Abstract
Purpose
To test the hypothesis that authors who play key scientific roles in oncology clinical trials, and who therefore have increased influence over the design, analysis, interpretation or reporting of trials, are more likely than those who do not play such roles to have financial ties to industry.
Methods
Data were abstracted from all trials (n = 235) of drugs or biologic agents published in the Journal of Clinical Oncology between January 1, 2006 and June 30, 2007. Article-level data included sponsorship, age group (adult v pediatric), phase, single versus multicenter, country (United States v other), and number of authors. Author-level data (n = 2,927) included financial ties (eg, employment, consulting) and performance of key scientific roles (ie, conception/design, analysis/interpretation, or manuscript writing). Associations between performance of key roles and financial ties, adjusting for article-level covariates, were examined using generalized linear mixed models.
Results
One thousand eight hundred eighty-one authors (64%) reported performing at least one key role, and 842 authors (29%) reported at least one financial tie. Authors who reported performing a key role were more likely than other authors to report financial ties to industry (adjusted odds ratio [OR], 4.3; 99% CI, 3.0 to 6.0; P < .0001). The association was stronger among trials with, compared with those without, industry funding (OR, 5.0 [99% CI, 3.4 to 7.5] v OR, 2.5 [99% CI, 1.3 to 4.8]), but was present regardless of sponsorship.
Conclusion
Authors who perform key roles in the conception and design, analysis, and interpretation, or reporting of oncology clinical trials are more likely than authors who do not perform such roles to have financial ties to industry.
INTRODUCTION
Financial relationships between investigators and industry have prompted widespread concern about the potential for conflicts of interest.1–6 A conflict of interest denotes a circumstance “…in which financial considerations may compromise, or have the appearance of compromising, an investigator's professional judgment in conducting or reporting research.”7 Financial relationships may take several forms, including industry funding of research or personal financial ties (eg, employment, consulting, or honoraria) between investigators and companies. Financial ties are common, with 14% to 70% of authors of articles or of meeting abstracts disclosing financial relationships to industry.8–15
A major criticism of industry-investigator relationships is that such relationships might foster bias in study conclusions. Numerous systematic reviews and meta-analyses indicate that industry-funded studies are more likely than other studies to favor novel interventions.16–27 The mechanisms underlying these differences and their significance remain to be clarified. For example, compared with other funders, industry sponsors may insist on a higher prior probability favoring the novel intervention before committing resources to a trial, fostering efficiency in therapeutic development without necessarily causing ethical problems. However, industry-funded study reports also appear to draw more favorable qualitative conclusions, controlling for quantitative results, than do reports of studies funded by other sources.28–30 Other potential mechanisms include differential publication bias and use of weak control groups in some industry-supported trials.6,19,31
Although extensive evidence demonstrates an association between industry sponsorship and conclusions favoring the novel intervention, little is known about the relationship between investigators' personal financial ties and study outcomes. A study of placebo-controlled trials in psychiatry demonstrated an association between the existence of personal financial ties and conclusions favorable to industry, an effect that was confined to the subset of industry-sponsored trials.32 Other studies show qualitatively similar results.33,34 Analogous data in oncology are unavailable.
An investigator's ability to affect the outcome or reporting of a study depends, in part, on the roles that she played in the trial. Participation in intellectually central study activities, such as conception and design, analysis and interpretation, or manuscript drafting, affords substantial influence over the outcome of the study or the presentation of results. Involvement in other study activities, such as recruitment or data collection, although essential to success, entails less influence over outcomes. To our knowledge, no studies address whether authors' financial ties vary systematically according to their study roles. In this analysis, we tested the hypothesis that authors with key scientific roles are more likely to have financial relationships with industry than authors without key roles. We also asked whether such an association, if it exists, varies by funding source.
METHODS
Study Population
Trained research assistants hand-searched all issues of the Journal of Clinical Oncology (JCO) published between January 1, 2006 and June 30, 2007 to identify eligible articles. JCO was selected because it publishes granular detail on authorship contributions and financial ties,35 the main variables of interest for this analysis. Articles were eligible if they reported the results of a clinical trial evaluating the safety or efficacy of a drug or biologic agent in human subjects. The drug or biologic agent could be administered alone, with other chemotherapy or biologic agents, or with other therapeutic modalities (eg, chemoradiotherapy). Furthermore, the intervention could be directed at prevention, anticancer treatment, or supportive care. We limited articles to those evaluating drugs or biologic agents because studies of other types of interventions (ie, procedural, psychosocial) are unlikely to have industry sponsorship, potentially confounding comparisons between industry-sponsored and other trials.
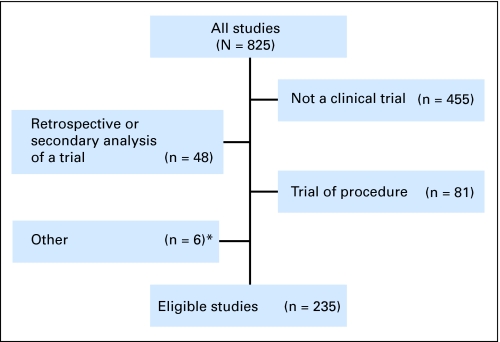
Studies were excluded if they were not clinical trials; were retrospective or secondary analyses; contained two or more trials; were group authored; or compared different schedules of a single intervention (Figure 1). The final sample included 235 studies, involving 2,927 authors (because some individuals contributed to more than one study, these authors represent 2,554 unique individuals). Because data were publicly available, the study was not considered human subjects research under 45CFR46.102(f)(2). This study had no external funding.
Fig 1.
Outcome of manuscript review process. (*) Report contained two or more trials, trial was authored by a group, and/or trial was comparing different schedules of interventions.
Data Collection
Research assistants abstracted article- and author-level data from eligible articles. Article-level data included sponsorship (industry, government, foundation, unspecified), number of centers (single, multi-), age group (adults, children), location of corresponding author (United States, other), phase, and number of authors. Author-level data included financial ties, if any, to industry and authorship contributions.
During the study period, JCO required authors to “… disclose any relationships with commercial entities that may have a direct bearing on the relevant subject matter.”36 Categories provided in the disclosure form included employment, leadership, consultant/advisor, equity, honoraria, research funding, expert testimony, and other remuneration.
JCO also required authors to specify the roles they played in study execution and reporting. Categories included: conception and design; financial support; administrative support; provision of study material or patients; collection and assembly of data; data analysis and interpretation; manuscript writing; and final manuscript approval. Information related to financial relationships and authorship roles, printed after each article, was recorded for each author.
Data Analysis: Variables
The dependent variable was whether or not an author disclosed one or more financial ties to industry. The primary independent variable was whether or not an author reported playing a key study role. Authors were defined as playing a key role if they reported involvement in conception and design, analysis and interpretation, or manuscript writing.
As an alternative to the key role variable, we also determined which authors met the International Committee of Medical Journal Editors (ICMJE) criteria for authorship. The JCO Information for Contributors37 references the ICMJE authorship criteria, which state that “…[e]ach author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content.”38 In addition, “[a]uthorship credit should be based on 1) substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; 2) drafting the article or revising it critically for important intellectual content; and 3) final approval of the version to be published. Authors should meet conditions 1, 2, and 3.”38 We calculated the proportion of authors who satisfied these criteria. Because JCO does not separately report critical revision for important intellectual content, all authors received credit for criterion 2.
Statistical Methods
χ2 tests were used to examine univariate associations between performance of a key role and having financial ties to industry. Logistic regression models were then used to confirm the association, after adjusting for covariates. To account for clustering of authors within articles, odds ratios (OR) were estimated using generalized linear mixed models. Analyses assumed independence between authors who were listed on different articles. Article-level covariates included in the initial model were: industry sponsorship (yes, no), corresponding author location (United States, other), number of centers (single, multi), trial phase (I or I/II, II, II/III or III, unspecified), age group (adult, pediatric), and number of authors. Covariates that were not significantly associated with the outcome were sequentially deleted from the model using a manual backwards stepwise regression procedure based on likelihood ratio tests. As univariate analyses suggested that the association depended on industry sponsorship, an additional interaction term between key role and industry sponsorship was included. Because this interaction term was statistically significant, we also report separate subgroup analyses for those trials that did and did not receive industry sponsorship. All covariates included in the final model were retained in these subgroup-specific models.
In addition to the main analysis described above, we conducted three sensitivity analyses. First, because it may seem self-evident that authors who performed key roles are more likely than other authors to receive research funds, the model was refit using a revised financial ties variable that excluded research funding. Second, we refit the model after excluding sponsor employees from the analysis. Third, we used satisfaction of ICMJE authorship criteria, rather than the study-defined key role variable, as the main independent variable.
To reduce the possibility of type 1 error due to making multiple comparisons, we used two-sided α lower than .01 as the criterion of statistical significance and report 99% CIs. Although the sample size was based on the number of articles published during the 18-month period of data collection, power calculations for univariate comparisons indicated that 1,050 authors would provide 80% power to detect an OR of 1.5 for the outcome, assuming 70% of authors would report playing key roles. We also verified post hoc that the observed sample gave adequate power in the multivariate analysis to detect an increased odds of financial ties among authors with, compared to those without, key roles. In this calculation, we assumed that 60% of authors played key roles; that 40% of authors with key roles had financial ties; a mean cluster size of 12; and an intraclass correlation coefficient of 0.2 among authors of the same article. On the basis of these assumptions, 2,026 authors allowed the detection of an OR of 2 with 90% power.39 Because the outcome (financial ties) was common, ORs should be interpreted as relative odds, not relative risks.
RESULTS
Characteristics of Study Articles
Of 235 articles, 52% reported industry funding, 42% reported government funding, and 24% reported foundation funding (Table 1). Most (80%) were multicenter trials, included adult participants (91%), and had a United States corresponding author (66%). Phase II trials constituted the largest group (42%). The mean number of authors was 12.5.
Table 1.
Article Characteristics (n = 235)
| Characteristic | No. | % |
|---|---|---|
| Sponsorship* | ||
| Industry | 123 | 52 |
| Government | 98 | 42 |
| Foundation | 57 | 24 |
| Sponsorship not specified | 27 | 12 |
| No. of centers | ||
| Single | 46 | 20 |
| Multi | 189 | 80 |
| Age group of participants | ||
| Adult | 213 | 91 |
| Pediatric | 22 | 9 |
| Country† | ||
| United States | 154 | 66 |
| Other | 81 | 35 |
| Phase | ||
| I or I/II | 41 | 18 |
| II | 97 | 42 |
| II/III or III | 64 | 27 |
| Not specified | 32 | 14 |
| Total mean No. of authors | 12.5 | |
| Standard deviation | 4.0 | |
| Range | 4-21 | |
Articles may have more than one type of sponsor.
Country of corresponding author.
Authorship Contributions
Of 2,927 authors, 64% reported performing at least one key role (Table 2). Specifically, 38% reported involvement in conception and design, 46% reported involvement in data analysis and interpretation, and 40% reported involvement in manuscript drafting. Sixty percent of authors satisfied ICMJE authorship criteria.
Table 2.
Authorship Contributions (n = 2,927)
| Contribution | No. | % |
|---|---|---|
| Conception and design | 1,101 | 38 |
| Financial support | 92 | 3 |
| Administrative support | 389 | 13 |
| Provision of study materials or patients | 1,870 | 64 |
| Collection and assembly of data | 1,387 | 47 |
| Data analysis and interpretation | 1,340 | 46 |
| Manuscript writing | 1,176 | 40 |
| Final approval of the manuscript | 2,324 | 79 |
| Any key role* | 1,881 | 64 |
| Fulfills ICMJE authorship criteria† | 1,760 | 60 |
Abbreviation: ICMJE, International Committee of Medical Journal Editors.
Coded as yes if the author reported involvement in conception and design, data analysis and interpretation, or manuscript writing.
Coded as yes if the author participated in conception and design, or collection and assembly of data, or analysis and interpretation of data; and the author gave final approval of the manuscript. ICMJE requirements for authorship also include a third criterion, drafting of the manuscript or critical revision of the manuscript for important intellectual content. Because Journal of Clinical Oncology does not report critical revision of the manuscript as a separate role, all authors were given credit for this criterion in determining fulfillment of ICMJE authorship criteria.
Authors' Financial Ties
Twenty-nine percent of authors reported at least one financial tie to industry (Table 3). The most common categories were research funding (12%), honoraria (11%), and consulting (10%). Virtually all authors who disclosed stock ownership were also employees (data not shown). Among industry-sponsored trials, 669 (44%) of 1,518 authors reported at least one tie to a commercial entity. Of these, 94% reported at least one tie to the study sponsor. Among authors of non–industry-sponsored trials, 173 (12%) of 1,409 reported ties to a commercial entity.
Table 3.
Authors' Self-Reported Financial Ties to Industry
| Type of Relationship | Financial Ties |
|||||
|---|---|---|---|---|---|---|
| All Studies (n = 2,927) |
Non–Industry- Sponsored Studies (n = 1,409) |
Industry- Sponsored Studies* (n = 1,518) |
||||
| No. | % | No. | % | No. | % | |
| Employment | 207 | 7 | 17 | 1 | 190 | 13 |
| Honorarium | 324 | 11 | 85 | 6 | 239 | 16 |
| Consultant | 300 | 10 | 79 | 6 | 221 | 15 |
| Stock ownership | 182 | 6 | 11 | 1 | 171 | 11 |
| Research funding | 336 | 12 | 58 | 4 | 278 | 18 |
| Expert testimony | 17 | 1 | 3 | 0 | 14 | 1 |
| Any financial tie† | 842 | 29 | 173 | 12 | 669‡ | 44 |
Includes studies with mixed industry and nonindustry funding.
Coded as yes if the author reported one or more of the above financial relationships.
Of these, 629 authors (41% of all authors of industry-sponsored studies) had at least one financial tie to the study sponsor.
Relationships Between Authorship Contributions and Financial Ties
In univariate analyses, authors who reported playing key roles in study design, analysis, or manuscript drafting were more likely than other authors to have financial relationships to industry (Table 4). This association was present when trials with or without industry sponsorship were considered separately.
Table 4.
Relationship Between Performance of a Key Role and Presence of Financial Ties
| Performance of a Key Role | No. of Authors | Financial Tie |
||||
|---|---|---|---|---|---|---|
| Yes |
No |
P* | ||||
| No. | % | No. | % | |||
| All trials | 2,927 | |||||
| Yes | 666 | 35 | 1,215 | 65 | < .0001 | |
| No | 176 | 17 | 870 | 83 | ||
| Industry-sponsored trials | 1,518 | |||||
| Yes | 532 | 55 | 432 | 45 | < .0001 | |
| No | 137 | 25 | 417 | 75 | ||
| Non–industry-sponsored trials | 1,409 | |||||
| Yes | 134 | 15 | 783 | 85 | < .0001 | |
| No | 39 | 8 | 453 | 92 | ||
Unadjusted P values based on χ2 analyses. Analyses do not account for clustering of authors within articles.
Next, we performed multivariate analyses to adjust for article-level covariates (Table 5). These analyses confirmed that authors who reported performing key roles were more likely than other authors to report financial ties (adjusted OR, 4.3; 99% CI, 3.0 to 6.0; P < .0001). The association was stronger among trials with than among those without industry sponsorship (P = .0038 for the interaction between funding source and performance of key roles), but was present regardless of sponsorship.
Table 5.
Multivariate Analyses of Relationships Between Performance of Key Authorship Roles and Self-Reported Financial Ties to Industry
| Model | No. of Authors | Adjusted Odds of Reporting Financial Ties | 99% CI | P |
|---|---|---|---|---|
| All studies | 2,927 | 4.3 | 3.0 to 6.0 | < .0001 |
| Subgroup analyses | ||||
| Industry-sponsored studies | 1,518 | 5.0 | 3.4 to 7.5 | < .0001 |
| Non–industry-sponsored studies | 1,409 | 2.5 | 1.3 to 4.8 | .0002 |
| Sensitivity analyses | ||||
| Exclusion of research funding from the financial ties composite dependent variable* | 2,927 | 4.4 | 3.1 to 6.3 | < .0001 |
| Exclusion of sponsor employees from the analysis | 2,742 | 3.6 | 2.5 to 5.1 | < .0001 |
| Satisfaction of ICMJE authorship criteria rather than key role as the independent variable† | 2,927 | 3.6 | 2.6 to 5.0 | < .0001 |
NOTE. Each cell reports the results of a separate generalized linear mixed model (PROC NLMIXED in PC-SAS) that tests the association between existence of a financial tie and performance of at least one key authorship role. All models adjust for age of study participants (adult v pediatric), and corresponding author address (United States v other). Models that include all studies also adjust for source of funding (industry v nonindustry).
Abbreviation: ICMJE, International Committee of Medical Journal Editors.
We repeated the main model after removing research funds from the financial ties composite variable.
We used satisfaction of ICMJE authorship criteria, rather than the study-defined key-role variable, as the main independent variable of interest.
In sensitivity analyses, the association between performance of a key role and presence of financial ties to industry persisted when research funding was excluded from the outcome variable (OR, 4.4; 99% CI, 3.1 to 6.3; P < .0001), as well as when sponsor employees were removed from the analysis (OR, 3.6; 99% CI, 2.5 to 5.1; P < .0001). Finally, authors who satisfied ICJME criteria were more likely than other authors to report financial ties (OR, 3.6; 99% CI, 2.6 to 5.0; P < .0001).
DISCUSSION
We examined the relationship between authorship contributions and financial ties to industry among authors of clinical trials published in JCO between January 1, 2006, and June 30, 2007. Consistent with our hypothesis, authors who reported participating in the intellectually central roles of conception and design, analysis and interpretation, or manuscript drafting were more likely than other authors to report financial ties. This relationship was present among both industry-sponsored and non–industry-sponsored trials, although it was stronger among industry-sponsored trials. To our knowledge, this association has not previously been demonstrated either within or outside the oncology setting.
Consistent with previous studies, a substantial proportion of authors reported financial ties to industry. Furthermore, authors of industry-sponsored studies were more likely than authors of non–industry-sponsored studies to report financial ties.40 Although our data do not address the reason for this difference, we suspect that it is related to the likelihood that authors of an article reporting an industry-sponsored trial view particular financial ties as “having a direct bearing on the relevant subject matter,” rather than to differences between groups in the underlying prevalence of financial ties.36 JCO requires disclosure of “financial and other relationships with entities that have investment, licensing, or other commercial interests in the subject matter under consideration.”37 In light of this requirement, authors of non–industry-sponsored trials may appropriately view many of their financial relationships as irrelevant to the subject matter at hand. Given this difference between industry- and non–industry-sponsored trials, studies of authors' financial relationships should be interpreted in light of the mix of sponsorship within the study sample, and future analyses of authors' personal financial relationships should be stratified by type of sponsorship.
Is the strong relationship between playing key intellectual roles and having financial ties to industry—even after excluding research funding—cause for concern? Although authors with the ability to influence study design, analysis, or reporting are more likely than other authors to have financial ties, the implications of this observation depend largely on the underlying mechanisms. One possibility is that industry sponsors select authors with whom they have cultivated financial relationships to play key roles, perhaps because they view such authors as more willing to favor their interests in designing, interpreting or reporting the study. Alternately, senior investigators with stronger reputations or greater experience may be more likely than other investigators both to have financial ties to industry and to play key roles. In other words, seniority, reputation, and experience may represent unmeasured confounders that account for the apparent relationship between having financial ties and performing key intellectual roles. Although the former explanation would be cause for concern, the latter may be less problematic. Although our cross-sectional data do not resolve the question of causal mechanism, two observations support the latter explanation. First, we observed an association between performing key roles and having financial ties in nonindustry-sponsored as well as in industry-sponsored trials. Second, a recent report commissioned by the U.S Food and Drug Administration found that “standing [US Food and Drug Administration] advisory committee members with higher overall measures of expertise were more likely than other standing advisory committee members to have been granted waivers for financial conflicts of interest.”41 We suspect that this relationship between expertise and the existence of financial ties noted among US Food and Drug Administration committee members also holds true among clinical trial investigators. Whatever the underlying mechanism, the increased prevalence of financial ties among authors who participate in study design, conduct or reporting has particular implications for JCO, given the Journal's recent prohibition of most financial relationships between principal investigators and study sponsors.42
Our data also illuminate authorship practices in oncology trials. Over the past decade, there has been considerable attention to ensuring that authorship credit accurately reflects those individuals who made “substantive intellectual contributions to a published study.”38 There is wide agreement that “ghost authors” (ie, individuals who made substantive contributions but did not receive authorship credit) and “honorary authors” (ie, individuals who received authorship credit but did not make substantive contributions) both raise concerns about scientific integrity.43 Few studies, however, have characterized the contributions reported by authors of published articles.44–46 We found that only 60% of authors satisfied the ICMJE's authorship criteria, even using a liberal definition that credited all authors with participation in drafting the manuscript or critically revising it for important intellectual content. These results suggest the need for education and discussion about authorship practices within the cancer research community. Finally, because “provision of study materials or patients” was the most common contribution reported after “final approval of the manuscript,” the editors of JCO and the ICMJE should clarify whether or not this contribution is sufficient to satisfy the ICMJE's first authorship criterion.
Our study has several limitations. First, the data were derived from articles published in a single oncology journal during a limited time frame. It is conceivable that patterns of association between financial ties and authorship contributions may differ across disciplines or oncology journals. It is also possible, though we suspect unlikely, that the associations we observed may not generalize to periods either before or after the window of data collection. Second, data on financial ties and on authorship contributions were ultimately derived from author self-report during the submission process, either directly or through the corresponding author. Thus we cannot exclude the possibility of under- or over-reporting of financial ties, or of inaccurate reporting of authorship contributions. Independent confirmation of authors' financial ties would lend confidence to these results. However, such data collection would be challenging and is beyond the scope of the current investigation. Furthermore, misclassification by authors of their financial ties (for example, misreporting of research funding as consulting income) could bias the associations we observed. Third, it is possible that multiple instances of authorship by an individual author could have influenced our results. However, any effect was likely small given that few authors appeared more than once in the data set. Finally, as noted previously, the data do not directly address the causal mechanisms underlying the observed association between authorship contributions and financial relationships.
In conclusion, authors who play central intellectual roles in the conception and design, analysis and interpretation, or reporting of oncology trials are more likely than other authors to report financial relationships with industry. This association is present regardless of sponsorship, although it is stronger in industry-sponsored trials. These data do not prove that authors' financial ties are problematic, nor do they necessarily reflect efforts by sponsors to influence investigators' work. They do, however, suggest an urgent need to clarify the mechanisms underlying these relationships and to determine what, if any, influence they have on the outcomes and reporting of cancer clinical trials.
Acknowledgment
We are grateful to Julie S. Najita, PhD, for her invaluable statistical guidance. We thank Norman Daniels, Cary Gross, Tom McGuire, Joe Newhouse, and Jane Weeks for their invaluable comments on an earlier draft of this manuscript.
Footnotes
See accompanying editorial on page 1281
Supported by National Cancer Institute Grant No. 2R25CA092203-08 (S.L.R.).
Presented in part in poster format at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30 to June 3, 2008.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Steven Joffe, Genzyme Corporation (C) Stock Ownership: None Honoraria: None Research Funding: Monika K. Krzyzanowska, Astra Zeneca, Exelixis, Roche Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Susannah L. Rose, Steven Joffe
Financial support: Steven Joffe
Administrative support: Steven Joffe
Collection and assembly of data: Steven Joffe
Data analysis and interpretation: Susannah L. Rose, Monika K. Krzyzanowska, Steven Joffe
Manuscript writing: Susannah L. Rose, Steven Joffe
Final approval of manuscript: Susannah L. Rose, Monika K. Krzyzanowska, Steven Joffe
REFERENCES
- 1.Bodenheimer T. Uneasy alliance–clinical investigators and the pharmaceutical industry. N Engl J Med. 2000;342:1539–1544. doi: 10.1056/NEJM200005183422024. [DOI] [PubMed] [Google Scholar]
- 2.DeAngelis CD. The influence of money on medical science. JAMA. 2006;296:996–998. doi: 10.1001/jama.296.8.jed60051. [DOI] [PubMed] [Google Scholar]
- 3.Djulbegovic B, Angelotta C, Knox KE, et al. The sound and the fury: Financial conflicts of interest in oncology. J Clin Oncol. 2007;25:3567–3569. doi: 10.1200/JCO.2007.11.9800. [DOI] [PubMed] [Google Scholar]
- 4.Morin K, Rakatansky H, Riddick FA, Jr, et al. Managing conflicts of interest in the conduct of clinical trials. JAMA. 2002;287:78–84. doi: 10.1001/jama.287.1.78. [DOI] [PubMed] [Google Scholar]
- 5.Korn D. Conflicts of interest in biomedical research. JAMA. 2000;284:2234–2237. doi: 10.1001/jama.284.17.2234. [DOI] [PubMed] [Google Scholar]
- 6.Saul S. Experts conclude Pfizer manipulated studies. The New York Times. 2008 Oct 8;:B4. [Google Scholar]
- 7.Report on Individual and Institutional Financial Conflict of Interest. Task Force on Research Accountability. Washington, DC: Association of American Universities; 2001. [Google Scholar]
- 8.Buchkowsky SS, Jewesson PJ. Industry sponsorship and authorship of clinical trials over 20 years. Ann Pharmacother. 2004;38:579–585. doi: 10.1345/aph.1D267. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal D, Campbell EG, Causino N, et al. Participation of life-science faculty in research relationships with industry. N Engl J Med. 1996;335:1734–1739. doi: 10.1056/NEJM199612053352305. [DOI] [PubMed] [Google Scholar]
- 10.Boyd EA, Bero LA. Assessing faculty financial relationships with industry: A case study. JAMA. 2000;284:2209–2214. doi: 10.1001/jama.284.17.2209. [DOI] [PubMed] [Google Scholar]
- 11.George B, Simhan S, Lee JA, et al. Conflicts of interest among articles published in the J Clin Oncol. J Clin Oncol. 2007;25:355s. abstr 6633. [Google Scholar]
- 12.Bekelman JE, Li Y, Gross CP. Scope and impact of financial conflicts of interest in biomedical research: A systematic review. JAMA. 2003;289:454–465. doi: 10.1001/jama.289.4.454. [DOI] [PubMed] [Google Scholar]
- 13.Gross CP, Gupta AR, Krumholz HM. Disclosure of financial competing interests in randomised controlled trials: Cross sectional review. BMJ. 2003;326:526–527. doi: 10.1136/bmj.326.7388.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampson LA, Joffe S, Fowler R, et al. Frequency, type, and monetary value of financial conflicts of interest in cancer clinical research. J Clin Oncol. 2007;25:3609–3614. doi: 10.1200/JCO.2006.09.3633. [DOI] [PubMed] [Google Scholar]
- 15.Johnston KL, Go RS. Financial conflicts of interest among ASCO Annual Meeting abstract authors, speakers, and planners. J Natl Cancer Inst. 2007;99:1415–1416. doi: 10.1093/jnci/djm108. [DOI] [PubMed] [Google Scholar]
- 16.Davidson RA. Source of funding and outcome of clinical trials. J Gen Intern Med. 1986;1:155–158. doi: 10.1007/BF02602327. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170:477–480. [PMC free article] [PubMed] [Google Scholar]
- 18.Als-Nielsen B, Chen W, Gluud C, et al. Association of funding and conclusions in randomized drug trials: A reflection of treatment effect or adverse events? JAMA. 2003;290:921–928. doi: 10.1001/jama.290.7.921. [DOI] [PubMed] [Google Scholar]
- 19.Djulbegovic B, Lacevic M, Cantor A, et al. The uncertainty principle and industry-sponsored research. Lancet. 2000;356:635–638. doi: 10.1016/S0140-6736(00)02605-2. [DOI] [PubMed] [Google Scholar]
- 20.Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: An exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry. 2006;163:185–194. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- 21.Jorgensen AW, Hilden J, Gotzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: Systematic review. BMJ. 2006;333:782–785. doi: 10.1136/bmj.38973.444699.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjaergard LL, Als-Nielsen B. Association between competing interests and authors' conclusions: Epidemiological study of randomised clinical trials published in the BMJ. BMJ. 2002;325:249–252. doi: 10.1136/bmj.325.7358.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lexchin J, Bero LA, Djulbegovic B, et al. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. BMJ. 2003;326:1167–1170. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ridker PM, Torres J. Reported outcomes in major cardiovascular clinical trials funded by for-profit and not-for-profit organizations: 2000-2005. JAMA. 2006;295:2270–2274. doi: 10.1001/jama.295.19.2270. [DOI] [PubMed] [Google Scholar]
- 25.Tungaraza T, Poole R. Influence of drug company authorship and sponsorship on drug trial outcomes. Br J Psychiatry. 2007;191:82–83. doi: 10.1192/bjp.bp.106.024547. [DOI] [PubMed] [Google Scholar]
- 26.Yank V, Rennie D, Bero LA. Financial ties and concordance between results and conclusions in meta-analyses: Retrospective cohort study. BMJ. 2007;335:1202–1205. doi: 10.1136/bmj.39376.447211.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Booth CM, Cescon DW, Wang L, et al. Evolution of the randomized controlled trial in oncology over three decades. J Clin Oncol. 2008;26:5458–5464. doi: 10.1200/JCO.2008.16.5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rochon PA, Gurwitz JH, Simms RW, et al. A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drugs in the treatment of arthritis. Arch Intern Med. 1994;154:157–163. [PubMed] [Google Scholar]
- 29.Friedberg M, Saffran B, Stinson TJ, et al. Evaluation of conflict of interest in economic analyses of new drugs used in oncology. JAMA. 1999;282:1453–1457. doi: 10.1001/jama.282.15.1453. [DOI] [PubMed] [Google Scholar]
- 30.Nieto A, Mazon A, Pamies R, et al. Adverse effects of inhaled corticosteroids in funded and nonfunded studies. Arch Intern Med. 2007;167:2047–2053. doi: 10.1001/archinte.167.19.2047. [DOI] [PubMed] [Google Scholar]
- 31.Johansen HK, Gotzsche PC. Problems in the design and reporting of trials of antifungal agents encountered during meta-analysis. JAMA. 1999;282:1752–1759. doi: 10.1001/jama.282.18.1752. [DOI] [PubMed] [Google Scholar]
- 32.Perlis RH, Perlis CS, Wu Y, et al. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. Am J Psychiatry. 2005;162:1957–1960. doi: 10.1176/appi.ajp.162.10.1957. [DOI] [PubMed] [Google Scholar]
- 33.Stelfox HT, Chua G, O'Rourke K, et al. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med. 1998;338:101–106. doi: 10.1056/NEJM199801083380206. [DOI] [PubMed] [Google Scholar]
- 34.Friedman LS, Richter ED. Relationship between conflicts of interest and research results. J Gen Intern Med. 2004;19:51–56. doi: 10.1111/j.1525-1497.2004.30617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haller DG. JCO and the public trust. J Clin Oncol. 2006;24:323–324. doi: 10.1200/JCO.2005.05.1714. [DOI] [PubMed] [Google Scholar]
- 36.Journal of Clinical Oncology. Author Disclosure Declaration (Version 091707), 2007. http://jco.ascopubs.org/misc/jco_disclosure_contribution.pdf.
- 37.Journal of Clinical Oncology. Authorship contributions: Information for contributors, 2008. http://jco.ascopubs.org/ifc/index.dtl.
- 38.International Committee of Medical Journal Editors. Authorship and Contributorship: Uniform Requirements for Manuscripts Submitted to Biomedical Journals: Writing and Editing for Biomedical Publication, 2007. http://www.icmje.org/#author.
- 39.Donner A, Klar ND. New York, NY: Oxford University Press; 2000. Design and Analysis of Cluster Randomization Trials in Health Research. [Google Scholar]
- 40.Riechelmann RP, Wang L, O'Carroll A, et al. Disclosure of conflicts of interest by authors of clinical trials and editorials in oncology. J Clin Oncol. 2007;25:4642–4647. doi: 10.1200/JCO.2007.11.2482. [DOI] [PubMed] [Google Scholar]
- 41.Ackerly N, Eyraud J, Mazzotta M. 2007. Measuring conflicts of interest and expertise on FDA advisory committees. Office of Policy, Planning and Preparedness, U.S. Food and Drug Administration. http://www.fda.gov/oc/advisory/ergcoireport.pdf. [Google Scholar]
- 42.American Society of Clinical Oncology. Revised conflict of interest policy. J Clin Oncol. 2006;24:519–521. doi: 10.1200/JCO.2005.04.8926. [DOI] [PubMed] [Google Scholar]
- 43.Ross JS, Hill KP, Egilman DS, et al. Guest authorship and ghostwriting in publications related to rofecoxib: A case study of industry documents from rofecoxib litigation. JAMA. 2008;299:1800–1812. doi: 10.1001/jama.299.15.1800. [DOI] [PubMed] [Google Scholar]
- 44.Hwang SS, Song HH, Baik JH, et al. Researcher contributions and fulfillment of ICMJE authorship criteria: Analysis of author contribution lists in research articles with multiple authors published in radiology. Radiology. 2003;226:16–23. doi: 10.1148/radiol.2261011255. [DOI] [PubMed] [Google Scholar]
- 45.Bates T, Anic A, Marusic M, et al. Authorship criteria and disclosure of contributions: Comparison of 3 general medical journals with different author contribution forms. JAMA. 2004;292:86–88. doi: 10.1001/jama.292.1.86. [DOI] [PubMed] [Google Scholar]
- 46.Baerlocher MO, Newton M, Gautam T, et al. The meaning of author order in medical research. J Investig Med. 2007;55:174–180. doi: 10.2310/6650.2007.06044. [DOI] [PubMed] [Google Scholar]