The TRβ-selective agonist GC-24, unlike GC-1, is not active on the brain, whereas the TRα-selective CO23 is active in brain but shows no TR isoform selectivity in vivo.
Abstract
Thyroid hormone analogs with selective actions through specific thyroid hormone receptor (TR) subtypes are of great interest. They might offer the possibility of mimicking physiological actions of thyroid hormone with receptor subtype or tissue specificity with therapeutic aims. They are also pharmacological tools to dissect biochemical pathways mediated by specific receptor subtypes, in a complementary way to mouse genetic modifications. In this work, we studied the in vivo activity in developing rats of two thyroid hormone agonists, the TRβ-selective GC-24 and the TRα-selective CO23. Our principal goal was to check whether these compounds were active in the rat brain. Analog activity was assessed by measuring the expression of thyroid hormone target genes in liver, heart, and brain, after administration to hypothyroid rats. GC-24 was very selective for TRβ and lacked activity on the brain. On the other hand, CO23 was active in liver, heart, and brain on genes regulated by either TRα or TRβ. This compound, previously shown to be TRα-selective in tadpoles, displayed no selectivity in the rat in vivo.
The physiological actions of thyroid hormones are mediated in part by interaction of T3 with nuclear receptors [thyroid hormone receptors (TRs)] and regulation of gene expression (for a recent review, please see Ref. 1). There are two receptor subtypes in mammals, TRα and TRβ, and three isoforms, TRα1, TRβ1, and TRβ2, encoded by two distinct genes, Thra and Thrb. Results from knockout mice lacking either receptor subtype have shown overlapping as well as specific functions in vivo (2–5). The different roles in vivo of TRα1 and TRβ are mostly due to differences in cellular or tissue expression, with some exceptions (6–8). In this respect, TRβ1 is the predominant receptor subtype in liver, whereas in the heart and brain, the predominant receptor subtype is TRα1, which accounts for up to 80–85% of total T3 binding (2, 9).
Selective targeting of TRβ has great therapeutic interest, in view of the role that TRβ plays in lipid metabolism (10, 11). TRβ-selective agonists are able to reduce blood levels of cholesterol, triglycerides, and lipoprotein(a) and may also reduce body weight (BW), without affecting the heart. The metabolic actions of several compounds, such as GC-1, GC-24, KB-141, and KB-2115 (12–15), have been extensively studied, although little attention has been paid to their effects on the brain (8, 16–19). Given the involvement of TRα1 in behavior (20, 21), TRα1 targeting in the brain might be of value in the treatment of anxiety and other psychiatric disorders. T3 analogs able to be transported and act on the brain would also be valuable agents in the treatment of patients with monocarboxylate transporter 8 (MCT8) mutations (22). The goal of this work was to examine whether the TRβ-selective T3 agonist GC-24 (12, 19, 23) and the TRα1-selective agonist, CO23 (19, 24, 25), are active on T3 targets in the brain.
Materials and Methods
Wistar rats bred in our animal facilities were used. Animals were under light- and temperature-controlled conditions (12-h light, 12-h dark cycle and 22 ± 2 C) and had free access to food and water. Animal procedures were supervised by institutional Ethic Committee, and in agreement with directives from the European Union. Euthyroid, hypothyroid, and hypothyroid rat pups treated with T3 or the analogs were used. Hypothyroidism was induced by administering 0.02% 2-mercapto-1-methylimidazol (Sigma Chemical Co, St. Louis, MO) and 1% KClO4 in the drinking water to the dams from gestational d 9 until the end of the experiment on postnatal d (P)16. T3 was administered to the hypothyroid pups at a daily dose of 22.5 pmol/g of BW (15 ng/g) in PBS containing 0.1% BSA. GC-24 and CO23 were administered to the pups in the same solution also as single daily ip injections at the doses specified for each experiment. Treatments were started on P10, and the last dose was administered on P15. Twenty-four hours after the last injection, the animals were anesthetized and decapitated for PCR assays or perfused with 4% paraformaldehyde in 0.1 m phosphate buffer for in situ hybridization.
RNA preparation and real-time PCR was performed as described (26), using TaqMan probes (Applied Biosystems, Foster City, CA) for genes previously shown to be sensitive to thyroid hormones: type 1 deiodinase (Dio1, Rn00572183_m1), glutathion-S-transferase (Gsta3, Rn00579867_m1), sarcoplasmic reticulum ATPase or sarco/endoplasmic reticulum calcium transporting ATPase (Atp2a2, Rn00568762_m1), myosin heavy chain α (Myh6, Rn00568304_m1) and myosin heavy chain β (Myh7, Rn00568328_m1), hairless (Hr, Rn00577605_m1), RevErbAα (Nr1d1, Rn00595671_m1), neurotrophin 3 (Ntf3, Rn00579280_m1), reelin (Reln, Rn00589609_m1), synaptotagmin 12 (Syt12, Rn00593706_m1), RC3/neurogranin (Nrgn, Rn00480741_m1), and ras homolog enriched in striatum (Rasd2, Rn00592054_m1). TRβ was also measured using a TaqMan probe (Thrb, Rn00562044_m1), and TRα1 was measured using SYBRGreen quantitative PCR with the forward primer 5′AGCTGCTGATGAAGGTGACTGA3′ and reverse primer 5′TGAGGCTTTAGACTTCCTGATCCT3′.
Total plasma cholesterol was measured using the Infinity Cholesterol kit supplied by Thermo Electron (Thermo Fisher Scientific, Auburn, AL). In situ mRNA hybridization analysis for Nrgn, Rasd2 in the cerebrum, and Hr in the cerebellum was performed using methods previously described in detail (8). Primary neuronal cultures were established as previously described (26).
Data were analyzed with the GraphPad Prism 5 software (GraphPad, San Diego, CA). For the comparisons between groups, we used one-way ANOVA with Tukey′s test as post hoc test. All results are expressed as mean ± sem. Statistical comparisons shown in most panels are made to the hypothyroid group, so as to evaluate the effect of hypothyroidism vs. the control and the effect of treatments vs. the hypothyroid. Results on multiple comparisons of all groups are provided in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
Results
GC-24 is a TRβ-selective compound in vivo without effects on the brain
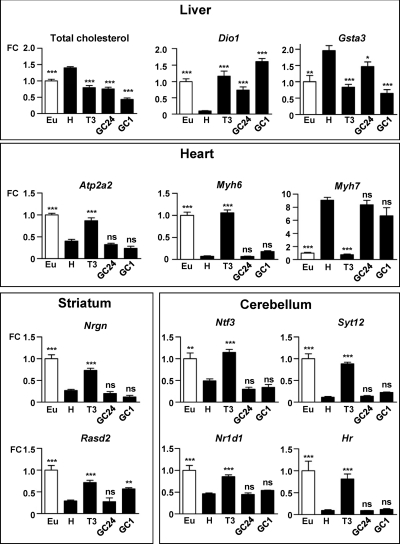
T3 or GC-24 was administered to hypothyroid pups at the dose of 22.5 pmol/g, and thyroid hormone responses were studied in liver, heart, striatum, and cerebellum (Fig. 1). In agreement with the TRβ selectivity, GC-24 was active in liver but not in heart. First, plasma cholesterol, which in hypothyroid pups was elevated by 40% with respect to controls, decreased by 45% after treatment with T3 or GC-24. For comparison, we also tested the TRβ selective compound GC-1 at the same molar dose. Treatment of hypothyroid rats with this compound reduced plasma cholesterol by 70%. As T3 target genes, we measured the expression of Dio1, an up-regulated gene (27), and Gsta3, a down-regulated gene (28). Dio1 was decreased in hypothyroid animals to less than 10% of control values. It then increased after treatment of hypothyroid rats with T3 (12-fold), GC-24 (8-fold), or GC-1 (16-fold). Conversely, Gsta3 was increased almost 2-fold in untreated hypothyroid livers and was decreased by T3 (58%), GC-24 (25%), and GC-1 (68%). We found that GC-1 was more potent than T3 or GC-24 on liver targets.
Fig. 1.
Effects of T3 and the TR synthetic agonists GC-24 and GC-1 on liver, heart, striatum, and cerebellum. Black bars, Each compound was administered to hypothyroid rats (H) as one daily injection of 22.5 pmol/g from P10 to P15. Open bar, Euthyroid animals. On P16, several end points of thyroid hormone action were measured. Typical TRβ responses, such as plasma cholesterol and liver Dio1 and Gsta3 mRNAs, were sensitive to hypothyroidism and drug administration. TRα-mediated responses, such as heart Atp2a2, Myh6, and Myh7, were sensitive to T3 but insensitive to GC-1 or GC-24 treatment. Thyroid hormone-regulated striatal and cerebellar genes were insensitive to GC-24. Statistical comparisons are made to the hypothyroid group, so as to evaluate the difference between euthyroid (open bar) and hypothyroid animals and the effect of treatments vs. the untreated hypothyroid animals. See Supplemental Table 1 for multiple cross comparisons. Eu, Untreated euthyroid rats (n = 5); H, untreated hypothyroid rats (n = 5); T3, T3-treated H rats (n = 5); GC-24, GC-24-treated H rats (n = 7); GC-1, GC-1-treated H rats (n = 4). *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. ns, Not significant; FC, fold change.
In the heart, the thyroid hormone responsive genes Atp2a2 (29) and Myh6 (30) were decreased by hypothyroidism by 60 and 90%, respectively. T3 treatment of hypothyroid rats induced these genes by 2- and 15-fold, respectively, but neither GC-24 nor GC-1 were active. Myh7, which increased 9-fold after hypothyroidism, decreased to euthyroid levels after T3, but GC-1 nor GC-24 were active. As for the brain, we measured the effects on the striatum and the cerebellum. In the striatum, hypothyroidism reduced the expression of Nrgn and Rasd2 by 75%, and T3 administration resulted in a 2.5-fold increase of both genes, as shown before (8). Also, as reported previously (8), GC-1 increased Rasd2 expression but was without effect on Nrgn. In contrast, GC-24 was without effect on either gene. These results confirm previous findings that regulation of Nrgn by T3 was mediated by TRα1, whereas Rasd2 was also under the influence of TRβ (8).
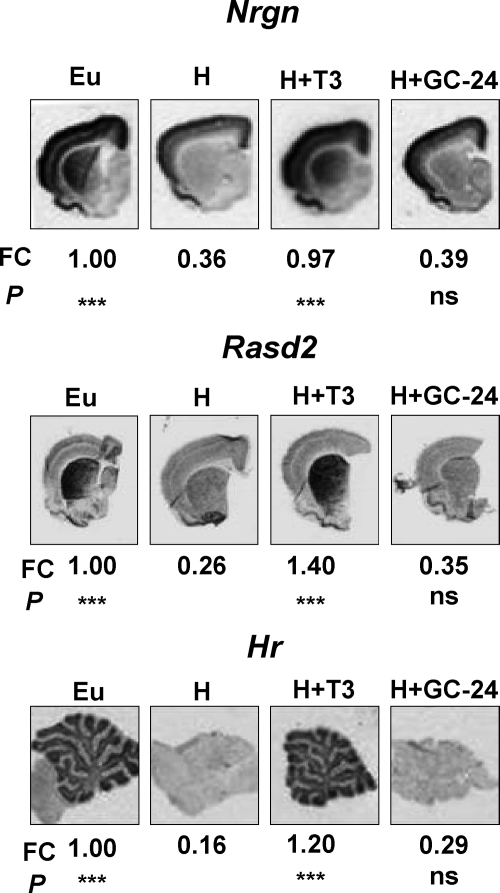
Known target genes of thyroid hormone in the cerebellum, such as of Ntf3 (31), Syt12, Hr (32), and Nr1d1 (8), were not influenced by treatment with GC-1 or GC-24. The lack of effect of GC-24 in comparison with T3 was also confirmed by in situ hybridization (Fig. 2) and by analyzing its effect on migration of cerebellar granule cells (data not shown).
Fig. 2.
In situ hybridization for Nrgn, Rasd2 in the cerebrum, and Hr in the cerebellum in euthyroid rats, hypothyroid rats (H), and hypothyroid rats treated with one daily injection of 45 pmol/g BW T3 or GC-24 for 6 d. The fold change (FC) and statistical significance (P) of differences respect to the hypothyroid sample are given after the quantification of slices from three animals per group. Eu, Untreated euthyroid; ns, not significant.
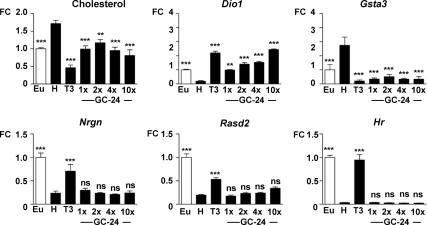
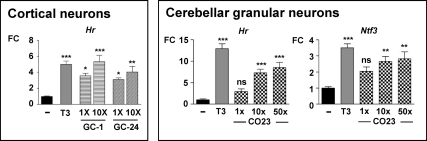
In view of the lack of effect of GC-24 on Rasd2, in contrast to GC-1, we checked the effect of higher doses. GC-24 was administered to the hypothyroid pups in incremental doses of 22.5, 45, 90, and 225 pmol/g for 6 d and compared with 22.5 pmol/g of T3. The results are shown in Fig. 3. GC-24 induced liver responses, such as cholesterol reduction, Dio1 up-regulation, and Gsta3 down-regulation at all the doses, whereas it had no effect on Nrgn and Rasd2 in the striatum or Hr in the cerebellum. In addition, cerebellar Rln mRNA, which is down-regulated by T3 through TRβ (8), was unaltered by GC-24 treatment (Supplemental Fig. 1). Despite the lack of action in vivo, GC-24, as well as GC-1, was able to induce Hr in cerebral cortex neurons in culture (Fig. 4).
Fig. 3.
Effects of high doses of GC-24. Black bars, GC-24 was administered to hypothyroid rats (H) as single daily injections for 6 d at the doses of 22.5, 45, 90, and 225 pmol/g BW. These doses represent 1×, 2×, 4×, and 10× the T3 dose used, 22.5 pmol/g BW. Although GC-24 was active on the liver, there was no effect of on striatal or cerebellar genes. Statistical comparisons are made to the hypothyroid group, so as to evaluate the difference between euthyroid (open bar) and hypothyroid animals, and the effect of treatments vs. the hypothyroid animals. See Supplemental Table 1 for multiple cross comparisons. Eu, Untreated euthyroid rats; H, untreated hypothyroid rats. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n = 6 in all groups. ns, Not significant; FC, fold change.
Fig. 4.
Effects of agonists of TR on primary cultures of cortical and cerebellar neurons. Primary cultures were established from neonatal rat cortex (left panel) or cerebellum (right panel) and incubated in the absence or presence of T3 1× (10 nm), GC-1, GC-24, or CO23 at the concentrations indicated expressed as fold over the T3 concentration. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n = 6 in all groups. ns, Not significant; FC, fold change.
CO23 acts through TRα and TRβ in vivo and is active in the brain
In preliminary experiments (data not shown), a dose of CO23 of 22.5 pmol/g had no effect on plasma cholesterol, liver Dio1 and Gsta3, heart Myh6, Myh7, and Atp2a2, or cerebellum Hr and Ntf3. The same dose of T3 normalized all these parameters, in a similar way as described in Fig. 1. The relative activity of CO23, compared with T3, was then evaluated in cerebellar granule neurons in primary cultures (Fig. 4). Addition of 10 nm T3 to the cultures increased Hr mRNA almost by 20-fold and Ntf3 by 3-fold. CO23 was used at incremental doses of 10, 100, and 500 nm. Significant responses were obtained with the two higher doses. The results suggested that CO23 was at least 50 times less active than T3 on isolated neurons.
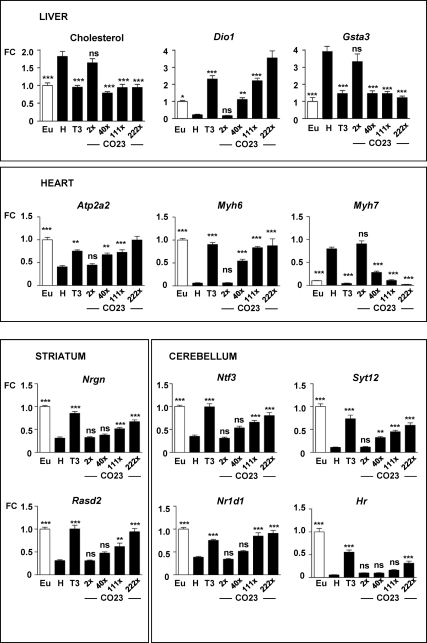
Based on the above data, we used CO23 for in vivo experiments at doses of 0.04, 0.8, 2.5, and 5.0 nmol/g, and the results are shown in Fig. 5. After administration to hypothyroid rats, cholesterol was not changed by the lower dose, whereas the 0.8 nmol/g dose had a similar effect as 22.5 pmol/g T3 (≈50% cholesterol reduction). Higher CO23 doses were equally effective. T3 increased Dio1 mRNA 10-fold after administration to hypothyroid rats. CO23 had no effect at the lowest dose of 0.04 nmol/g and increased Dio1 mRNA 5-, 10-, and 15-fold at the doses of 0.8, 2.5, and 5 nmol/g, respectively. Therefore, for liver Dio1, a dose of 2.5 nmol/g of CO23 was equivalent to 22.5 pmol/g of T3. The effect on Gsta3 was similar than on cholesterol, with the dose of 0.8 nmol/g having a similar effect as 22.5 pmol T3 (65% Gsta3 mRNA reduction over hypothyroid levels).
Fig. 5.
Effect of CO23 on liver, heart, striatum, and cerebellum. Black bars, Hypothyroid rats were given one daily dose of 22.5 pmol/g BW T3 for 6 d or incremental doses of CO23: 0.04, 0.8, 2.5, and 5.0 nmol/g BW (2×, 40×, 111×, and 222×, respectively, the molar dose of T3 used in the experiment). Statistical comparisons were made to the hypothyroid group, so as to evaluate the difference between euthyroid (open bar) and hypothyroid animals and the effect of treatments vs. the hypothyroid animals. See Supplemental Table 1 for multiple cross comparisons. Eu, Untreated euthyroid rats; H, untreated hypothyroid rats; n = 8 in all groups. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001. ns, Not significant; FC, fold change.
In the heart, 2.5 nmol/g of CO23 was equivalent to 22.5 pmol/g of T3 on Atp2a2 induction (1.8-fold in each case), and the effect was still higher (2.4-fold) with the highest dose. For the myosin heavy chains, 2.5 nmol/g of CO23 was also equivalent to 22.5 pmol/g T3 in the induction of Myh6 (14-fold). T3 treatment decreased by 95% the elevated levels of Myh7 mRNA of hypothyroid rats. Although 0.8 nmol/g of CO23 induced a 65% decreased, it required the highest dose to elicit a similar effect than 22.5 pmol T3.
In the brain, the lowest effective dose of CO23 was 0.8 nmol for Syt12 induction (3-fold). For Nrgn, Rasd2, Ntf3, and Nr1d1, the lowest dose that resulted in significant induction was 2.5 nmol/g. The least sensitive gene was Hr, which required the highest dose of CO23 with about half the effect of T3 (5.5- vs. 9.7-fold). In the cerebellum, Rln was insensitive to CO23 (Supplemental Fig. 1).
Finally we considered the possibility that some of the observations related to the effects of T3 analog administration to hypothyroid pups was at least partially influenced by altered expression of the TRs by hypothyroidism. Therefore, we measured the relative amounts of TRα1 and TRβ mRNAs in the striatum of euthyroid and hypothyroid pups. Hypothyroidism increased TRα1 mRNA by 48% (P = 0.02) and decreased TRβ mRNA by 25% (P = 0.013) in relation to the euthyroid values (Supplemental Fig. 2).
Discussion
In this work, we compared the pharmacological activity of two thyroid hormone analogs, GC-24 and CO23, previously described as being TRβ- and TRα1-selective compounds, respectively (12, 23–25). Our primary interest was to analyze the effects of these compounds in the rat brain, which is often neglected in studies on the biological activity of thyroid hormone analogs.
As previously reported, GC-24 was found to have TRβ selectivity in vivo. Thyroid hormone-sensitive liver responses, such as plasma cholesterol, and expression of Dio1 and Gsta3 genes were sensitive to GC-24. In contrast to the liver, the heart genes Atp2a2, Myh6, and Myh7 were not sensitive to GC-24 or GC-1, as was expected, because heart responses to thyroid hormone are mediated in large part by TRα1 (33).
In contrast to the liver, GC-24 was not active in the brain. We have previously shown that GC-1 has limited activity in brain (8, 17, 18), especially on genes that in the cerebellum are expressed in the granular cells, which express predominantly TRα1. GC-24 was also not active on cerebellar genes, but GC-1 and GC-24 differed in the induction of striatal Rasd2. In an earlier study (8), we found that GC-1 was able to induce Rasd2 in the striatum but not Nrgn, even if these two genes are expressed in the same kind of cells, the medium γ-aminobutyric acid-ergic interneurons. We confirmed this result and show here that in contrast to GC-1, GC-24 was not active on Rasd2. In addition, GC-24 had no activity on cerebellar Rln, a gene also sensitive to GC-1 (8). The results indicate that GC-24 has no activity in the brain, which is likely due to restricted entry through the blood-brain barrier. Another factor that may contribute to the lack of activity of TRβ-selective compounds in the brain is the increase of the TRα1/TRβ ratio that occurs in hypothyroidism. An increase of TRα1 expression of 40% and decrease TRβ of 25%, as found in this work, may facilitate TRα1 responses, especially in the setting of restricted brain accumulation of the agonists.
As reported previously by Ocasio and Scanlan (24), CO23 showed no preference for TR subtype in a competitive 125I-T3 binding assay in vitro. Despite this, it displayed TRα-selective properties in cultured cells and in vivo. In cultured cells, it had 5-fold higher activity through TRα over TRβ in the activation of a direct repeat 4 thyroid responsive element in U2OS and HeLa cells (24). In vivo studies on the comparative actions of T3, GC-1, and CO23 on metamorphosis showed that CO23 had similar effects as T3 on hind limb growth but required 7-fold higher concentrations (16, 24). CO23 was much less active in tail, gills, and head resorption. CO23 had also similar effects as T3 on cell proliferation in the ventricular and subventricular zones during neurogenesis, whereas GC-1 and GC-24 had no effect (16). It was also observed that the combination of CO23 and GC-1 was able to mimic T3 action completely. These observations support the TRα selectivity of CO23.
Despite its TRα subtype selectivity on tadpoles, when administered to rats, CO23 was active in tissues expressing preferentially either TRα1 or TRβ. For example, CO23 was found to activate thyroid hormone-responsive liver and heart genes in a similar way as T3, unlike the TRβ-selective GC-1, which acts similarly to T3 in the liver and lacks activity on the heart. These differences in TR selectivity might be related to differences in tissue distribution of the T3 analogs. For example, the accumulation of GC-1 in liver is 17-fold of that in the heart, and this contributes to the in vivo organ selectivity (10, 11). High doses of CO23 needed to be used in vivo, due to the fact that the relative potency of CO23, in comparison with T3, was relatively low. In cultured cerebellar granular cells, it was estimated that the potency of CO23 was more than 50-fold lower that of T3. In vivo, high doses 100- to 200-fold of that of T3 had to be used. Therefore, it may be argued that by using high doses of the agonist, we increase the chance of having an effect in liver through activation of TRα1, if CO23 accumulates preferentially in this organ.
We have no data on tissue CO23 distribution. However, it seems unlikely that the effects of CO23 on plasma cholesterol and liver Dio1 are due to preferential accumulation of CO23 in the liver and subsequent activation of TRα1. The cholesterol response to thyroid hormone is totally dependent on TRβ. TRβ knockout mice are resistant to T3-induced reduction in cholesterol, a defect that cannot be rescued by overexpressing TRα1 by 6-fold in the liver (34). On the other hand, Dio1 expression shows a zonal distribution in the liver, with low mRNA concentration around the periportal area and increasing toward the pericentral area, where it reaches the highest level. This pattern of expression follows closely that of TRβ1 (35). In addition, only extremely high doses of T3 administered to TRβ-deficient mice were able to weakly induce Dio1 (36). Therefore, we think that the liver responses to CO23 are due to TRβ1 stimulation.
Taken together, the response of liver, heart, and brain suggests that CO23 was similarly active on TRα1 and TRβ, but a subtle preference for TRα1 cannot be discarded. In fact, the lack of response of Rln may suggest that CO23 entry into the brain is not as efficient as in the liver, precluding the activation of TRβ. Despite this, our results indicate that CO23 was able to be transported through the blood-brain barrier and to cross the neuronal cell membrane. Although we did not identify the specific transporter involved (37), it is unlikely that it uses the Mct8 transporter, which has extremely tight selectivity for T4 and T3. The reason for the apparent lack of receptor subtype selectivity of CO23 in rodents as compared with metamorphosing tadpoles is unknown at present. The lack of TR selectivity in mammalian tissues would make this compound less interesting as a therapeutic agent. On the other hand, its ability to cross the blood-brain barrier would make it worth to investigate its capacity to act on the brain in the absence of the specific T4 and T3 transporter Mct8 (22).
Acknowledgments
We thank Eulalia Moreno for technical work.
This work was supported by Ministry of Science of Spain Grants SAF2008-01168 and SAF2008-00429E, the European Union Integrated Project “CRESCENDO,” the Center for Biomedical Research on Rare Diseases, the Instituto de Salud Carlos III (J.B.), and the National Institutes of Health Grant DK-52798 (to T.S.S.).
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 745
- Atp2a2
- Sarcoplasmic reticulum ATPase
- BW
- body weight
- Dio1
- type 1 deiodinase
- Gsta3
- glutathion-S-transferase
- Hr
- hairless
- Mct8 or MCT8
- monocarboxylate transporter 8
- Myh6
- myosin heavy chain α
- Myh7
- myosin heavy chain β
- Nrgn
- RC3/neurogranin
- Nr1d1
- RevErbAα
- Ntf3
- neurotrophin 3
- P
- postnatal day
- Rasd2
- Rhes
- Syt12
- synaptotagmin 12
- TR
- thyroid hormone receptor.
References
- 1. Cheng SY, Leonard JL, Davis PJ. 2010. Molecular aspects of thyroid hormone actions. Endocr Rev 31:139–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ercan-Fang S, Schwartz HL, Oppenheimer JH. 1996. Isoform-specific 3,5,3′-triiodothyronine receptor binding capacity and messenger ribonucleic acid content in rat adenohypophysis: effect of thyroidal state and comparison with extrapituitary tissues. Endocrinology 137:3228–3233 [DOI] [PubMed] [Google Scholar]
- 3. Forrest D, Hanebuth E, Smeyne RJ, Everds N, Stewart CL, Wehner JM, Curran T. 1996. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor β: evidence for tissue-specific modulation of receptor function. EMBO J 15:3006–3015 [PMC free article] [PubMed] [Google Scholar]
- 4. Wallis K, Sjögren M, van Hogerlinden M, Silberberg G, Fisahn A, Nordström K, Larsson L, Westerblad H, Morreale de Escobar G, Shupliakov O, Vennström B. 2008. Locomotor deficiencies and aberrant development of subtype-specific GABAergic interneurons caused by an unliganded thyroid hormone receptor α1. J Neurosci 28:1904–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wikström L, Johansson C, Saltó C, Barlow C, Campos Barros A, Baas F, Forrest D, Thorén P, Vennström B. 1998. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J 17:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barca-Mayo O, Liao XH, Alonso-Sampedro M, Di Cosmo C, Refetoff S, Weiss RE. Impairment of type 3 deiodinase (D3) contributes to the hypersensitivity to thyroid hormone (TH) of TRα knock out (TRaKO) mice. 92nd Annual Meeting of The Endocrine Society, San Diego, CA, 2010, p. S2541 (Abstract OR41-3) [Google Scholar]
- 7. Chiamolera MI, Matsumoto S, Ortiga-Carvalho TM, Wondisford FE. Fundamentally different roles for thyroid hormone receptor isoforms in thyroid hormone pituitary feedback. 92nd Annual Meeting of The Endocrine Society, San Diego, CA, 2010, p. S2542, (Abstract OR41-4) [Google Scholar]
- 8. Manzano J, Morte B, Scanlan TS, Bernal J. 2003. Differential effects of triiodothyronine and the thyroid hormone receptor β-specific agonist GC-1 on thyroid hormone target genes in the brain. Endocrinology 144:5480–5487 [DOI] [PubMed] [Google Scholar]
- 9. Gloss B, Trost S, Bluhm W, Swanson E, Clark R, Winkfein R, Janzen K, Giles W, Chassande O, Samarut J, Dillmann W. 2001. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142:544–550 [DOI] [PubMed] [Google Scholar]
- 10. Baxter JD, Webb P, Grover G, Scanlan TS. 2004. Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol Metab 15:154–157 [DOI] [PubMed] [Google Scholar]
- 11. Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. 2007. Targeting thyroid hormone receptor-β agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 104:15490–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Borngraeber S, Budny MJ, Chiellini G, Cunha-Lima ST, Togashi M, Webb P, Baxter JD, Scanlan TS, Fletterick RJ. 2003. Ligand selectivity by seeking hydrophobicity in thyroid hormone receptor. Proc Natl Acad Sci USA 100:15358–15363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. 2004. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-L-thyronine. Endocrinology 145:1656–1661 [DOI] [PubMed] [Google Scholar]
- 14. Grover GJ, Mellström K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennström B, Mookhtiar K, Horvath R, Speelman J, Egan D, Baxter JD. 2003. Selective thyroid hormone receptor-β activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA 100:10067–10072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. 2010. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med 362:906–916 [DOI] [PubMed] [Google Scholar]
- 16. Denver RJ, Hu F, Scanlan TS, Furlow JD. 2009. Thyroid hormone receptor subtype specificity for hormone-dependent neurogenesis in Xenopus laevis. Dev Biol 326:155–168 [DOI] [PubMed] [Google Scholar]
- 17. Morte B, Manzano J, Scanlan T, Vennström B, Bernal J. 2002. Deletion of the thyroid hormone receptor α1 prevents the structural alterations of the cerebellum induced by hypothyroidism. Proc Natl Acad Sci USA 99:3985–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morte B, Manzano J, Scanlan TS, Vennström B, Bernal J. 2004. Aberrant maturation of astrocytes in thyroid hormone receptor α1 knockout mice reveals an interplay between thyroid hormone receptor isoforms. Endocrinology 145:1386–1391 [DOI] [PubMed] [Google Scholar]
- 19. Ribeiro MO. 2008. Effects of thyroid hormone analogs on lipid metabolism and thermogenesis. Thyroid 18:197–203 [DOI] [PubMed] [Google Scholar]
- 20. Guadaño-Ferraz A, Benavides-Piccione R, Venero C, Lancha C, Vennström B, Sandi C, DeFelipe J, Bernal J. 2003. Lack of thyroid hormone receptor α1 is associated with selective alterations in behavior and hippocampal circuits. Mol Psychiatry 8:30–38 [DOI] [PubMed] [Google Scholar]
- 21. Venero C, Guadaño-Ferraz A, Herrero AI, Nordström K, Manzano J, de Escobar GM, Bernal J, Vennström B. 2005. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor α1 can be ameliorated by T3 treatment. Genes Dev 19:2152–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Cosmo C, Liao XH, Dumitrescu AM, Weiss RE, Refetoff S. 2009. A thyroid hormone analog with reduced dependence on the monocarboxylate transporter 8 for tissue transport. Endocrinology 150:4450–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martínez L, Nascimento AS, Nunes FM, Phillips K, Aparicio R, Dias SM, Figueira AC, Lin JH, Nguyen P, Apriletti JW, Neves FA, Baxter JD, Webb P, Skaf MS, Polikarpov I. 2009. Gaining ligand selectivity in thyroid hormone receptors via entropy. Proc Natl Acad Sci USA 106:20717–20722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ocasio CA, Scanlan TS. 2006. Design and characterization of a thyroid hormone receptor α (TRα)-specific agonist. ACS Chem Biol 1:585–593 [DOI] [PubMed] [Google Scholar]
- 25. Ocasio CA, Scanlan TS. 2008. Characterization of thyroid hormone receptor α (TRα)-specific analogs with varying inner- and outer-ring substituents. Bioorg Med Chem 16:762–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ceballos A, Belinchon MM, Sanchez-Mendoza E, Grijota-Martinez C, Dumitrescu AM, Refetoff S, Morte B, Bernal J. 2009. Importance of monocarboxylate transporter 8 for the blood-brain barrier-dependent availability of 3,5,3′-triiodo-L-thyronine. Endocrinology 150:2491–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zavacki AM, Ying H, Christoffolete MA, Aerts G, So E, Harney JW, Cheng SY, Larsen PR, Bianco AC. 2005. Type 1 iodothyronine deiodinase is a sensitive marker of peripheral thyroid status in the mouse. Endocrinology 146:1568–1575 [DOI] [PubMed] [Google Scholar]
- 28. Caubín J, Iglesias T, Bernal J, Muñoz A, Márquez G, Barbero JL, Zaballos A. 1994. Isolation of genomic DNA fragments corresponding to genes modulated in vivo by a transcription factor. Nucleic Acids Res 22:4132–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rohrer D, Dillmann WH. 1988. Thyroid hormone markedly increases the mRNA coding for sarcoplasmic reticulum Ca2+-ATPase in the rat heart. J Biol Chem 263:6941–6944 [PubMed] [Google Scholar]
- 30. Lompré AM, Nadal-Ginard B, Mahdavi V. 1984. Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem 259:6437–6446 [PubMed] [Google Scholar]
- 31. Lindholm D, Castrén E, Tsoulfas P, Kolbeck R, Berzaghi Mda P, Leingärtner A, Heisenberg CP, Tessarollo L, Parada LF, Thoenen H. 1993. Neurotrophin-3 induced by tri-iodothyronine in cerebellar granule cells promotes Purkinje cell differentiation. J Cell Biol 122:443–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson CC. 1996. Thyroid hormone-responsive genes in developing cerebellum include a novel synaptotagmin and a hairless homolog. J Neurosci 16:7832–7840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Johansson C, Göthe S, Forrest D, Vennström B, Thorén P. 1999. Cardiovascular phenotype and temperature control in mice lacking thyroid hormone receptor-β or both α1 and β. Am J Physiol 276:H2006–H2012 [DOI] [PubMed] [Google Scholar]
- 34. Gullberg H, Rudling M, Saltó C, Forrest D, Angelin B, Vennström B. 2002. Requirement for thyroid hormone receptor β in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol 16:1767–1777 [DOI] [PubMed] [Google Scholar]
- 35. Zandieh Doulabi B, Platvoet-ter Schiphorst M, van Beeren HC, Labruyere WT, Lamers WH, Fliers E, Bakker O, Wiersinga WM. 2002. TR(β)1 protein is preferentially expressed in the pericentral zone of rat liver and exhibits marked diurnal variation. Endocrinology 143:979–984 [DOI] [PubMed] [Google Scholar]
- 36. Amma LL, Campos-Barros A, Wang Z, Vennström B, Forrest D. 2001. Distinct tissue-specific roles for thyroid hormone receptors β and α1 in regulation of type 1 deiodinase expression. Mol Endocrinol 15:467–475 [DOI] [PubMed] [Google Scholar]
- 37. Friesema EC, Jansen J, Milici C, Visser TJ. 2005. Thyroid hormone transporters. Vitam Horm 70:137–167 [DOI] [PubMed] [Google Scholar]