High fat hyperphagia occurs in MC4R+/− mouse, is elicited by saturated and monounsaturated fats, and may result from enhanced reward rather than defective satiety.
Abstract
Defective melanocortin signaling causes hyperphagic obesity in humans and the melanocortin-4 receptor knockout mouse (MC4R−/−). The human disease most commonly presents, however, as haploinsufficiency of the MC4R. This study validates the MC4R+/− mouse as a model of the human disease in that, like the MC4R−/−, the MC4R+/− mouse also exhibits a sustained hyperphagic response to dietary fat. Furthermore, both saturated and monounsaturated fats elicit this response. N-acylphosphatidylethanolamine (NAPE) is a signaling lipid induced after several hours of high-fat feeding, that, if dysregulated, might explain the feeding behavior in melanocortin obesity syndrome. Remarkably, however, MC4R−/− mice produce elevated levels of NAPE and are fully responsive to the anorexigenic activity of NAPE and oleoylethanolamide. Interestingly, additional differences in N-acylethanolamine (NAE) biochemistry were seen in MC4R−/− animals, including reduced plasma NAE levels and elevated hypothalamic levels of fatty acid amide hydrolase expression. Thus, while reduced expression of NAPE or NAE does not explain the high-fat hyperphagia in the melanocortin obesity syndrome, alterations in this family of signaling lipids are evident. Analysis of the microstructure of feeding behavior in response to dietary fat in the MC4R−/− and MC4R+/− mice indicates that the high-fat hyperphagia involves defective satiation and an increased rate of food intake, suggesting defective satiety signaling and enhanced reward value of dietary fat.
The melanocortin-4 receptor (MC4R) is involved in coordinated regulation of both energy intake and energy expenditure. Prior analyses of effects of central melanocortin signaling on energy intake showed that administration of melanocortin agonists in rodents reduces intake of normal chow by decreasing meal size (1, 2). Additionally, the MC4R appears to have a specific role in regulating homeostatic responses to dietary fat. MC4R knockout (MC4R−/−) mice exhibit a profound fat-induced hyperphagia (3, 4). When wild-type (WT) mice are switched to a high-fat chow, they actually reduce the volume of intake to retain a daily intake that is nearly isocaloric. In contrast, the MC4R−/− mouse actually increases the volume of intake (3). MC4R−/− also exhibit a defective thermogenic response to dietary fat (3, 5) and a defective satiety response to cholecystokinin (CCK) (6), a gut peptide released in response to dietary fat and protein. Pharmacological inhibition of the MC4R increases the reward value of fat but not carbohydrate rich foods (7), and injection of the MC4R agonist melanotan II into the amygdala of rats reduces preference for high-fat chow in a meal preference paradigm (8). Little is known, however, regarding the mechanisms by which central melanocortin circuits sense dietary fat ingestion. Defective sensing of CCK demonstrated in the MC4R knockout might have some impact on homeostatic responses to dietary fat, because CCK release is induced primarily by fat and protein (9, 10). However, CCK and CCK receptor knockout animals do not exhibit the high-fat hyperphagia seen in the MC4R−/− animal (11, 12). Thus, the mechanisms by which the central melanocortin circuitry senses dietary fat remain to be determined.
N-acylphosphatidylethanolamines (NAPEs) are lipid signaling molecules secreted into circulation from the small intestine in response to ingested fat, and administration of C16:0 NAPE decreases food intake in rodents (13). Hydrolysis of NAPEs by NAPE phospholipase D (NAPE-PLD) produces a family of N-acylethanolamines (NAEs) (14), including the well-known endocannabinoid anandamide, and its derivative oleoylethanolamide (OEA), that regulate a variety of physiological processes including food intake (15). Thus, derivatives of NAPE, such as anandamide, may either be orexigenic (16) or anorexigenic, as in the case of OEA (17). In this study, we also tested the hypothesis that the high-fat hyperphagia of MC4R−/− mice may attributable to dysregulation of NAPE and/or NAE expression or response, by testing the expression and response to NAPE and OEA. Enzymes involved in processing these lipids are also implicated in energy homeostasis. For example, deletion of fatty acid amide hydrolase (FAAH), the enzyme required for hydrolyzing acid ethanolamides such as anandamide and OEA, causes obesity and increases the reward value of fat in mice (18). Thus, we also examined expression of FAAH, and NAPE-PLD, an enzyme involved in the synthesis of NAEs from NAPEs, which is also expressed in the central nervous system (19).
Materials and Methods
Animals
Aged-matched melanocortin 4 receptor knockout (MC4R−/−), heterozygous (MC4R+/−), and WT mice derived from the original colony (20) on a C57BL/6J genetic background were obtained from breeding colonies maintained at Vanderbilt University (Nashville, TN). The strain had previously been backcrossed onto the C57BL/6J background for more than 10 generations. MC4R-τ-Sapphire transgenic (MC4R-GFP) male mice (21) from the Vanderbilt colony were used for immunohistochemistry (IHC). All animals had ad libitum access to food and water in 12-h light, 12-h dark cycle under controlled temperature and humidity. Sim1 heterozygous and WT control animals were maintained in the Yale Animal Resources Center (YARC) and had ad libitum access to Harlan 2018S chow (Harlan, Indianapolis, IN). Experiments were approved by the Animal Care and Use Committee of Vanderbilt University and Yale University.
Diets
Purina rodent diet 5001(LabDiet, PMI Nutrition International Inc., Brenwood, MO) or Picolab rodent diet 20 (LabDiet, PMI Nutrition International Inc.) were used as control diets for D12492; 60% Kilocalorie (Kcal) from fat (Research Diets, New Brunswick, NJ) as indicated. The Purina 5001 diet and the PicoLab 20 diet are nutritionally very similar (see Table 1), and this change was only initiated because of a change in availability in the housing facility. Custom made isocaloric high-saturated fatty acid (SFA, 45% Kcal from fat) and high-monounsaturated fatty acid (MUFA, 45% Kcal from fat) diets were purchased from Research Diets (Research Diets). D12328 low-fat diet (Research Diets) was used as a control diet for the custom made high-fat diets. Macronutrient composition of all diets is listed in Table 1.
Table 1.
Composition of diets
| SFA | MUFA | D12492 | D12328 | Purina 5001 | Picolab | |
|---|---|---|---|---|---|---|
| Kcal % | ||||||
| Carbohydrate | 38.6 | 38.6 | 20 | 73.1 | 58 | 62 |
| Protein | 16.4 | 16.4 | 20 | 16.4 | 28.5 | 24.6 |
| Fat | 45 | 45 | 60 | 10.5 | 13.5 | 13.2 |
| Kcal per gram | 5.05 | 5.05 | 5.24 | 4.07 | 3.02 | 3.07 |
| Relative gram amounts | Fat and carbohydrate from a variety of nonpurified plant and animal sources | Fat and carbohydrate from a variety of nonpurified plant and animal sources | ||||
| Lard | 0 | 0 | 245 | 0 | ||
| Soybean oil | 25 | 25 | 25 | 25 | ||
| Coconut oil | 253 | 0 | 0 | 40 | ||
| Olive oil | 0 | 253 | 0 | 0 | ||
| Maltodextrin 10 | 170 | 170 | 125 | 170 | ||
| Corn starch | 356 | 356 | 0 | 835 | ||
| Sucrose | 0 | 0 | 68.8 | 0 | ||
| Fatty acid profile | ||||||
| C12, lauric | 120.4 | 0 | 0 | 19 | ||
| C14, Myristic | 45.5 | 0 | 2.2 | 7.2 | ||
| C16, Palmitic | 24.6 | 34.5 | 58.7 | 6.1 | ||
| C18, Stearic | 27.8 | 7 | 33.5 | 5.2 | ||
| C18:1, Oleic | 8.1 | 187 | 106.8 | 6.4 | ||
| C18:2, Linoleic | 13.5 | 39.4 | 34.4 | 13.4 | ||
| C18:3, Linolenic | 2 | 4 | 4.4 | 2 |
Food intake and body weight
To determine whether the hyperphagic response to dietary fat could be observed in MC4-R+/−, daily food intake and weekly body weight were measured in 6-month-old female WT, MC4R+/−, and MC4R−/− mice housed in groups (four to five per cage) and maintained on Purina 5001 for 2 wk before being switched to high-fat D12492 for 2 wk. Food intake was measured at 1300 h by collecting the weight of food remaining in the stainless steel feeder in the roof of the cage and adjusting for spillage.
To evaluate effects of different types of fatty acids on food intake and body weight, 6- to 7-wk-old female WT, MC4R+/−, and MC4R−/− mice were housed individually and given free access to D12328 and water for 1 wk before the start of the experiment. Food intake was measured daily at 1300 h for 1 wk of D12328 and 1 wk of either SFA or MUFA diets. Food spillage was counted and daily food intake was rectified by that amount. Body weight before and after the experiment was measured. Food efficiency is ratio of body weight gain per food intake consumed during 1-wk period of low-fat and high-fat diet feeding.
Meal pattern analysis
Meal pattern was evaluated in 3-month-old male WT, MC4R+/−, and MC4R−/− mice fed Picolab rodent diet 20 and D12492 using a comprehensive lab animal monitoring system (CLAMS, Columbus Instruments, Columbus, OH). Mice were acclimated to the monitoring chambers for 2 d followed by data collection for 24 h. Both diets were presented in powder form. Meal size was determined for any feeding bout of greater than 0.02 g. A meal was said to be terminated when a bout of feeding was followed by 10 min with no measurable intake. The food bout was an episode of uninterrupted feeding of at least 0.02 g.
Intraperitoneal injection of C16:0 NAPE
Individually housed animals maintained on Picolab rodent diet 20 were injected ip once daily for 1 wk with 0.9% sodium chloride (Hospira, Inc., Lake Forest, IL) to acclimate them to the experimental protocol. Immediately before lights off, free feeding 7-wk-old weight-matched female WT, MC4R+/−, and MC4R−/− littermates were injected ip with vehicle (0.9% sodium chloride with solutol HS 15, 12:1 ratio) or 100 mg/kg C16:0 NAPE dissolved in vehicle. Food intake was monitored at 6 and 16 h after injection. The same dose of NAPE was injected ip into 4-month-old female WT and MC4R−/− mice maintained on D12492 for 3 wk, and food intake was measured at 4, 6, 12, and 24 h.
Intraperitoneal injection of OEA
Before dark phase, ad libitum–fed age- and weight-matched animals maintained on Picolab rodent diet 20 were injected ip with vehicle solution (sterile saline with solutol HS 15) or 50 mg/kg OEA (Cayman Chemical, Ann Arbor, MI) dissolved in the vehicle solution. Food intake was monitored for 24 h. All animals were acclimated to handling and injection protocol before the day of experiment.
Intraperitoneal injection of NAPE in Sim1+/− mice
WT or Sim1+/− male mice were fasted overnight and treated with vehicle (physiological saline with 5% Tween 80 and 5% polypropylene glycol) or 250 mg/kg C16:0 NAPE. Overnight food intake was then recorded at the indicated intervals.
Double label IHC
Nine-wk-old MC4R-GFP male mice maintained on Purina 5001 were injected ip once daily with 0.9% sodium chloride for 1 wk before the start of the experiment to acclimate them to the experimental protocol. Overnight fasted mice were injected ip either with vehicle or 500 mg/kg C16:0 NAPE. Three transgenic mice were used in each group. At 60 min after injection, animals were deeply anesthetized by 0.2% Avertin, injected ip, and transcardially perfused with 0.9% saline with heparin and then followed by ice-cold 4% paraformaldehyde in 0.1 m PBS (pH 7.4). Brains were removed and postfixed in 4% paraformaldehyde for 6 h at room temperature. Brains were immersed in 30% sucrose in PBS at 4 C. Free-floating 30-μm coronal brain sections were cut and rinsed three times with PBS and blocked with 5% nonfat dry milk in PBS containing 0.05% Triton (PBST) for 1 h at room temperature with shaking, and then incubated with 1:10,000 polyclonal rabbit anti c-fos antibody (Ab-5; Calbiochem, EMD Bioscience Inc, La Jolla, CA) in 5% milk in PBST overnight at 4 C and followed with 1:500 Alexa Fluor 594 donkey antirabbit (Invitrogen, Molecular Probes, Eugene, OR). The sections were rinsed with PBS and then incubated in 1:500 goat anti-GFP FITC antibody (ab6662–100, ABcam, Inc., Cambridge, MA) for 30 min at room temperature. Anatomical parameters were defined according to the Franklin and Paxinos mouse brain atlas. All images were acquired using a fluorescent microscope (Zeiss Imager Z1, Carl Zeiss MicroImaging, LLC, Thornwood, NY). Double labeled neurons were defined as those cells exhibiting red nuclear fluorescence above background, conforming to the shape of the GFP positive cell bodies.
Gene expression analysis
Animals were maintained on Picolab rodent diet 20 and water. Whole hypothalamus was dissected from free feeding 19- to 20-wk-old female WT and MC4R−/− littermates at 1300–1500 h, quickly frozen in dry ice, and kept at −80 C. Hypothalamic blocks were outlined rostrally by the decussation of the optic chiasma, caudally by the mammillary bodies, laterally by the optic tract, and dorsally by the apex of the third ventricle. Total RNA was extracted from hypothalamic tissues using a RNAeasy lipid mini kit (Qiagen Sciences, Inc., Germantown, MD) according to the manufacturer's instruction. The total RNA was treated with RNase-free DNase (Qiagen Sciences, Inc.). cDNA synthesis from 1 μg of total RNA was performed according to manufacturer's instruction using Iscript cDNA synthesis kit (Bio-Rad laboratories).
The following TaqMan gene assays (Applied Biosystems, Inc, Foster City, CA) were used: Assay ID, Mm00724596_m1 for mouse NAPE-PLD; Assay ID, Mm00515684_m1 for mouse FAAH; and Assay ID, Mm00607939_s1 for mouse β-actin. Samples were run in a 20-μl reaction volume containing 10 μl of 2 × TaqMan Universal PCR Master Mix (Applied Biosystems), 0.5 μl Taqman gene expression assay, 0.5 μl Rnase-free water, and 9 μl cDNA solution. Duplicate measurements of each sample were performed in a 96-well plate. Thermal cycling conditions for real-time PCR were set as follows: 95 C, 10 min, after that 95 C, 15 sec for denaturing step and 60 C, 1 min for annealing step for 40 cycles using a Stratagene Mx3000p (Stratagene, La Jolla, CA). The PCR efficiency for each individual sample was determined by using the LinRegPCR quantitative PCR data analysis program as described previously (22).
Western blotting for NAPE-PLD and FAAH protein
To confirm the real time PCR results, we performed Western blotting. Whole hypothalamus from WT and MC4R−/− mice was homogenized on ice in an ice-cold RIPA buffer [(150 mm NaCl, 50 mm Tris, 1 mm EDTA, 1% triton × 100, 0.1% SDS, 0.5% sodium deoxycholate (pH 8.0)] with protease inhibitor (Complete EDTA-free protease inhibitor cocktail tablets, Roche Diagnostics GmbH, Mannheim, Germany) and centrifuged at 20,000 × g for 30 min at 4 C. Supernatants were diluted 1:10 in the lysis buffer, and a BCA protein assay was performed (Pierce Chemical, Rockford, IL) according to company protocol to determine protein concentration. BSA (A9647-100G, Sigma-Aldrich, Co., St. Louis, MO) was used to generate the standard curve. Fifty micrograms of total protein suspended in 20 μl of loading buffer containing NuPAGE 4 × LDS Sample buffer (Cat No. NP007, Invitrogen, Carlsbad, CA) in lysis buffer, boiled 10 min at 95 C, was electrophoresed at 200 V in a 10% Tris-glycine sodium dodecyl sulfate polyacrylamide gel (PAGEr Gold Precast gels, cat No. 58502, Lonza, Rockland, ME) and electroblotted onto pure nitrocellulose membrane (PROTRAN, PerkinElmer life and Analytical Sciences, Boston, MA) at 200 mA for 1.5 h at 4 C. Precision Plus dual color ladder (Bio-Rad, Madrid, Spain) was included on each gel. FAAH Western ready control (Cat No. 10010182, Cayman Chemical, Ann Arbor, MI) was used as positive control for FAAH antibody. The membranes were blocked in 50:50 Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) in 0.1 m PBS for 1 h at room temperature. Immunoblotting was performed with 1:1000 polyclonal rabbit NAPE-PLD antibody (ab77474, ABcam, Inc., Cambridge, MA) or 1:500 polyclonal rabbit FAAH antibody (Cat No. 101600, Cayman Chemical) in blocking buffer with 0.05% Tween20 at 4 C overnight on a shaking platform. Monoclonal mouse β-tubulin IgG1 (E7, Developmental Studies Hybridoma Bank at University of Iowa, IA City, IA) at a final concentration of 1:5000 was used as loading control. The blots were rinsed three times for 2 min each with TTBS [(20 mm Tris base, 137 mm NaCl, 0.1% Tween 20 (pH 7.6)] and probed with IRDye 680 infrared secondary antibody, donkey antimouse IgG at a final concentration of 1:15,000, and IRDye 800CW donkey antirabbit IgG at a final concentration of 1:15,000 (LI-COR Biosciences, Lincoln, NE) for 1 h at room temperature. The blots were rinsed six times with TTBS for 5 min each and last two times for 5 min each with 0.1 m PBS buffer. The blots were scanned on a LiCor Imaging System and analyzed using Odyssey software. To determine changes in NAPE-PLD and FAAH protein levels between WT and MC4R−/− mice, targeted protein levels were normalized to β-tubulin levels within the same lane.
FAAH enzyme activity assay
FAAH activity assays were performed according to published methods (23). Briefly, hypothalamii were homogenized in 20 mm HEPES (pH 7.8) containing 10% glycerol, 150 mm NaCl, and 1% triton X-100 on ice, centrifuged at 13,000 × g, 4 C for 10 min. Supernatant was collected. Five micoliters of sample in 15 μl of lysis buffer were added to 175 μl of reaction buffer; 125 mm Tris (pH 9.0), and 1 mm EDTA containing 1 μm FAAH substrate Arachidonoyl m-nitroaniline (Cat No. 90059, Cayman Chemical, Ann Arbor, MI) in a 96-well plate. The measurements were performed in duplicate. A standard curve generated from duplicate measurement of serial dilutions of human recombinant FAAH (Cat No. 10010183, Cayman Chemical, Ann Arbor, MI) in homogenizing buffer was included in every plate. The plate was incubated at 37 C for 30 min. The reactions were measured at absorbance 410 nm and normalized to protein concentration as determined by BCA protein assay.
Liquid chromotography tandem mass spectrometry (LC/MS/MS) analysis
Plasma samples from 3-month-old female MC4R−/− and WT littermates were used to evaluate level of NAPE and NAE species. Samples were collected from free-feeding mice maintained on Picolab rodent diet 20 and D12492 for 48 h. Animals were anesthetized with 2% Avertin. Blood was collected by decapitation. Plasma was separated by centrifuging at 5,000 × g, 4 C for 5 min. All LC/MS/MS methods and analyses were performed as described previously (13).
Statistical analysis
All values were expressed as means ± sem. An unpaired two-tailed Student's t test was used to compare two test groups. One-way ANOVA followed by a Tukey post test was used for multiple comparisons. Statistical analysis was performed using Graphpad PRISM 5 software (GraphPad Software Inc., San Diego, CA). Differences were considered significant at P < 0.05.
Results
Effect of MC4R haploinsufficiency on dietary fat intake
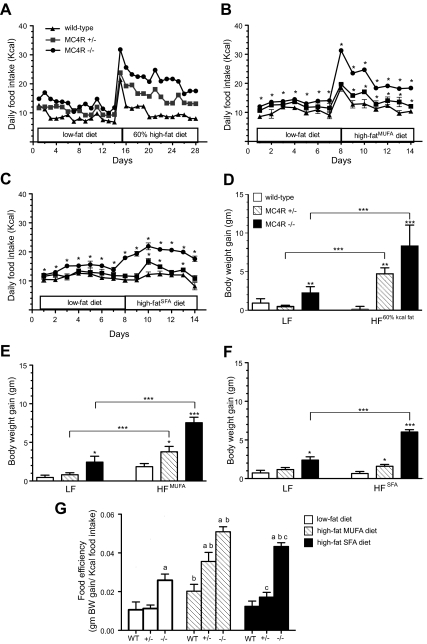
When fed standard laboratory chow (Purina 5001) 6-month-old female MC4R−/− mice had significantly greater average daily food intake relative to age and sex-matched WT mice, while MC4R+/− mice exhibited an intermediate phenotype (Fig. 1A). Switching to a high-fat chow (60% Kcal from fat, D12492) produced an acute spike in intake in all genotypes, followed by a sustained hyperphagic response to the increased dietary fat in MC4R+/−, and MC4R−/− mice but not in WT. Energy intake of MC4R+/− mice was intermediate to WT and MC4R−/− levels. This phenomenon did not appear to be age- or gender-dependent, as the same effect of the 60% diet was observed in 15- to 18-wk-old male mice (Supplemental Fig. 1A published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org/). To determine whether fatty acid types play a role in the high-fat hyperphagia phenotype, we examined high-fat diets formulated to contain primarily monounsaturated or saturated fats. Both high-fat diets caused sustained hyperphagia in MC4R+/− and MC4R−/− mice, although the initial burst of hyperphagia was not observed with high-SFA diet (Fig. 1, B and C). Hyperphagia in response to the high-MUFA and SFA chows was also observed using 15- to 18-wk-old male mice MC4R−/− but was not as robust as the effect seen in older female mice (Supplemental Fig. 1, B and C). Low-fat control diet (Purina 5001) fed MC4R−/− mice but not MC4R+/− mice gained significantly more body weight relative to WT during the study period (Fig. 1D). However, a significant difference in % body fat was apparent in MC4R+/− vs. WT mice on low-fat control diet (Supplemental Fig. 2). High-fat chow feeding produced an increase in adipose mass and % body fat in WT and MC4R+/− mice but did not increase lean mass significantly in either genotype over the study period body (Supplemental Fig. 2). Furthermore, weight gain during high-fat diet feeding was affected by genotype, with an intermediate effect of haploinsufficiency of the MC4R on the high-fat (60% Kcal from fat; D12492) and MUFA diets (Fig. 1, D–F). Significant but less striking weight gain was seen in the MC4R+/− mice on the SFA diet. Food efficiency of MC4R−/− mice was significantly greater than those of WT on all diets but further increased on the MUFA and SFA diets. The MUFA diet feeding significantly increased food efficiency of all genotypes relative to low-fat diet group (Fig. 1G). The SFA diet significantly increased food efficiency in the MC4R−/− and slightly increased food efficiency of MC4R+/− mice (Fig. 1G).
Fig. 1.
Characterization of high-fat hyperphagia attributable to loss of one or more alleles of the MC4R. A, Means of daily energy intake from group housed WT (n = 6), MC4R+/− (n = 16), and MC4R−/− (n = 4) female mice demonstrated a gene dosage effect in response to high-fat diet feeding. Food intake of (B) individually housed female mice fed high-fat diets containing primarily MUFA (*, P < 0.05 vs. WT, one-way ANOVA, WT = 7, MC4R+/− = 6, MC4R−/− = 7) and (C) individually housed female mice fed high-fat diets containing primarily SFA (*, P < 0.05 vs. WT, one-way ANOVA, WT = 8, MC4R+/− = 6, MC4R−/− =10). D12328 was used as low-fat control. Graphs show body weight gain of (D) 6-month-old female animals for 2 wk, (E) 6- to 7-wk-old female mice fed MUFA diet, and (F) 6- to 7-wk-old female mice fed SFA diet for 7 d (*, P < 0.05; **, P < 0.01; ***, P < 0.001). G, Feed efficiency of 6- to 7-wk-old female mice maintained on low-fat D12328, MUFA, or SFA for 1 wk. (a, P < 0.05 vs. WT; b, P < 0.05 vs. same genotype fed low-fat diet; c, P < 0.05 vs. same genotype fed MUFA, one-way ANOVA). Data are expressed as mean ± sem. Statistical analysis is not available for data in A as animals were group housed.
Effect of the MC4R on the microstructure of food intake
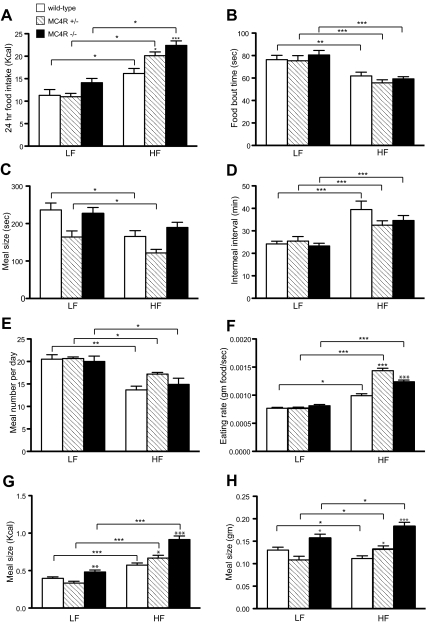
Loss of the MC4R caused an increase in 24-h food intake in response to high-fat diet (60% Kcal from fat; D12492) that was inversely proportional to gene dosage in 3-month-old male mice used to study the microstructure of meals (Fig. 2A). This effect did not appear to be affected by age or gender, as it was observed in 7- to 8-wk-old female mice as well (Supplemental Fig. 3); indeed high-fat chow did not produce any significant increase in 24-h intake in WT 7- to 8-wk-old females. Surprisingly, in light of the profound high-fat hyperphagia in MC4R−/− and MC4R+/− mice, high-fat diet significantly decreased food bout times (Fig. 2B) and meal times (Fig. 2C) in all genotypes, regardless of age or gender (Supplemental Fig. 3) compared with low-fat chow (PicoLab 20). High-fat diet also significantly prolonged the intermeal interval in all genotypes, with the exception of the MC4R−/− 7- to 8-wk-old females (Supplemental Fig. 3), although the increase was comparably smaller in MC4R−/− and MC4R+/− mice, relative to WT mice, suggesting defective satiation in both MC4R−/− and MC4R+/− mice (Fig. 2D). Indeed, when average satiety ratios were calculated (intermeal interval in min. per meal size) using meal size in either kcals or grams (Supplemental Tables 1 and 2), the satiating value of calories from fat is proportionate to MC4R gene dosage.
Fig. 2.
Effect of dietary fat on the microstructure of food intake in WT, MC4-R+/−, and MC4-R−/− mice. A, Twenty-four–hour food intake of 3-month-old male mice fed Picolab rodent diet 20 (LF) or D12492 (HF) was monitored by CLAMS (*, P < 0.05; ***, P < 0.001, one-way ANOVA, WT LF = 7, WT HF = 8, MC4R+/− LF = 8, MC4R+/− HF = 8, MC4R−/− LF = 7, MC4R−/− HF = 9). B, Average duration of each feeding bout. C, Duration that animals consumed each meal. Animals (B) and (C) were evaluated during 6 h after light off (*, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA). D, Intermeal interval. E, Number of meals. Data in D and E were obtained from the 24-h period on d 3 (***, P < 0.001; *, P < 0.05; **, P < 0.01). F, Eating rate, indicated as gram food consumed per second, was significantly higher in HF-fed MC4R+/− and MC4R−/− mice vs. WT (*, P < 0.05; ***, P < 0.001, one-way ANOVA). G, Meal size, in Kcal, was greater in MC4R−/− vs. WT (*, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA). H, Meal size, in g, was significantly decreased in WT animals switched to HF diet, whereas MC4R+/− and MC4R−/− mice increased meal size in response to HF diet (*, P < 0.05; ***, P < 0.001, one-way ANOVA). All data were represented as mean ± sem.
On a low-fat diet, MC4R+/− and MC4R−/− mice displayed normal meal number per day, and high-fat diet significantly reduced meal number in all genotypes (Fig. 2E). Remarkably, eating rate was significantly increased in high-fat diet–fed MC4R+/− and MC4R−/− mice compared with WT (Fig. 2F), irrespective of gender or age (Supplemental Fig. 3). High-fat–fed WT mice significantly increased energy per meal compared with low-fat diet–fed group (Fig. 2G) but showed reduced meal size (in grams) in response to high-fat feeding (Fig. 2H). Furthermore, MC4R−/− mice showed significantly greater meal size compared with WT in both low-fat and high-fat diet–fed groups. MC4R+/− mice exhibited larger meal size compared with WT only in high-fat diet–fed group (Fig. 2, G and H), and only in the older male mice (compare Figs. 2, G and H with Supplemental Fig. 3, G and H). The data in Fig. 2 are also displayed as percent change in each parameter as a function of chow (Supplemental Fig. 4). As can be seen, despite the reduced length of feeding bouts or meals and the increased intermeal intervals in response to high-fat feeding, heterozygous or homozygous loss of the MC4R results in increased energy intake, both by mass and total Kcals, as a consequence of an increased rate of eating during each individual feeding bout or meal and a decreased satiating effect of calories from fat (Fig. 2; Supplemental Figs. 3 and 4; Supplemental Tables 1 and 2).
NAPE and NAE levels in the MC4R−/− mouse
Because MC4R−/− mice exhibit high-fat hyperphagia and reduced satiation in response to a high-fat meal (Fig. 2D; Supplemental Tables 1 and 2), we tested the hypothesis that these mice may exhibit either defective production or defective response to NAPE. To characterize the contributions of MC4R to control of NAPE and NAE production, we used LC/MS/MS to quantitate these families of lipids in plasma from animals maintained on Picolab rodent diet 20 or switched for 48 h to the 60% Kcal fat diet D12492. Total plasma NAPE was similar in both genotypes on low-fat chow. Total plasma NAPE was elevated in both WT and MC4R−/− mice after high-fat feeding, and the increase in NAPE was greater in the MC4R−/− mice (Table 2). Plasma NAE levels were not regulated by diet in WT or MC4R−/− mice and were reduced markedly in MC4R−/− mice (Table 3). Taken together, these data suggest that MC4R plays a role in regulation of peripheral NAPE and NAE levels.
Table 2.
Plasma NAPE (41) level (μM) of free feeding mice maintained on low-fat and high-fat diet
| WT-LF | WT-HF | KO-LF | KO-HF | |
|---|---|---|---|---|
| C22_5 | 0.160 ± 0.0155 | 0.159 ± 0.0145 | 0.170 ± 0.0096 | 0.179 ± 0.0088 |
| C22_6 | 0.521 ± 0.0728 | 0.374 ± 0.057 | 0.530 ± 0.0609 | 0.429 ± 0.0302 |
| C20_2 | 0.187 ± 0.0067 | 0.223 ± 0.023 | 0.184 ± 0.0114 | 0.248 ± 0.0096# |
| C20_3 | 0.169 ± 0.0022 | 0.216 ± 0.0260 | 0.173 ± 0.0083 | 0.247 ± 0.0120# |
| C20_4 | 0.434 ± 0.0144 | 0.533 ± 0.0677 | 0.465 ± 0.0254 | 0.653 ± 0.0540# |
| C20_5 | 0.337 ± 0.0304 | 0.375 ± 0.05 | 0.369 ± 0.0281 | 0.447 ± 0.0333 |
| C18_0 | 1.911 ± 0.0679 | 2.586 ± 0.278 | 2.067 ± 0.1175 | 3.373 ± 0.1950*# |
| C18_1 | 2.645 ± 0.2268 | 3.579 ± 0.3845 | 2.971 ± 0.2505 | 4.736 ± 0.2576*# |
| C18_2 | 2.172 ± 0.1713 | 2.481 ± 0.3046 | 2.271 ± 0.1936 | 3.344 ± 0.1992* |
| C18_3 | 1.007 ± 0.0665 | 1.006 ± 0.1424 | 1.003 ± 0.0778 | 1.278 ± 0.0751 |
| C16_0 | 3.128 ± 0.1032 | 2.899 ± 0.2960 | 3.414 ± 0.1618 | 3.442 ± 0.1404 |
| C16_1 | 2.317 ± 0.1541 | 2.106 ± 0.2285 | 2.589 ± 0.1848 | 2.519 ± 0.1125 |
| Total | 14.99 ± 0.8893 | 16.45 ± 1.799 | 16.21 ± 0.9281 | 20.90 ± 1.1020*# |
NAPEs were determined by LC/MS/MS. Values are expressed as mean ± sem.
, P < 0.05 compared with WT-HF;
, P < 0.05 compared with KO-LF, one-way ANOVA, WT = 5, MC4R−/− = 7.
Table 3.
Plasma NAE level (μM) of free feeding mice maintained on low-fat diet and high-fat diet
| WT-LF | WT-HF | KO-LF | KO-HF | |
|---|---|---|---|---|
| C22_5 | 0.1609 ± 0.0155 | 0.1595 ± 0.0145 | 0.1700 ± 0.0096 | 0.1792 ± 0.0088 |
| C22_6 | 0.0282 ± 0.0051 | 0.0209 ± 0.0018 | 0.0178 ± 0.0019* | 0.0143 ± 0.0012* |
| C20_2 | 0.0152 ± 0.0004 | 0.0185 ± 0.0001# | 0.0084 ± 0.0008* | 0.0118 ± 0.0010*# |
| C20_3 | 0.0117 ± 0.0008 | 0.0159 ± 0.0005# | 0.0069 ± 0.0009* | 0.0103 ± 0.0009*# |
| C20_4 | 0.0237 ± 0.0029 | 0.0294 ± 0.0017 | 0.0144 ± 0.0026 | 0.0199 ± 0.0019* |
| C20_5 | 0.0181 ± 0.0020 | 0.0186 ± 0.0007 | 0.0101 ± 0.0014* | 0.0125 ± 0.0009* |
| C18_0 | 0.1149 ± 0.0084 | 0.1354 ± 0.0094 | 0.0616 ± 0.0064* | 0.0863 ± 0.0079*# |
| C18_1 | 0.1632 ± 0.0089 | 0.1741 ± 0.0133 | 0.0878 ± 0.0076* | 0.1107 ± 0.0089* |
| C18_2 | 0.1174 ± 0.0038 | 0.1098 ± 0.0062 | 0.0601 ± 0.0065* | 0.0700 ± 0.0062* |
| C18_3 | 0.0502 ± 0.0016 | 0.0441 ± 0.0020 | 0.0248 ± 0.0026* | 0.0265 ± 0.0025* |
| C16_0 | 0.1351 ± 0.0093 | 0.1292 ± 0.0046 | 0.0802 ± 0.0091* | 0.0861 ± 0.0065* |
| C16_1 | 0.1034 ± 0.0037 | 0.0937 ± 0.0046 | 0.0619 ± 0.0065* | 0.0622 ± 0.0049* |
| Total | 0.9422 ± 0.0344 | 0.9493 ± 0.0403 | 0.6046 ± 0.0417* | 0.6902 ± 0.0427* |
NAEs were determined by LC/MS/MS. Values are expressed as mean ± sem.
, P < 0.05 compared with WT maintained on the same diet;
, P < 0.05 compared with the same genotype maintained on different diet, one-way ANOVA, WT = 5, MC4R−/− = 7.
MC4R knockout mice are fully sensitive to exogenous NAPE administration
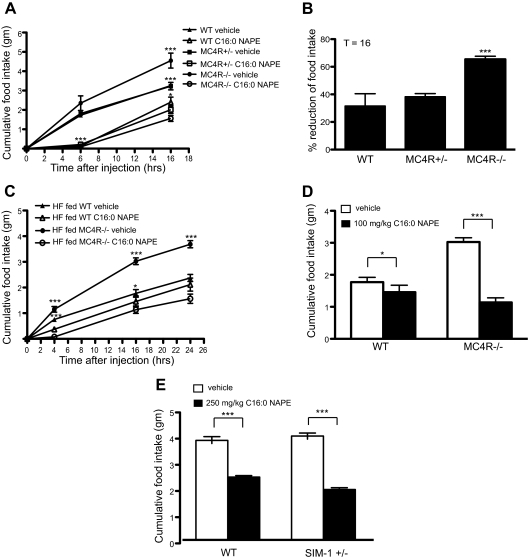
Because the MC4R−/− mice increased plasma NAPE levels after 48 h of high-fat feeding, we next tested to see whether these mice had a defective anorexigenic response to NAPE. For this experiment, we examined the effect of NAPE administration on food intake of age- and weight-matched 7-wk-old female mice maintained ad libitum on a low-fat diet (PicoLab 20). We found that MC4R−/− mice were fully sensitive to anorexigenic effects of an ip injection of NAPE relative to MC4R+/− (Fig. 3A). Total 16-h food intake after NAPE injection of MC4R−/− mice was dramatically decreased (65.4 ± 2.203%) compared with vehicle group. NAPE induced reduction of food consumption in WT (31.5 ± 8.9%) and MC4R+/− (38.2 ± 2.3%) mice were statistically equivalent (Fig. 3B). We further investigated effect of NAPE administration on food intake of high-fat diet–fed female MC4R−/− mice. NAPE reduced food intake in both high-fat diet (60% Kcal from fat; D12492) fed WT and MC4R−/− mice (Fig. 3C). However, effect of NAPE on 16-h food intake was significantly greater in MC4R−/− mice compared with WT (Fig. 3D). Indeed, dose–response analysis of the ability of NAPE to reduce 24-h food intake in WT and MC4R−/− mice showed a trend toward increased responsiveness of MC4R−/− mice to the compound (Supplemental Fig. 5).
Fig. 3.
Response to exogenous NAPE administration in MC4R−/− and SIM-1+/− mice. A, NAPE decreased food intake in age- and weight-matched female mice as compared with vehicle groups on normal chow (***, P < 0.001 vs. NAPE injected same genotype group; *, P < 0.05 vs. vehicle injected WT, t test, WT = 7, MC4R+/− = 7, MC4R−/− = 10). B, Percent reduction of food intake from data in A. At 16 h, MC4R−/− mice showed greater cumulative reduction of food intake after 100 mg/kg NAPE injection compared with WT and MC4R+/− mice (***, P < 0.001, one-way ANOVA). C, 100 mg/kg NAPE ip injection reduced food intake in high-fat diet–fed 4-month-old female mice (***, P < 0.001; *, P < 0.05 vs. NAPE injected group, t test WT = 7, MC4R−/− = 8). D, Cumulative food intake at 16 h after injection from C. High-fat diet–fed MC4R−/− mice showed hypersensitivity to NAPE at 16 h after injection (***, P < 0.001; *, P < 0.05, t test). E, Age- and weight-matched male SIM-1+/− mice showed greater reduction of cumulative food intake at 21 h after 250 mg/kg NAPE injection compared with vehicle-injected group (***, P < 0.001, t test, WT vehicle = 6, WT NAPE = 7, SIM-1+/− vehicle = 5, SIM-1+/− NAPE = 5). All data are presented as mean ± sem.
Heterozygous sim1 mutant mice are hypersensitive to exogenous NAPE administration
Heterozygous sim1 (SIM1+/−) mutant mice have been previously shown to share phenotypic similarities with MC4R−/− mice (24). We also tested to see whether SIM1+/− mice were responsive to the anorexigenic effects of NAPE. As with the MC4R−/− mice, SIM1+/− mice were more sensitive to ip injection of NAPE than WT at 21 h after injection (Fig. 3E).
MC4R−/− mice are more sensitive to exogenous OEA administration
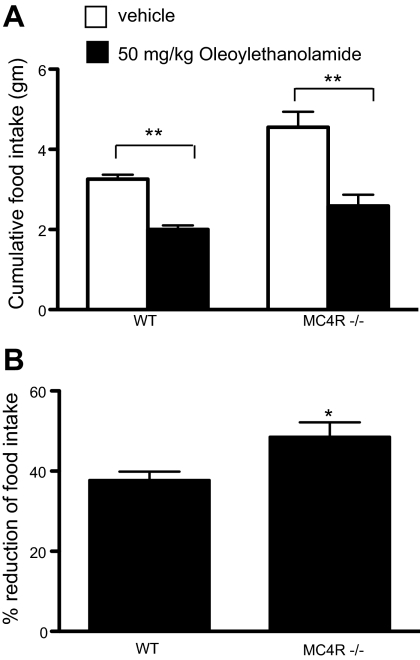
NAPE is a precursor for NAE. To determine whether MC4R−/− mice also are hypersensitive to NAE, we injected MC4R−/− mice ip with OEA, a species of NAE known to cause reduction in food intake in mice. We found that OEA reduced food intake in both WT and MC4R−/− mice (Fig. 4A). OEA-injected MC4R−/− mice exhibited a 48.6% ± 3.6 decrease in food intake, whereas WT mice exhibited a 37.8% ± 2.1 decrease in food intake (Fig. 4B).
Fig. 4.
Response to OEA administration in MC4R−/− mice. A, At 19 h after OEA injection, MC4R−/− mice showed greater reduction of cumulative food intake compared with WT (**, P < 0.01, t test, WT = 7; MC4R−/− = 10). B, Percent reduction of food intake from A is shown as mean ± sem (*, P < 0.05, t test).
NAPE administration enhances activity of a small population of MC4R expressing neurons
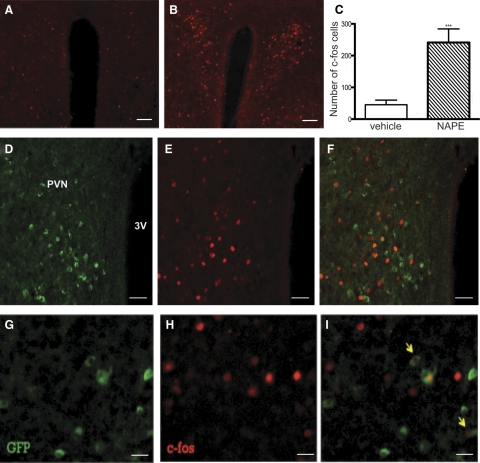
To determine whether NAPE administration affects neuronal activity of MC4R expressing neurons in the paraventricular nucleus (PVN) of the hypothalamus, we performed IHC to examine c-fos as an indirect marker for neuronal activity. MC4R-positive PVN neurons, known to be critical for the regulation of energy homeostasis (25), were identified using a transgenic mouse strain with GFP under the control of the MC4R promoter (21). NAPE increases c-fos–positive cells in PVN as previously shown (13) (Fig. 5, A–C). Double GFP and c-fos IHC analyses on PVN from MC4R-GFP mice treated with either vehicle or NAPE (n = 3 per group) showed that, in NAPE-injected mice, there were 8.9% ± 1.2 of GFP neurons (269 cells from three mice) expressing c-fos, and 4.6% ± 0.6 of detectable c-fos cells (513 cells from three mice) expressed GFP, indicating MC4R expressing neurons (Fig. 5, D–I). In vehicle-injected mice, no GFP neurons expressed c-fos (c-fos 81 cells, GFP 158 cells from three mice). These data show that NAPE activates only a small subpopulation of MC4R expressing neurons in PVN.
Fig. 5.
Activation of MC4R-GFP neurons by exogenous C16:0 NAPE administration. IHC of c-fos (red) in the PVN of overnight fasted male MC4R-GFP mice followed by either (A) vehicle or (B) 500 mg/kg C16:0 NAPE ip injection. Scale bar, 100 μm. C, NAPE increased c-fos–positive cells (***, P < 0.001, t test). GFP (green; D) and c-fos (red; E) positive cells and (F) double immunostaining of GFP and c-fos in the PVN are displayed. Scale bar, 50 μm. Panels G–I show higher-magnification images of double-fluorescent IHC for MC4R-GFP and c-fos. Scale bar, 10 μm. Arrows point to neurons showing double immunostaining.
Disruption of MC4R gene alters FAAH expression and activity in hypothalamus
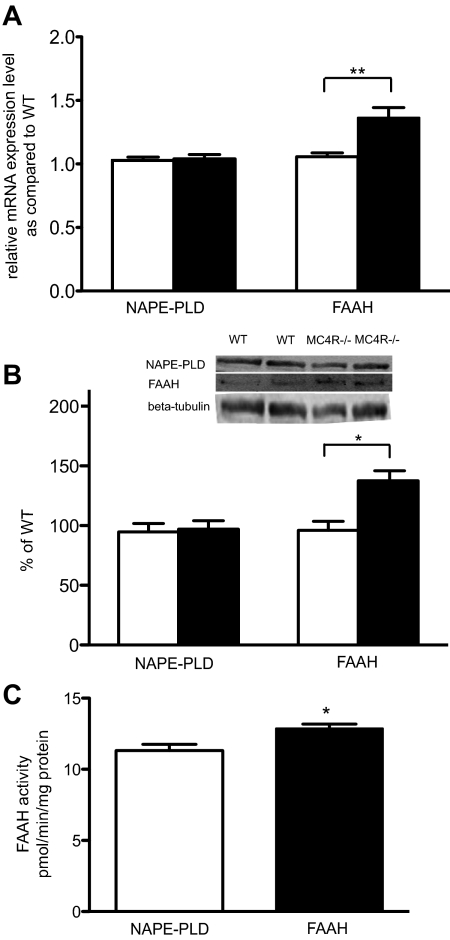
NAPE-PLD and FAAH enzymes are known to mediate the production and degradation of endogenous NAEs. Given the altered serum levels of NAE in the MC4R−/− mice, we tested for aberrant expression of these enzymes. We first investigated gene expression of NAPE-PLD and FAAH in hypothalamus using quantitative real-time PCR. The data showed that free feeding 19- to 20-wk-old female MC4R−/− mice showed a significant increase of FAAH mRNA compared with WT (Fig. 6A). Nevertheless, there was no significant difference in NAPE-PLD mRNA expression between WT and MC4R−/− mice (P = 0.8) (Fig. 6A).
Fig. 6.
Disruption of MC4R altered FAAH expression and activity in hypothalamus. A, Graphs show relative NAPE-PLD and FAAH mRNA expression in hypothalamus determined by quantitative real-time PCR (**, P < 0.01, t test, WT = 9, MC4R−/− = 13) and (B) NAPE-PLD and FAAH protein levels, normalized to WT (*, P < 0.05, t test, WT = 7, MC4R−/− = 9). C, FAAH enzyme activity was determined in hypothalamus (*, P < 0.05, t test, WT = 4, MC4R−/− = 6). All data are mean ± sem.
To confirm the quantitative real-time PCR data, we further performed Western blot analysis. Levels of NAPE-PLD and FAAH protein in hypothalamus of age-matched animals were assessed. We found that FAAH protein was significantly higher in hypothalamus of MC4R−/− mice compared with WT, yet level of NAPE-PLD protein was not significantly different between WT and MC4R−/− mice (Fig. 6B). Based on detected elevation of FAAH mRNA and protein in hypothalamus of MC4R−/− mice, we then hypothesized that activity of FAAH enzyme was higher in hypothalamus of MC4R−/− mice compared with WT. As expected, MC4R−/− mice displayed significantly increased FAAH enzyme activity compared with wild-type control (Fig. 6C).
Discussion
In the first publication on the phenotype of the MC4R knockout mouse, it became apparent that, in contrast to most GPCRs, deletion of the MC4R exhibited a gene dosage effect, with heterozygotes exhibiting an intermediate rate of weight gain and an intermediate rate of increase in linear growth (20). The discovery that haploinsufficiency caused early onset morbid obesity in humans demonstrated the physiology of the system to be conserved (26, 27). MC4R haploinsufficiency causes up to 5% of severe early onset obesity in children, and with an allele frequency in the general population of 0.1% may be one of the most common Mendelian disorders in humans. Remarkably, although many different physiological functions have now been ascribed to the MC4R using the mouse as a model system (28), the vast majority of these have been characterized in the homozygous knockout mice. As a consequence of the human disorder resulting from haploinsufficiency, characterization of the physiological consequences of melanocortin haploinsufficiency in the mouse may be of more direct medical relevance.
The melanocortin obesity syndrome in the mouse produces both hyperphagia and metabolic defects that lead to obesity. Multiple metabolic defects have been characterized in the mouse, and recently reduced autonomic tone has been observed in humans as well (29). Indeed, mild obesity is still observed when MC4R−/− mice are pair fed to WT levels of food intake (30). Nonetheless, the melanocortin obesity syndrome is characterized in mouse (20) and human (26, 27) by a readily measurable hyperphagia. In the mouse, this hyperphagia appears to be particularly triggered by dietary fat, and the animals exhibit multiple defective homeostatic responses to dietary fat. These include defects in intake (3) and diet-induced thermogenesis (3, 31), even when fat content is only increased from 12 to 25 Kcal %. Because of the potential opportunity for nutritional intervention in melanocortin obesity syndrome in children, we characterized the hyperphagic response to dietary fat in the MC4R haploinsufficient mouse.
In the C57BL/6J mouse strain used for the studies in this report, animals exhibit an acute 24 h hyperphagia when switched high-fat chow (60% of Kcal from fat; D12492) and return to near isocaloric intake by d 6 (Fig. 1A). Loss of one or both alleles of the MC4R also produces the acute 24 h hyperphagia, but in contrast to WT mice loss of one MC4R allele produces a hyperphagia sustained for up to 14 d, and loss of two alleles produces approximately twice the degree of hyperphagia. The fat in the D12492 chow is constituted primarily with lard and thus represents a mixture of saturated and monounsaturated fats. We also tested high-fat diets formulated primarily with saturated fats and monounsaturated fats (Fig 1, C–G). Both saturated and monounsaturated diets produced significant hyperphagia in 6-month-old female MC4R−/− and MC4R+/− mice. The hyperphagia in the haploinsufficient mouse was not as profound with these diets as the lard-based diet, however the latter formulations were 45% fat vs. 60%, so it is not possible to determine whether either of these fat types produce less hyperphagia than a lard-based diet.
Interestingly, the purified low-fat chow (D12328), matched for protein content to the formulated high-fat chows, did not produce any significant increase in weight gain or increase in food efficiency over the 7-d study period in MC4R+/− mice compared with WT mice (Fig. 1G). In contrast, all three high-fat diets increase weight gain in MC4R+/− mice. Weight gain in the heterozygous animals appears intermediate between WT and MC4R−/− mice with the high-fat 60% Kcal fat diet and MUFA diet but appears less pronounced in the MC4R+/− mice on the SFA diet. However, because there are also high levels of saturated fat in the 60% fat diet, this difference may be attributable to reduced palatability and intake of saturated fats derived from coconut oil in the SFA diet. In summary, the MC4R haploinsufficient mouse exhibits hyperphagia and rapid weight gain in response to a variety of dietary fats yet remains relatively normophagic over this short study period on a isocaloric low-fat diet.
Based on the observation that MC4R−/− mice exhibit resistance to the satiety factor CCK, a protein and fat-induced satiety factor, we anticipated that MC4R−/− and MC4R+/− mice would exhibit signs of defective satiety and/or satiation, such as larger size of meals or a decreased intermeal interval when hyperphagia was induced by high-fat feeding. We were surprised to observe that feeding bouts and meals were reduced in length, and intermeal intervals were increased in length in MC4R+/− and MC4R−/− animals, as animals were switched from low-fat (Picolab rodent diet20) to high-fat (D12492) chow. MC4R+/− and MC4R−/− animals actually eat fewer meals when placed on high-fat chow. However, the intermeal interval increases less in MC4R+/− and MC4R−/− animals than WT, and in concert with the increased intake in MC4R+/− and MC4R−/− this yields a significant drop in the satiety ratio, the ability of food to maintain satiation. These data argue that while some aspects of satiety and satiation remain intact in the MC4R+/− and MC4R−/− animals, the MC4R mediates a critical gene dosage effect on the satiating effects of dietary fat. Remarkably, the MC4R+/− and MC4R−/− animals also eat faster than WT mice, but only on high-fat chow. For example, WT, MC4R+/−, and MC4R−/− animals eat 29.1, 86.8, and 52.63 percent faster, respectively, when placed on high-fat chow. Thus, these animals have a compound defect in that they rapidly consume more calorie dense food and are less sensitive to its satiating properties.
Because the primary difference in food intake in response to high-fat diets in the MC4R+/− and MC4R−/− animals relative to WT occurs after a shared acute hyperphagia of 24 h, we were quite intrigued with the discovery of NAPE as a potential factor regulating the response to dietary fat in that NAPE is not produced acutely after fat intake but rather peaks in the serum after 4 h in the mouse (13).
Consistent with the data from microstructural analysis of food intake in response to high-fat diet, however, we observed that MC4R−/− mice produced NAPE in response to high-fat feeding and exhibited an anorexigenic response to NAPE. Rather than exhibiting a WT response, however, the MC4R−/− exhibited a greater production of total NAPE in response to high-fat feeding, perhaps attributable to the increased consumption of fat. Furthermore, the MC4R−/− mice exhibited a full anorexigenic response to ip administration of NAPE and even trended toward enhanced sensitivity. Interestingly, we also observed a decreased serum concentration of total NAE, a lipid species derived from NAPE, and enhanced sensitivity to anorexigenic activity of OEA, a species of NAE. NAE is hydrolyzed by FAAH. Reduction in serum NAE levels was consistent with levels of hypothalamic FAAH mRNA, protein, and enzyme activity, which were all increased in the MC4R−/−. The responsiveness of MC4R−/− to NAPE was supported by studies of the activity of PVN MC4R neurons to NAPE administration. Only a very small percentage of MC4R neurons in the PVN exhibited c-fos immunoreactivity in response to NAPE, and this could be interpreted to mean that NAPE is not dependent on activation of MC4R signaling for its anorexigenic activity.
A growing body of literature supports a role for melanocortin signaling in reward and food preference. The MC4R and MC3R are expressed in brain regions involved in reward, such as the amygdala, accumbens, and ventral tegmental area (32, 33). Several observations suggest that blockade of melanocortin signaling stimulates fat intake (34–36), increasing the reward value, preference, and consumption of high-fat vs. high-carbohydrate–containing food. In rats, intracerebroventricular administration of the MC4R/MC3R antagonist, AgRP, increased operant responding for a fat but not a sucrose reinforcer in a progressive ratio paradigm (7). Preference for high-fat chow stimulated by administration of a μ opioid receptor agonist is blunted in the AgRP knockout mouse (37). Finally, stimulation of high-fat feeding in satiated rats by μ agonist injection into the accumbens is blunted by lateral ventricle administration of the MC3R/MC4R agonist, MTII (38).
These data, along with data presented here showing normal or even enhanced anorexigenic action of NAPE, and data on the microstructure of meals indicating a reduction in meal length and increase in intermeal interval in MC4R+/− and MC4R−/− animals in response to dietary fat argue for additional mechanisms of hyperphagia in these animals. While previous data had previously demonstrated a defect in satiety resulting from MC4R blockade (4, 6), we propose that the hyperphagia resulting from loss or haploinsufficiency of the MC4R is compounded by an enhanced reward response to fat. Because μ opioids have been well documented to stimulate preference for palatable foods and fat intake (39, 40), a simple model might involve dysregulation of the normal signal provided by the μ agonist β-endorphin, and the MC4R agonist α-MSH, coreleased throughout the CNS by POMC nerve terminals. In this model, loss of the α-MSH signal through deletion of the MC4R removes a critical counterbalance to the β-endorphin signal, producing high-fat hyperphagia, as described here.
Because these findings also pertain to MC4R haploinsufficient mice, these data also have potential implications for treatment of MC4R haploinsufficient children. Because these children may present with obesity as young as 6 months of age, nutritional interventions involving significantly reducing calories from fat may have a significant impact on the rate of weight gain.
Acknowledgments
We thank Meghan Rowland and Savannah Williams for excellent technical assistance.
This work was supported by National Institutes of Health Grant RO1 DK070332 (to R.D.C.), RO1 DK-40936 (to G.I.S.), a grant from the Robert C. and Veronica Atkins Foundation (to R.D.C.) and by a University Development Commission Scholarship, Ministry of University Affairs, Thailand (to D.S.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- CCK
- Cholecystokinin
- FAAH
- fatty acid amide hydrolase
- IHC
- immunohistochemistry
- LC/MS/MS
- liquid chromotography tandem mass spectrometry
- MC4R
- melanocortin-4 receptor
- MUFA
- monounsaturated fatty acid
- NAE
- N-acylethanolamine
- NAPE
- N-acylphosphatidylethanolamine
- NAPE-PLD
- NAPE phospholipase D
- OEA
- oleoylethanolamide
- PVN
- paraventricular nucleus
- SFA
- saturated fatty acid
- WT
- wild type.
References
- 1. Azzara AV, Sokolnicki JP, Schwartz GJ. 2002. Central melanocortin receptor agonist reduces spontaneous and scheduled meal size but does not augment duodenal preload-induced feeding inhibition. Physiol Behav 77:411–416 [DOI] [PubMed] [Google Scholar]
- 2. Zheng H, Patterson LM, Phifer CB, Berthoud HR. 2005. Brain stem melanocortinergic modulation of meal size and identification of hypothalamic POMC projections. Am J Physiol Regul Integr Comp Physiol 289:R247–R258 [DOI] [PubMed] [Google Scholar]
- 3. Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. 2001. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 4:605–611 [DOI] [PubMed] [Google Scholar]
- 4. Sutton GM, Duos B, Patterson LM, Berthoud HR. 2005. Melanocortinergic modulation of cholecystokinin-induced suppression of feeding through extracellular signal-regulated kinase signaling in rat solitary nucleus. Endocrinology 146:3739–3747 [DOI] [PubMed] [Google Scholar]
- 5. Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. 2007. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148: 1550–1560 [DOI] [PubMed] [Google Scholar]
- 6. Fan W, Ellacott KL, Halatchev IG, Takahashi K, Yu P, Cone RD. 2004. Cholecystokinin-mediated suppression of feeding involves the brainstem melanocortin system. Nat Neurosci 7:335–336 [DOI] [PubMed] [Google Scholar]
- 7. Tracy AL, Clegg DJ, Johnson JD, Davidson TL, Benoit SC. 2008. The melanocortin antagonist AgRP (83–132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav 89:263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boghossian S, Park M, York DA. Melanocortin activity in the amygdala controls appetite for dietary fat 2010 Am J Physiol Regul Integr Comp Physiol 298:R385–R393 [DOI] [PubMed] [Google Scholar]
- 9. Maljaars PW, Symersky T, Kee BC, Haddeman E, Peters HP, Masclee AA. 2008. Effect of ileal fat perfusion on satiety and hormone release in healthy volunteers. Int J Obes (Lond) 32:1633–1639 [DOI] [PubMed] [Google Scholar]
- 10. Sufian MK, Hira T, Miyashita K, Nishi T, Asano K, Hara H. 2006. Pork peptone stimulates cholecystokinin secretion from enteroendocrine cells and suppresses appetite in rats. Biosci Biotechnol Biochem 70:1869–1874 [DOI] [PubMed] [Google Scholar]
- 11. Lo CM, King A, Samuelson LC, Kindel TL, Rider T, Jandacek RJ, Raybould HE, Woods SC, Tso P. 2010. Cholecystokinin knockout mice are resistant to high-fat diet-induced obesity. Gastroenterology 138:1997–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Donovan MJ, Paulino G, Raybould HE. 2007. CCK(1) receptor is essential for normal meal patterning in mice fed high fat diet. Physiol Behav 92:969–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillum MP, Zhang D, Zhang XM, Erion DM, Jamison RA, Choi C, Dong J, Shanabrough M, Duenas HR, Frederick DW, Hsiao JJ, Horvath TL, Lo CM, Tso P, Cline GW, Shulman GI. 2008. N-acylphosphatidylethanolamine, a gut- derived circulating factor induced by fat ingestion, inhibits food intake. Cell 135:813–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han S, Chen X, Cox B, Yang CL, Wu YM, Naes L, Westfall T. 1998. Role of neuropeptide Y in cold stress-induced hypertension. Peptides 19:351–358 [DOI] [PubMed] [Google Scholar]
- 15. Di Marzo V, Ligresti A, Cristino L. 2009. The endocannabinoid system as a link between homoeostatic and hedonic pathways involved in energy balance regulation. Int J Obes (Lond) 33(Suppl 2):S18–S24 [DOI] [PubMed] [Google Scholar]
- 16. Williams CM, Kirkham TC. 1999. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 143:315–317 [DOI] [PubMed] [Google Scholar]
- 17. Lo Verme J, Gaetani S, Fu J, Oveisi F, Burton K, Piomelli D. 2005. Regulation of food intake by oleoylethanolamide. Cell Mol Life Sci 62:708–716 [DOI] [PubMed] [Google Scholar]
- 18. Tourino C, Oveisi F, Lockney J, Piomelli D, Maldonado R. 2010. FAAH deficiency promotes energy storage and enhances the motivation for food. Int J Obes (Lond) 34:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Egertova M, Simon GM, Cravatt BF, Elphick MR. 2008. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol 506:604–615 [DOI] [PubMed] [Google Scholar]
- 20. Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. 1997. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141 [DOI] [PubMed] [Google Scholar]
- 21. Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. 2003. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23:7143–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. 2009. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patricelli MP, Cravatt BF. 2001. Characterization and manipulation of the acyl chain selectivity of fatty acid amide hydrolase. Biochemistry 40:6107–6115 [DOI] [PubMed] [Google Scholar]
- 24. Holder JL, Jr, Zhang L, Kublaoui BM, DiLeone RJ, Oz OK, Bair CH, Lee YH, Zinn AR. 2004. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am J Physiol Endocrinol Metab 287:E105–E113 [DOI] [PubMed] [Google Scholar]
- 25. Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB. 2005. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- 26. Vaisse C, Clement K, Guy-Grand B, Froguel P. 1998. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genetics 20:113–114 [DOI] [PubMed] [Google Scholar]
- 27. Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O'Rahilly S. 1998. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet 20:111–112 [DOI] [PubMed] [Google Scholar]
- 28. Cone RD. 2005. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- 29. Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O'Rahilly S, Farooqi IS. 2009. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360:44–52 [DOI] [PubMed] [Google Scholar]
- 30. Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. 2000. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA 97:12339–12344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. 2007. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148:1550–1560 [DOI] [PubMed] [Google Scholar]
- 32. Mountjoy KG, Mortrud MT, Low MJ, Simerly RB, Cone RD. 1994. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol Endo 8:1298–1308 [DOI] [PubMed] [Google Scholar]
- 33. Roselli-Rehfuss L, Mountjoy KG, Robbins LS, Mortrud MT, Low MJ, Tatro JB, Entwistle ML, Simerly RB, Cone RD. 1993. Identification of a receptor for gamma melanotropin and other proopiomelanocortin peptides in the hypothalamus and limbic system. Proc Natl Acad Sci USA 90:8856–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hagan MM, Rushing PA, Benoit SC, Woods SC, Seeley RJ. 2001. Opioid receptor involvement in the effect of AgRP- (83–132) on food intake and food selection. Am J Physiol Regul Integr Comp Physiol 280:R814–R821 [DOI] [PubMed] [Google Scholar]
- 35. Koegler FH, Schaffhauser RO, Mynatt RL, York DA, Bray GA. 1999. Macronutrient diet intake of the lethal yellow agouti (Ay/a) mouse. Physiol Behav 67:809–812 [DOI] [PubMed] [Google Scholar]
- 36. Samama P, Rumennik L, Grippo JF. 2003. The melanocortin receptor MCR4 controls fat consumption. Regul Pept 113:85–88 [DOI] [PubMed] [Google Scholar]
- 37. Barnes MJ, Argyropoulos G, Bray GA. 2010. Preference for a high fat diet, but not hyperphagia following activation of mu opioid receptors is blocked in AgRP knockout mice. Brain Res 1317:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zheng H, Townsend RL, Shin AC, Patterson LM, Phifer CB, Berthoud HR. 2010. High-fat intake induced by mu-opioid activation of the nucleus accumbens is inhibited by Y1R-blockade and MC3/4R-stimulation. Brain Res 1350:131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang M, Gosnell BA, Kelley AE. 1998. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther 285:908–914 [PubMed] [Google Scholar]
- 40. Gosnell BA, Krahn DD. 1993. The effects of continuous morphine infusion on diet selection and body weight. Physiol Behav 54:853–859 [DOI] [PubMed] [Google Scholar]
- 41. Lawrence CB, Snape AC, Baudoin FM, Luckman SM. 2002. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143:155–162 [DOI] [PubMed] [Google Scholar]