per follicle (Fig. 5G). The D1D2KO were comparable with Wt mice in all histological parameters listed above. The histological study of M8KO mice was reported elsewhere (13).
The thyroidal TH content, both Tg and non-Tg TH, was measured in all eight genotypes of mice (Fig. 5, H and I). All Mct8-deficient mice showed a significant increase in T4 and T3 content, both as Tg TH (1.5- to 2.6-fold) and non-Tg TH (2- to 3.8-fold) relative to the Wt and their Mct8-sufficient littermates (P < 0.001). No significant difference was found in D1KO, D2KO, and D1D2KO relative to Wt mice, except for the slight increase of Tg T3 in D1D2KO mice.
Discussion
The aim of this study was to gain further insight into the pathophysiology of the complex phenotype of Mct8 deficiency, in particular, to understand how specific types of deiodinases interplay with TH transport to modify cellular TH bioavailability and the phenotype of Mct8 deficiency. To this purpose, we generated mice with combined Mct8 and deiodinase deficiencies (M8D1KO, M8D2KO, and M8D1D2KO). A summary of the main findings is shown in Table 3.
Table 3.
Summary of findings in Mct8-deficient mice
| M8KO | M8D1KO | M8D2KO | M8D1D2KO | |
|---|---|---|---|---|
| Serum tests of thyroid function | ||||
| TSH | ↑ | NL to ↑ | ↑↑ | ↑↑ to↑↑↑ |
| T4 | ↓ | ↑ | ↓ | ↑↑ |
| T3 | ↑ | NL | ↑↑ | ↑ |
| rT3 | ↓ | NL | ↓ | ↑ |
| Growth | NL to ↓ | ↓ | ↓↓ | ↓↓ |
| Brain TH action | ↓↓ | ↓ | ↓↓↓ | ↓↓ to ↓↓↓ |
| Liver TH action | ↑ | NL to ↑ | ↑ to↑↑ | ↑↑ |
| Thyroid weight | ↑↑ | ↑ | ↑↑ | ↑↑↑ |
Results are relative to those in Wt mice. NL, Normal (unchanged); ↑, increased; ↓, decreased.
T3 transport into the brain of M8KO mice is severely impaired. This, and possibly the reduced T3 access to D3-containing cells in brain, such as neurons, would cause retention of T3 in serum and consequently increase the T3 supply to tissues having other TH transporters, such as those in liver and kidneys. The elevated hepatic and renal T3 content in M8KO mice would trigger D1 expression and further increase serum T3. Meanwhile, the impaired feedback to the HPT axis keeps the serum TSH elevated, which stimulates TH production. The consequences will be the following: 1) the elevated serum T3 would produce peripheral tissue thyrotoxicosis; 2) the high D1 activity in liver and kidney will increase T4 metabolism and cause TH loss peripherally, such as the loss in the kidney (12); and 3) this TH loss would further deplete T4 supply to the brain. In this context, removing D1 could break down this chain of events and make available more serum T4 to the brain. Because T4 is less dependent than T3 on Mct8 for entry into brain, a beneficial effect would be expected, as evidenced in our M8D1KO mice.
In M8D1KO mice, the serum T4 increased to the level of D1KO mice, T3 and rT3 normalized, and both T3 to T4 and T3 to rT3 ratios decreased to the levels of Wt animals. Thyroid gland weight slightly decreased to become intermediate to those of Wt and M8KO mice, in agreement with the corresponding serum TSH levels. Brain T3 content normalized, as did the Aldh1a1 mRNA content. D2 activity and hairless expression were also partially corrected. These data clearly indicate that in M8D1KO mice the consequences of the Mct8 defect were attenuated, at least as it concerns serum and brain parameters.
In contrast, M8D2KO mice maintained the pattern of low serum T4 and rT3 and high T3, characteristic of M8KO mice. Serum TSH was even higher due to an additional decrease in sensitivity of the HPT axis to T4 in the absence of D2. Although brain T3 content in M8D2KO mice was comparable with that of M8KO mice, the expression of all three TH-responsive genes tested was severely decreased in the former and was, in fact, the lowest among all eight genotypes. A recent study in young pups also showed that impairment of TH responsiveness was greater in M8D2KO than M8KO mice (27). This suggests that the brain of M8D2KO mice have further reduced TH action in cells in which these genes are expressed. Liver TH content, D1 activity, ME expression, and serum cholesterol and AP concentrations were comparable in M8D2KO and M8KO, whereas UCP2 expression was higher in M8D2KO than M8KO mice. Taken together these results indicate that absence of D2 aggravated the M8KO defect by further impairing HPT regulation and the expression of brain TH responsive genes and maintaining liver thyrotoxicosis that, if anything, was worse than in M8KO mice. Thus, although D2 is another 5′-deiodinating enzyme and could have potentially contributed to the elevated serum T3 level and T3 to T4 ratio in M8KO, it did not. This confirms the major role of D1 as contributor to the abnormally high serum T3 and T3 to T4 ratio. It also indicates that D2 converts T4 to T3 mainly locally and partially but effectively compensates the TH depletion in the brain of M8KO mice.
In M8D1D2KO mice, the marked insensitivity to TH in the hypothalamus and pituitary induced very high serum TSH levels. The latter produced not only thyroid gland hyperplasia but also elevated serum T4 and T3. This increase in T3 production appears to find its way into the brain as reflected in an increase in cerebral T3 content and action, which manifested in the normalization of Aldh1a1 expression. In fact, all parameters measuring brain TH effect in M8D1D2KO were less severely affected than in M8D2KO mice. These observations indicate that some circulating T3 can reach the brain of adult mice through transporters other than Mct8. Also, T4 may have a direct effect of its own as previously suggested (15). Meanwhile, the elevated serum TH levels of M8D1D2KO mice affected the peripheral tissues. Liver UCP2 expression and serum AP activity were the highest and cholesterol the lowest of all eight genotypes studied. This shows that the liver of M8D1D2KO mice is more thyrotoxic than that of M8KO mice. Marked decreases in both weight and length were observed in the M8D1D2KO and M8D2KO mice, with more dramatic reduction in weight. We suspect that increased energy expenditure due to TH excess is the cause of weight loss. Both liver and bone isoenzyme increases contributed to serum AP elevation, suggesting that bone may be also thyrotoxic. Further studies are needed to characterize these findings.
We found slightly elevated and normal brain T3 content in adult D2KO and D1D2KO mice, respectively. These findings, which were confirmed independently in the laboratories of two of the authors (V.A.G. and S.R.), contrast with previous reports showing a 50% decrease in brain T3 content of D2KO and D1D2KO pups at 15 d of age (15, 30). One possible explanation could be that the alternative TH transporters and/or adaptive mechanism was activated in adult mice. One such mechanism may be the reduced T3 degradation as suggested by the lower brain D3 activity compared with Wt mice. M8D2KO mice appear to have comparable cerebral T3 level with that of M8KO mice. Nevertheless, despite this, TH action assessed by the three TH responsive genes showed more severe deficiency in M8D2KO than M8KO mice. Overall, the gene expression patterns did not correlate precisely with brain T3 content. This could be explained by the regional and cellular differences in T3 content, depending on the efficiency of local T3 generation and the availability and redundancy of TH transport. Hairless is expressed in many brain areas and in different cell types such as, astrocytes, neurons, and oligodendrocytes (31). Significantly decreased expression was found both in M8KO and D2KO mice, whereas M8D2KO mice showed an additive decrease. This suggests that both direct T3 supply and its local generation play a role in hairless gene expression. Aldh1a1, which is 5 times more abundant in astrocyte (31), was normally expressed in both M8D1KO and M8D1D2KO mice compared with the high levels in their littermates, D1KO and D1D2KO, indicating that this correction could be D1 KO related. TH regulation of the Cbr2 gene showed another pattern. All Mct8-deficient mice had significant decreases in Cbr2 gene expression, whereas D1 and/or D2 deficiency showed no alteration. This implies that Cbr2-expressing cells are dependent on Mct8 for the supply of T3. It is of note that although global cerebral T3 content was normal in M8D1KO and M8D1D2KO mice, the decrease of Cbr2 and hairless expression was maintained, suggesting that the effect of Mct8 deficiency could not be completely overcome at the cell level.
Although Mct8 is abundantly expressed in liver, both direct and indirect evidences indicate that Mct8 plays no major role in the uptake of TH in this organ (9, 10). We therefore expected that the reduced local generation of T3 in D1 deficiency would reduce the thyrotoxic effect in liver. Whereas this occurred to a certain degree in the D1KO and D1D2KO mice, the manifestation of TH excess in the M8D1KO mice, as assessed by ME and UCP2 mRNA and serum AP, was not much different from that of M8KO mice. Although this result was unexpected, we noted that the liver to serum T3 ratio was higher in M8D1KO mice compared with their D1KO littermates (0.296 vs. 0.196). The same was true for M8D1D2KO compared with D1D2KO mice (0.271 vs. 0.160), implying relatively higher intracellular liver T3 content in Mct8-deficient mice. Because adult mouse liver has minimal, if any, D2 and D3 activity (32, 33) and D1 is deficient in these mice, our finding of increased T3 content in liver of M8D1KO and M8D1D2KO most likely reflects impaired hepatic T3 efflux because T3 uptake is normal in M8KO mice (9, 10).
Mct8 was found to be important in the TH secretion from thyroid gland (13, 14). One of the findings was the increase of thyroidal content of Tg and non-Tg TH (13). This effect is currently observed in all mice with combined deiodinases and Mct8 deficiency but not in mice deficient in deiodinases alone. These results confirm that the impaired TH release is due to lack of Mct8. It should be noted that in M8D1KO mice, serum T3 is comparable with Wt and only 16% higher than D1KO mice, but thyroidal Tg and non-Tg T3 are 100–200% higher than in Wt and D1KO mice, suggesting the impairment of T3 secretion. In fact, both thyroidal T4 and T3 secretion were impaired in Mct8 deficient mice as shown in our recent study (13). Our findings contradict those of Trajkovic-Arsic et al. (14), suggesting increased thyroidal T3 secretion, which could be due to supraphysiological TSH stimulation used in the study. The finding of Mct8-associated decrease of thyroidal TH secretion does not conflict with the observed increase serum T4 in M8D1KO mice. Indeed, the reduced D3-mediated degradation of T4 caused by Mct8 deficiency resulted in a much lower rT3 in M8D1KO than D1KO mice and maintained the T4 level despite the concomitant reduction in T4 secretion.
Another interesting finding is that D2-deficient mice responded less to the TSH effect on promoting thyroid gland growth. In fact, despite the markedly elevated serum TSH level, D2KO and D1D2KO mice have normal thyroid gland size with normal structure. This reduced responsiveness to TSH could not be explained by the serum TSH bioactivity. These findings suggest that D2 is involved in TSH-mediated thyroid gland enlargement. This could be important for the human thyroid, which exhibits notable D2 expression (34). It is also consistent with the regulation of D2 gene expression by the thyroid transcription factor 1 (35) and the altered D2 activities in the thyroid gland of humans with thyroid disease (34, 36–38). However, it is still unknown whether D2 is important for the mouse thyroid gland and whether D2 could directly or indirectly regulate thyroid gland growth.
In conclusion, the absence of D1 corrected the abnormalities of serum TH and TSH, characteristic of Mct8 deficiency, and partially corrected the brain depletion of TH. The absence of both D2 and Mct8 aggravated the manifestations of TH deprivation in brain and the impairment of the HPT axis feedback regulation. Of note is the abrogation of the beneficial effect of D1 absence in the M8D1D2KO mouse. This indicates that D1 is the main cause for the high T3 observed in Mct8 deficiency and contributes, in part, to the low serum T4 concentration. D2 is crucial for the local generation of T3 in brain and serves for the partial compensation of the Mct8 defect.
Acknowledgments
We thank Dr. Sara J. Minnich from Mayo Clinic Laboratories (Rochester, MN) for performing isoenzyme analysis of serum AP.
This work was supported in part by National Institutes of Health Grants 4R37-DK15070 and 2P60-DK020595 and the Abrams and Esformes Families Endowments. Special thanks are due for the funds donated by the Smile Foundation, with support from the Sherman family. A.M.D. was supported by Award T32DK007011 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP
- Alkaline phosphatase
- D
- iodothyronine deiodinase
- HPT
- hypothalamo-pituitary-thyroid
- KO
- knockout
- LoI
- low iodine diet
- MCT8 or M8
- monocarboxylate transporter 8
- ME
- malic enzyme
- MMI
- methimazole
- P
- postnatal day
- Tg
- thyroglobulin
- TH
- thyroid hormone
- UCP
- uncoupling protein
- Wt
- wild type.
References
- 1. Kopp P, Solis-Sainz JC. 2009. Thyroid hormone synthesis. In: Wondisford FE, Radovick S. eds. Clinical management of thyroid disease. Philadelphia: Saunders/Elsevier; 19–41 [Google Scholar]
- 2. Jansen J, Friesema EC, Milici C, Visser TJ. 2005. Thyroid hormone transporters in health and disease. Thyroid 15:757–768 [DOI] [PubMed] [Google Scholar]
- 3. Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. 2008. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev 29:898–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 5. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. 2003. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- 6. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. 2004. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. 2004. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- 8. Schwartz CE, Stevenson RE. 2007. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 21:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dumitrescu AM, Liao XH, Weiss RE, Millen K, Refetoff S. 2006. Tissue-specific thyroid hormone deprivation and excess in monocarboxylate transporter (mct) 8-deficient mice. Endocrinology 147:4036–4043 [DOI] [PubMed] [Google Scholar]
- 10. Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. 2007. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. 2009. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology 150:4450–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trajkovic-Arsic M, Visser TJ, Darras VM, Friesema EC, Schlott B, Mittag J, Bauer K, Heuer H. 2010. Consequences of monocarboxylate transporter 8 deficiency for renal transport and metabolism of thyroid hormones in mice. Endocrinology 151:802–809 [DOI] [PubMed] [Google Scholar]
- 13. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. 2010. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest 120:3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trajkovic-Arsic M, Müller J, Darras VM, Groba C, Lee S, Weih D, Bauer K, Visser TJ, Heuer H. 2010. Impact of monocarboxylate transporter-8 deficiency on the hypothalamus-pituitary-thyroid axis in mice. Endocrinology 151:5053–5062 [DOI] [PubMed] [Google Scholar]
- 15. Galton VA, Schneider MJ, Clark AS, St. Germain DL. 2009. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology 150:2957–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. 1999. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- 17. Perret J, Ludgate M, Libert F, Gerard C, Dumont JE, Vassart G, Parmentier M. 1990. Stable expression of the human TSH receptor in CHO-K1 cells and characterization of differentially expressing clones. Biochem Biophys Res Commun 171:1044–1050 [DOI] [PubMed] [Google Scholar]
- 18. Moeller LC, Kimura S, Kusakabe T, Liao XH, Van Sande J, Refetoff S. 2003. Hypothyroidism in thyroid transcription factor 1 haploinsufficiency is caused by reduced expression of the thyroid stimulating hormone receptor. Mol Endocrinol 17:2295–2302 [DOI] [PubMed] [Google Scholar]
- 19. Radonić A, Thulke S, Mackay IM, Landt O, Siegert W, Nitsche A. 2004. Guideline to reference gene selection for quantitative real-time PCR. Biochem Biophys Res Commun 313:856–862 [DOI] [PubMed] [Google Scholar]
- 20. Dumitrescu AM, Liao XH, Abdullah MS, Lado-Abeal J, Majed FA, Moeller LC, Boran G, Schomburg L, Weiss RE, Refetoff S. 2005. Mutations in SECISBP2 result in abnormal thyroid hormone metabolism. Nat Genet 37:1247–1252 [DOI] [PubMed] [Google Scholar]
- 21. Balzano S, Bergmann BM, Gilliland MA, Silva JE, Rechtschaffen A, Refetoff S. 1990. Effect of total sleep deprivation on 5′-deiodinase activity of rat brown adipose tissue. Endocrinology 127:882–890 [DOI] [PubMed] [Google Scholar]
- 22. Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St. Germain DL. 1999. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest 103:979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morreale de Escobar G, Pastor R, Obregon MJ, Escobar del Rey F. 1985. Effects of maternal hypothyroidism on the weight and thyroid hormone content of rat embryonic tissues, before and after onset of fetal thyroid function. Endocrinology 117:1890–1900 [DOI] [PubMed] [Google Scholar]
- 24. Morreale de Escobar G, Calvo R, Escobar del Rey F, Obregón MJ. 1994. Thyroid hormones in tissues from fetal and adult rats. Endocrinology 134:2410–2415 [DOI] [PubMed] [Google Scholar]
- 25. Kero J, Ahmed K, Wettschureck N, Tunaru S, Wintermantel T, Greiner E, Schütz G, Offermanns S. 2007. Thyrocyte-specific G (q)/G(11) deficiency impairs thyroid function and prevents goiter development. J Clin Invest 117:2399–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alonso M, Goodwin C, Liao X, Page D, Refetoff S, Weiss RE. 2007. Effects of maternal levels of thyroid hormone (TH) on the hypothalamus-pituitary-thyroid set point: studies in TH receptor beta knockout mice. Endocrinology 148:5305–5312 [DOI] [PubMed] [Google Scholar]
- 27. Morte B, Ceballos A, Diez D, Grijota-Martínez C, Dumitrescu AM, Di Cosmo C, Galton VA, Refetoff S, Bernal J. 2010. Thyroid hormone-regulated mouse cerebral cortex genes are differentially dependent on the source of the hormone: a study in monocarboxylate transporter-8- and deiodinase-2-deficient mice. Endocrinology 151:2381–2387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ricquier D. 1999. Uncoupling protein-2 (UCP2): molecular and genetic studies. Int J Obes Relat Metab Disord 23(Suppl 6):S38–S42 [DOI] [PubMed] [Google Scholar]
- 29. Fukuda H, Hirakawa T, Iritani N. 2007. Nutritional and hormonal regulation of uncoupling protein gene expression in rat adipocytes. J Nutr Sci Vitaminol (Tokyo) 53:426–431 [DOI] [PubMed] [Google Scholar]
- 30. Galton VA, Wood ET, St. Germain EA, Withrow CA, Aldrich G, St. Germain GM, Clark AS, St. Germain DL. 2007. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology 148:3080–3088 [DOI] [PubMed] [Google Scholar]
- 31. Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci 28:264–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwakkel J, Chassande O, van Beeren HC, Fliers E, Wiersinga WM, Boelen A. 2010. Thyroid hormone receptor α modulates lipopolysaccharide-induced changes in peripheral thyroid hormone metabolism. Endocrinology 151:1959–1969 [DOI] [PubMed] [Google Scholar]
- 33. Schneider MJ, Fiering SN, Thai B, Wu SY, St. Germain E, Parlow AF, St. Germain DL, Galton VA. 2006. Targeted disruption of the type 1 selenodeiodinase gene (dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology 147:580–589 [DOI] [PubMed] [Google Scholar]
- 34. Salvatore D, Tu H, Harney JW, Larsen PR. 1996. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest 98:962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gereben B, Salvatore D, Harney JW, Tu HM, Larsen PR. 2001. The human, but not rat, dio2 gene is stimulated by thyroid transcription factor-1 (TTF-1). Mol Endocrinol 15:112–124 [DOI] [PubMed] [Google Scholar]
- 36. Celi FS, Coppotelli G, Chidakel A, Kelly M, Brillante BA, Shawker T, Cherman N, Feuillan PP, Collins MT. 2008. The role of type 1 and type 2 5′-deiodinase in the pathophysiology of the 3,5,3′-triiodothyronine toxicosis of McCune-Albright syndrome. J Clin Endocrinol Metab 93:2383–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Staveren WC, Solís DW, Delys L, Venet D, Cappello M, Andry G, Dumont JE, Libert F, Detours V, Maenhaut C. 2006. Gene expression in human thyrocytes and autonomous adenomas reveals suppression of negative feedbacks in tumorigenesis. Proc Natl Acad Sci USA 103:413–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanou Y, Hishinuma A, Tsunekawa K, Seki K, Mizuno Y, Fujisawa H, Imai T, Miura Y, Nagasaka T, Yamada C, Ieiri T, Murakami M, Murata Y. 2007. Thyroglobulin gene mutations producing defective intracellular transport of thyroglobulin are associated with increased thyroidal type 2 iodothyronine deiodinase activity. J Clin Endocrinol Metab 92:1451–1457 [DOI] [PubMed] [Google Scholar]