Abstract
Purpose
Src family kinase (SFK) proteins are frequently activated in cancer and can coordinate tumor cell growth, survival, invasion, and angiogenesis. Given the importance of SFK signaling in cancer, known cooperation between SFK and epidermal growth factor receptor (EGFR) signaling, and efficacy of EGFR inhibitors, we performed a phase I trial combining dasatinib, an SFK and multikinase inhibitor, with erlotinib, an EGFR inhibitor, in patients with advanced non–small-cell lung cancer.
Patients and Methods
Patients received erlotinib for 1 week before addition of dasatinib; pharmacokinetics were performed after weeks 1 and 2. Four cohorts were examined, including twice-daily and daily dasatinib dosing. Responses were assessed after 8 weeks. Plasma levels of angiogenic markers (vascular endothelial growth factor [VEGF], interleukin-8, and basic fibroblast growth factor [bFGF]) were determined before and during treatment.
Results
Thirty-four patients were enrolled. The average duration of treatment was 73 days. The main adverse events include GI (diarrhea, anorexia, and nausea), skin rash, cytopenias, pleural effusions, and fatigue. No effect of escalating doses of dasatinib was observed on erlotinib pharmacokinetics. Two partial responses and one bone response were observed, and the disease control rate was 63%. Reductions in plasma VEGF and bFGF were observed, and reductions in VEGF correlated with disease control.
Conclusion
The combination of erlotinib and dasatinib is tolerable, with adverse effects consistent with the two agents. Disease control and inhibition of plasma angiogenesis markers were observed. Personalized strategies for deployment of SFK should receive further attention.
INTRODUCTION
The Src family of protein tyrosine kinases (SFK) are novel drug targets because of their ability to link signaling initiated by growth factor, integrin, and cytokine receptors on the surface of cells to their downstream effector signaling cascades.1 SFKs cooperate with multiple receptor tyrosine kinases, such as the epidermal growth factor receptor (EGFR), to modulate signaling, transform cells, and promote tumor growth.1–7 SFKs activate downstream signaling pathways, such as Ras/Raf/MAPK, PI3K/Akt, and STATs, that control tumor growth.8–10 Angiogenic growth factors, such as vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8), are downstream targets for c-Src, and small-molecule inhibitors of SFK can inhibit angiogenesis.11–17
Elevated SFK activity is found in human tumors, including lung cancer, resulting from diverse mechanisms.10,18–21 Small-molecule inhibitors of SFK have antitumor properties because they inhibit cell proliferation, survival, angiogenesis, and invasion.22–24 Dasatinib (BMS-354825) is a potent, orally available SFK inhibitor currently approved for imatinib-resistant chronic myelogenous leukemia (CML) and is being studied in numerous clinical trials of solid tumors. Dasatinib can exert antitumor effect in lung cancer cells, and preclinical data suggest that dasatinib and other SFK inhibitors can inhibit tumor growth and induce tumor cell death in lung cancer cell lines dependent on EGFR for growth and survival.21,24–26
Because of the importance of EGFR signaling in lung cancer, the beneficial effects of erlotinib (an EGFR inhibitor) on patient survival, cooperation between EGFR and Src proteins, and evidence of elevated Src activity in human lung cancers, we conducted a phase I trial of combined erlotinib and dasatinib in patients with advanced non–small-cell lung cancer (NSCLC).27 The primary objectives were to determine the safety and tolerability of this combination and recommend a phase II dose. Secondary objectives included characterizing the pharmacokinetics of both agents, assessing plasma angiogenic markers as pharmacodynamic markers, and estimating antitumor efficacy.
PATIENTS AND METHODS
Eligibility
Key inclusion criteria included diagnosis of advanced/metastatic (stage III [pleural metastasis] or IV) NSCLC, previous chemotherapy, progressive and measurable disease defined by the Response Evaluation Criteria in Solid Tumors (RECIST), Eastern Cooperative Oncology Group performance status of 0 to 1, no therapy for at least 14 days before study entry, and adequate bone marrow reserve and organ function. Exclusion criteria included prior treatment with any EGFR-targeting agent or dasatinib, treatment within the last 28 days with an experimental drug, untreated or progressive CNS metastasis, prolonged QTc interval (> 450 milliseconds), history of significant bleeding disorder unrelated to cancer, current use of drugs with risk of causing torsades de pointes, and chronic obstructive pulmonary disease or pleural effusions requiring chronic oxygen therapy. All patients gave written informed consent, and the protocol was approved by the Moffitt Scientific Review Committee and the University of South Florida Institutional Review Board.
Study Design
This was a dual-agent, open-label, phase I study. Three patients were treated per cohort for one cycle (28 days per cycle). Dose-limiting toxicity (DLT) was defined as grade 4 rash, grade 3 or 4 nausea/vomiting refractory to antiemetics, grade 3 or 4 diarrhea refractory to antidiarrhea medications, other grade 3 or 4 nonhematologic toxicity, grade 4 hematologic toxicity, or treatment-related death occurring in the first cycle. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria of Adverse Events version 3. In the absence of any DLT, three patients were treated in the subsequent cohort. Presence of DLT in a cohort required that another three patients be treated in the cohort for one cycle; if not DLTs occurred, then dose escalation continued. The recommended phase II dose was defined as the highest dose at which, at most, one of six patients experienced a DLT. No intrapatient dose escalation was permitted. Ten patients (total) were treated at the recommended phase II dose to confirm tolerability.
Treatment Delivery
The dose-escalation scheme was as follows: cohort 1, dasatinib 50 mg twice daily and erlotinib 100 mg once daily; cohort 2, dasatinib 50 mg twice daily and erlotinib 150 mg once daily; cohort 3, dasatinib 70 mg twice daily and erlotinib 150 mg once daily; and cohort 4, dasatinib 140 mg once daily and erlotinib 150 mg once daily. Dasatinib doses were chosen based on experience in CML and other solid tumors.28 Because erlotinib and dasatinib are metabolized through CYP3A4, patients received 1 week of erlotinib before dasatinib to establish erlotinib pharmacokinetics both with (day 15) and without (day 8) dasatinib, whereas dasatinib pharmacokinetics were assessed only once during erlotinib treatment (day 15).
Response Assessment
Tumor size was assessed via computed tomography or magnetic resonance imaging every 50 ± 7 days. Modified (up to five lesions) RECIST criteria were used to determine tumor response and disease progression.
Pharmacokinetics
Blood samples were collected on study days 8 and 15 before dose administration and at 0.5, 2, 4, 6, 8 to 10, and 24 hours after administration. Erlotinib plasma samples were prepared for liquid chromatography assay by protein precipitation and extraction.29,30 Linear regression was used to form the calibration curve using 1-year weighting. The assay has a linear range from 50 to 5,000 ng/mL. Recovery from plasma averaged greater than 88%. Inter- and intra-assay variability of the quality controls (QCs) was less than 10% coefficient of variation, with a relative mean error of less than 5%, displaying both precision and accuracy.
Dasatinib was extracted from plasma samples by reverse-phase, solid-phase extraction. Extracts were assayed for dasatinib by liquid chromatography tandem mass spectrometry using [13C4, 15N2]-dasatinib as internal standard.31,32 The standard curves were well fitted by a weighted (1/x2) quadratic equation over the concentration range 1.00 to 1,000 ng/mL. The between-run variability and within-run variability for the QCs were no greater than 3% and 7% coefficient of variation, respectively, with a relative mean error of ± 6%. The standard curve and QC data indicate that the method was precise and accurate. Raw concentration data were used to formulate pharmacokinetic data for erlotinib and dasatinib. Further details are available in the Appendix (online only).
Plasma Biomarker Analysis
Plasma samples were collected before treatment and on days 15 and 29 and stored at −80°C. Fluorokine MAP assay kits for VEGF (LUH293), IL-8 (LUH208), and bFGF (LUH233) and Base Kit A (LUH000) were obtained from R&D Systems (Minneapolis, MN) and analyzed on a Luminex 100 (Luminex Corporation, Austin, TX) using Masterplex QT (MiraBio, Alameda, CA). Standard curves were generated following manufacturer's instructions, and all samples were run in triplicate.
Statistical Analysis
Survival analysis was performed using Kaplan-Meier survival curves. Levels of VEGF, IL-8, and bFGF were averaged using a base 10 log transformation because they were closer to a normal distribution than were the raw data, as determined using the Anderson-Darling statistic. Descriptive statistics were calculated using this average score. Logistic regression was performed to estimate association between treatment responses with levels of treatment in three different time periods (day 0, 15, and 29) as well as with change in markers. The Wilcoxon signed rank test was used to test whether treatment affected levels of the markers at three time periods. All statistical analyses were performed using SAS (Version 9.1; SAS Institute, Cary, NC).
RESULTS
Patient Characteristics
Thirty-four patients were enrolled between June 2007 and February 2009. Patient characteristics are listed in Table 1. Half of the patients (50%) had adenocarcinoma, with the remainder having squamous cell histology (21%) or other NSCLC (29%). Twenty-one percent of patients had received three or more prior regimens of treatment. Only one patient was a lifetime never smoker (< 100 cigarettes in lifetime).
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| Median | 61 | |
| Range | 39-76 | |
| Sex | ||
| Male | 18 | 53 |
| Female | 16 | 47 |
| Race | ||
| White | 33 | 97 |
| Hispanic | 1 | 3 |
| African American | 0 | 0 |
| Asian | 0 | 0 |
| ECOG performance status | ||
| 0 | 24 | 71 |
| 1 | 10 | 29 |
| Smoking status | ||
| Never* | 1 | 3 |
| Quit | 30 | 88 |
| Active | 3 | 9 |
| Stage | ||
| IIIB | 2 | 6 |
| IV | 32 | 94 |
| Histology | ||
| Adenocarcinoma/BAC | 17 | 50 |
| Squamous | 7 | 21 |
| Other | 10 | 29 |
| No. of prior chemotherapy regimens | ||
| 1 | 16 | 47 |
| 2 | 11 | 32 |
| 3 | 3 | 9 |
| > 3 | 4 | 12 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; BAC, bronchioloalveolar carcinoma.
Never smoker is defined as having smoked < 100 cigarettes in lifetime.
We encountered no DLTs during cohorts 1 to 3. We enrolled a total of 13 patients with the recommended phase II dose of erlotinib 150 mg once daily and dasatinib 70 mg twice daily (cohort 3; four in escalation component and nine in expansion component). Cohort 4 consisted of limited phase II expansion with erlotinib 150 mg once daily combined with dasatinib 140 mg once daily to explore single-day dosing of dasatinib (n = 15). We did not explore schedules of dasatinib beyond a total daily dose of 140 mg. The average number of cycles (one cycle = 4 weeks) delivered was 2.9 cycles (range, one to 11 cycles).
Safety and Toxicity
Table 2 and the Data Supplement list grade 2 to 4 adverse events that are at least possibly related to study treatment for all cycles in patients receiving at least one cycle. Discontinuation during the first cycle, which occurred in seven patients, was a result of early death (n = 2), withdrawal of consent (n = 3), and rapid disease progression (n = 2), leaving 27 of the 34 patients fully evaluable for adverse events. One patient experienced sudden death (an acute cardiac or pulmonary event), and a second patient died of progressive bronchial tumor obstruction, infection, and respiratory compromise.
Table 2.
Adverse Events Across All Cycles of Treatment for All Patients Receiving at Least One Cycle of Treatment
| Adverse Event | Grade 2 |
Grade 3 |
Grade 4 |
Grade 2-4 (%) | All Grades (%) | |||
|---|---|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | No. of Patients | % | |||
| GI | ||||||||
| Colitis | 1 | 3 | 3 | 3 | ||||
| Diarrhea | 6 | 18 | 2 | 6 | 24 | 88 | ||
| Hemorrhage, GI/lower GI NOS | 1 | 3 | 3 | 3 | ||||
| Dehydration | 3 | 9 | 1 | 3 | 12 | 21 | ||
| Abdominal gas/bloating | 0 | 3 | ||||||
| Flatulence | 0 | 3 | ||||||
| Anorexia | 11 | 32 | 32 | 71 | ||||
| Dry mouth | 0 | 3 | ||||||
| Heartburn/dyspepsia | 0 | 3 | ||||||
| Nausea | 8 | 24 | 24 | 79 | ||||
| Taste alteration/dysgeusia | 0 | 27 | ||||||
| Vomiting | 6 | 18 | 18 | 44 | ||||
| Dermatologic | ||||||||
| Rash (acneiform) | 9 | 27 | 2 | 6 | 33 | 74 | ||
| Rash (desquamation) | 3 | 9 | 9 | 18 | ||||
| Pruritus | 1 | 3 | 3 | 9 | ||||
| Dry skin | 0 | 24 | ||||||
| Alopecia | 4 | 12 | 12 | 29 | ||||
| Nail changes | 1 | 3 | 3 | 9 | ||||
| Metabolic | ||||||||
| Hyponatremia | 1 | 3 | 3 | 15 | ||||
| Elevated BUN | 0 | 3 | ||||||
| Acidosis (metabolic) | 1 | 3 | 3 | 3 | ||||
| Hypoalbuminemia | 2 | 6 | 6 | 21 | ||||
| Elevated alkaline phosphatase | 0 | 3 | ||||||
| Elevated ALT | 0 | 3 | ||||||
| Elevated AST | 0 | 9 | ||||||
| Hyperbilirubinemia | 0 | 3 | ||||||
| Hypocalcemia | 1 | 3 | 3 | 6 | ||||
| Elevated creatinine | 1 | 3 | 3 | 3 | ||||
| Elevated chloride | 0 | 3 | ||||||
| Hyperkalemia | 0 | 3 | ||||||
| Hypokalemia | 0 | 3 | ||||||
| Low magnesium | 0 | 3 | ||||||
| Hematologic | ||||||||
| Anemia/hemoglobin | 8 | 24 | 2 | 6 | 30 | 53 | ||
| Platelets | 2 | 6 | 1 | 3 | 9 | 29 | ||
| Hyperglycemia | 1 | 3 | 3 | 12 | ||||
| Leukocytes | 2 | 6 | 6 | 12 | ||||
| Neutrophils/neutropenia | 0 | 9 | ||||||
| Infection | ||||||||
| Infection with normal ANC–lung (pneumonia) | 1 | 3 | 3 | 3 | ||||
| Opportunistic infection associated with ≥ grade 2 lymphopenia | 1 | 3 | 3 | 3 | ||||
| Colitis (infection) | 1 | 3 | 3 | 3 | ||||
| Lymphopenia | 12 | 35 | 5 | 15 | 1 | 3 | 53 | 65 |
| Mucositis (oral) | 1 | 3 | 3 | 27 | ||||
| Musculoskeletal | ||||||||
| Edema, hands | 0 | 3 | ||||||
| Muscle weakness (generalized) | 1 | 3 | 3 | 3 | ||||
| Neuropathy | ||||||||
| Sensory | 0 | 6 | ||||||
| Respiratory | ||||||||
| Dyspnea | 4 | 12 | 1 | 3 | 15 | 15 | ||
| Cough | 1 | 3 | 1 | 3 | 6 | 9 | ||
| Wheezing, bronchospasm | 0 | 3 | ||||||
| Pleural effusion | 6 | 18 | 18 | 35 | ||||
| Pain | ||||||||
| Throat | 1 | 3 | 3 | 6 | ||||
| Mouth | 1 | 3 | 3 | 3 | ||||
| Chest/thorax NOS | 0 | 3 | ||||||
| Joint | 0 | 3 | ||||||
| Muscle | 0 | 3 | ||||||
| Nail bed | 0 | 3 | ||||||
| Pain, abdomen NOS | 0 | 6 | ||||||
| Pain/headache | 1 | 3 | 3 | 12 | ||||
| Cardiac | ||||||||
| Cardiac general, tachycardia | 0 | 3 | ||||||
| Prolonged QTc interval | 1 | 3 | 3 | 3 | ||||
| Constitutional | ||||||||
| Fatigue | 15 | 44 | 44 | 74 | ||||
| Fever | 1 | 3 | 3 | 12 | ||||
| Weight loss | 2 | 6 | 6 | 15 | ||||
| Other | ||||||||
| Dizziness | 0 | 9 | ||||||
| Dry eye syndrome | 0 | 3 | ||||||
| Mood swings | 1 | 3 | 3 | 3 | ||||
| Rigors/chills | 0 | 3 | ||||||
| Hypothyroid | 0 | 3 | ||||||
| Blurred vision | 0 | 3 | ||||||
NOTE. Each patient is reported once, at maximum grade for each adverse event. One grade 5 sudden death occurred in cohort 3 and was considered as possibly related to study treatment.
Abbreviations: NOS, not otherwise specified; BUN, blood urea nitrogen; ANC, absolute neutrophil count.
The most common adverse events (Table 2) were GI intolerance, rash, anemia, pleural effusion, and fatigue. GI toxicities were common (diarrhea, 88%; anorexia, 71%; and nausea, 79%), but only two episodes of diarrhea were grade 3. Acneiform rash, a well-known adverse effect of erlotinib, was frequent, but only two events were grade 3. Hematologic toxicity consisted mainly of anemia (53%; two episodes of grade 3), thrombocytopenia (29%; one episode of grade 3), and lymphopenia (65%; six episodes of grade 3 or 4). Pleural effusions occurred in 35% of patients, but all were grade 2 or less; no patient required thoracentesis or pleurodesis to manage drug-related effusions. Finally, fatigue was common (74%), but all episodes were grade 1 to 2.
We compared the incidence of adverse events between the twice-daily and once-day dosing schedules for dasatinib (Appendix). The most common toxicities were fairly evenly balanced across the treatment schedules, adjusting for differences in average cycles on twice-daily or once-daily schedules. Although uncommon in both groups, hypoalbuminemia, alopecia, taste alteration, dyspnea, hyponatremia, and hyperglycemia were more frequent with twice-daily dosing.
Pharmacokinetics
To determine whether the steady-state pharmacokinetics of erlotinib were affected by the coadministration of dasatinib, we compared the pharmacokinetic parameters of erlotinib on days 8 (no dasatinib) and 15 (coadministration of dasatinib) in all cohorts. No such effect was detected because similar pharmacokinetic parameter estimates were obtained for day 8 and day 15 erlotinib levels. Table 3 lists the pharmacokinetic parameter estimates by cohort determined from the erlotinib plasma concentrations.
Table 3.
Pharmacokinetics of Erlotinib
| Cohort and Dose | No. of Patients | AUC(0–24) (μg × h/mL) |
Half-Life (hours) | Cmax (ug/mL) |
Tmax (hours) |
Clearance (L/h) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 8 |
Day 15 |
Day 8 |
Day 15 |
Day 8 |
Day 15 |
Day 8 |
Day 15 |
|||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| 1, 100 mg | 3 | 47.3 | 6.1 | 45.8 | 19.4 | ND | 2.8 | 0.2 | 2.5 | 0.7 | 2.7 | 1.2 | 2.2 | 1.8 | 2.1 | 0.3 | 2.4 | 0.8 |
| 2, 150 mg | 3 | 22.5 | 7.6 | 20.5 | 9.9 | ND | 1.4 | 0.2 | 1.4 | 0.8 | 4.7 | 3.1 | 5.5 | 5.9 | 7.3 | 2.6 | 8.7 | 4.4 |
| 3, 150 mg | 13 on day 8; 11 on day 15* | 49.1 | 28.9 | 51.8 | 29.9 | ND | 2.8 | 1.1 | 3.0 | 1.4 | 3.2 | 2.1 | 4.0 | 3.0 | 3.9 | 1.9 | 4.8 | 5.5 |
| 4, 150 mg | 14 on day 8; 13 on day 15* | 50.1 | 23.8 | 45.4 | 25.5 | ND | 2.9 | 1.3 | 3.0 | 1.3 | 3.2 | 2.1 | 3.9 | 6.1 | 4.1 | 3.1 | 4.2 | 1.9 |
NOTE. All patients who had day 8 and day 15 pharmacokinetic data are included (n = 13 in cohort 3; n = 14 in cohort 4).
Abbreviations: AUC(0–24), area under the curve from 0 to 24 hours; Cmax, maximum concentration; Tmax, time to maximum concentration; SD, standard deviation; ND, not determined.
Two patients from cohort 3 and one patient from cohort 4 were nonevaluable for the day 15 erlotinib pharmacokinetic sampling.
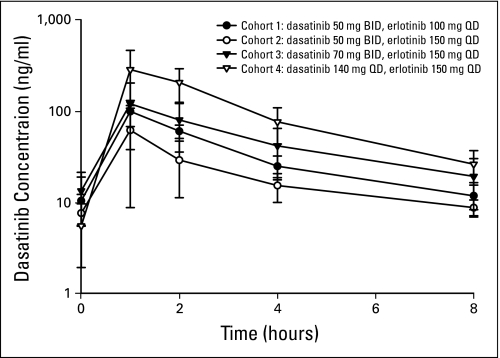
Dasatinib pharmacokinetic parameter estimates at day 15 (ie, during erlotinib exposure) are listed in Table 4. These parameter estimates take into account an 8-hour period of sampling as a result of twice-daily dosing of dasatinib in the first three cohorts. Area under the curve and maximum concentration are proportional to dose as the escalation occurred. Figure 1 is a graph of the time versus mean plasma concentration by cohort for the dasatinib pharmacokinetic sampling.
Table 4.
Pharmacokinetics of Dasatinib
| Cohort and Dose | No. of Patients | AUC(0–8) (ng × h/mL) |
Half-Life (hours) at Day 15 |
Cmax (ng/mL) |
Tmax (hours) |
Clearance (L/h) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 8 |
Day 15 |
Day 8 |
Day 15 |
Day 8 |
Day 15 |
Day 8 |
Day 15 |
||||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 1, 50 mg | 3 | ND | 293.1 | 65.0 | 3.7 | 0.6 | ND | 99.0 | 31.4 | ND | 1.0 | 0.0 | ND | 177.0 | 43.8 | ||||
| 2, 50 mg | 3 | ND | 172.9 | 96.4 | 6.0 | 2.9 | ND | 62.9 | 51.1 | ND | 2.0 | 1.7 | ND | 389.9 | 280.5 | ||||
| 3, 70 mg | 13 on day 8; 11 on day 15* | ND | 410.2 | 201.2 | 4.4 | 2.9 | ND | 127.0 | 71.6 | ND | 1.5 | 0.9 | ND | 238.9 | 196.1 | ||||
| 4, 140 mg | 14 on day 8; 13 on day 15* | ND | 865.5 | 374.8 | 2.7 | 1.0 | ND | 296.8 | 164.9 | ND | 1.2 | 0.4 | ND | 190.2 | 78.8 | ||||
NOTE. All patients who had day 8 and day 15 pharmacokinetic data are included (n = 13 in cohort 3; n = 14 in cohort 4).
Abbreviations: AUC(0–8), area under the curve from 0 to 8 hours; Cmax, maximum concentration; Tmax, time to maximum concentration; SD, standard deviation; ND, not determined.
Two patients from cohort 3 and one patient from cohort 4 were nonevaluable for the day 15 dasatinib pharmacokinetic sampling.
Fig 1.
Dasatinib pharmacokinetics. BID, twice daily; QD, once daily.
Efficacy
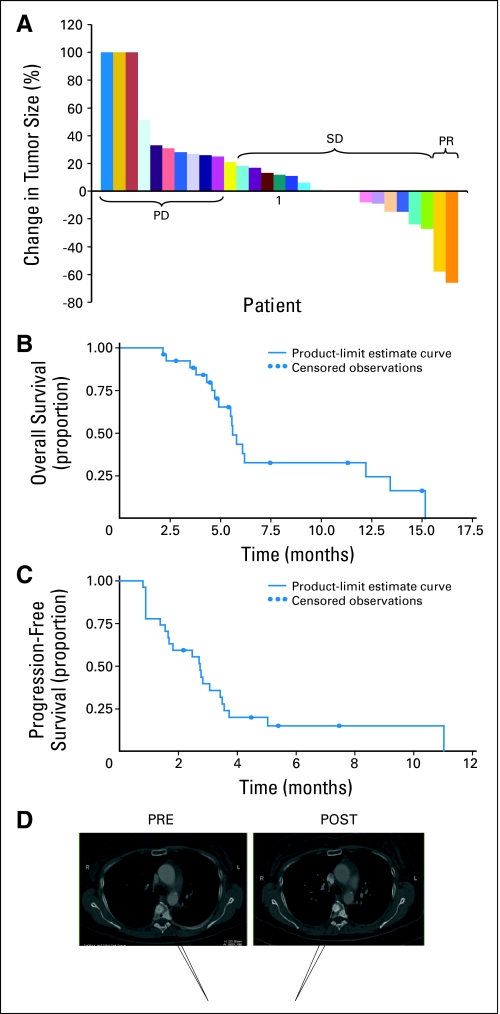
Response data are shown in Figure 2; 29 of 34 patients were evaluable for response. Reasons for lack of response assessment include death (n = 2) and withdrawal of consent (n = 3). The overall disease control rate (partial response plus stable disease) was 62% (18 of 29 patients). Median progression-free survival time was 2.7 months (95% CI, 1.6 to 3.4 months). Overall survival time was 5.6 months (95% CI, 4.9 to 12.2 months). We observed two partial responses, both in the twice-daily cohort. One patient was a man with adenocarcinoma harboring an activating EGFR mutation (del746E-750A). The second patient was a woman with squamous cell carcinoma (which did not harbor an activating EGFR mutation) with a durable ongoing (> 12 month) response. Another patient had stable disease, but multiple bone metastases increased in density, suggesting a responsive or healing effect on bone (Fig 2C). This patient's experience is interesting in light of known effects of Src on osteoclast function and raises the possibility of antiosteoclast effects of dasatinib promoting bone healing in response to metastasis.
Fig 2.
Efficacy. (A) Waterfall plot of response. New lesions = 100% increase. (B) Progression-free survival (PFS) and overall survival (OS). Circles indicate censored events. (C) Computed tomography images of metastatic bone lesions demonstrated increased intensity with restaging suggestive of a responsive or healing effect on bone (arrows). PD, progressive disease; SD, stable disease; PR, partial response; Pre, before therapy; Post, after therapy.
Plasma Pharmacodynamic Studies
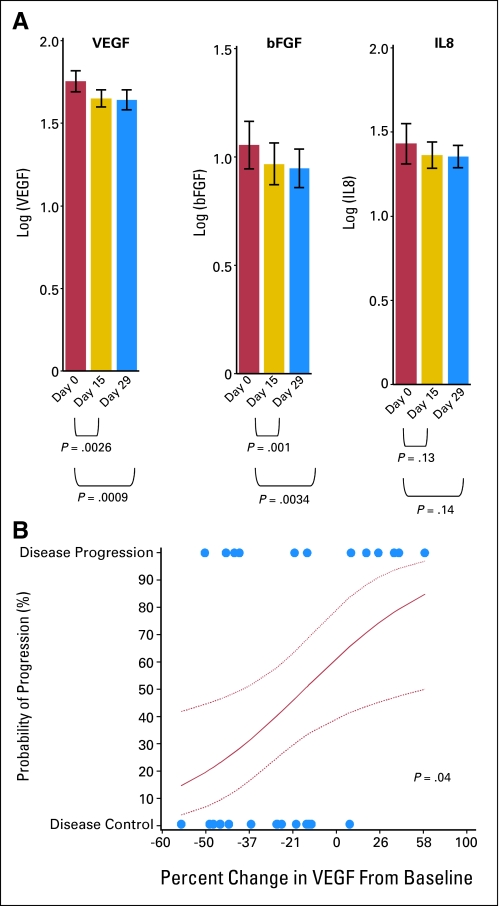
We examined the effect of treatment on plasma levels of VEGF, IL-8, and bFGF based on published reports of these factors being regulated by Src signaling as well as unpublished studies from our lab demonstrating reductions in VEGF and IL-8 gene expression in cells treated with dasatinib.11–17 Plasma was collected before treatment and on days 15 and 29. We found no correlation between pretreatment levels of any of these markers and treatment response. Treatment resulted in reduced levels of VEGF and bFGF on day 15 and day 29, but no statistically significant change was observed in IL-8 levels (Fig 3). Finally, we observed that reductions in VEGF on day 29 correlated with disease control (complete response + partial response + stable disease; P = .04; Fig 3B). Although preliminary, these results suggest that plasma biomarkers of Src-dependent angiogenic factors should be further explored in monitoring the effect of treatment.
Fig 3.
Plasma biomarkers. (A) Log10 levels of vascular endothelial growth factor (VEGF), interleukin-8 (IL-8), and basic fibroblast growth factor (bFGF) were evaluated before treatment and on day 15 and day 29. Each bar is based on 27 patient samples. (B) Circles indicate percent changes in average VEGF levels on day 29 from baseline. The red curve shows estimated probability of progression after two cycles, along with 90% confidence bounds (dotted curves) from logistic regression analysis.
DISCUSSION
Our study demonstrates that dasatinib can be safely combined with erlotinib; the major adverse events were GI events (diarrhea, nausea, and anorexia), skin rash, pleural effusions, anemia, lymphopenia, and fatigue. We aggressively managed skin rash and diarrhea at early onset, which allowed all patients to remain on therapy. Overall, the adverse events were consistent with those observed in larger studies of dasatinib and erlotinib27; no new combination-related effects were found.
Pleural effusions were observed in 13 patients (10 patients on the twice-daily schedule and three patients on the once-daily schedule) and all were ≤ grade 2. The majority of the effusions were apparent on the first restaging computed tomography scan, but some could have been related to disease progression as opposed to being purely drug induced. Our results agree with studies in patients with CML that found more effusions in twice-daily compared with once-daily schedules and early (median time of 5 weeks) development of effusions.33 Although dasatinib can cause exudative pleural effusions that, in some cases, can be severe, we did not encounter serious problems with drug-related pleural effusions at the doses in this trial.28,33,34 We frequently used short pulses of corticosteroids to treat respiratory complaints, and this could have blunted immune-mediated pleural effusions caused by dasatinib.35 The shorter duration of dasatinib treatment in our patients (approximately 10 weeks) compared with patients with CML (42 weeks) may have also limited incidence of pleural effusions.33 It is possible, although unlikely, that erlotinib somehow interferes with dasatinib-induced effusions.
In our unselected group of patients who were treated, not all of whom received phase II doses of erlotinib or dasatinib, the disease control rate was 62%, which is slightly better than the rate of 51% observed in an earlier phase II study of erlotinib.27,36 Of the two partial responses observed in our study, one patient had a documented activating EGFR mutation predictive of response to erlotinib.37–39 The other patient had response in the absence of an EGFR mutation. This second patient might have responded to erlotinib alone because response can occur in tumors with nonmutated EGFR; might have responded to dasatinib, implicating one or more of its target kinases; or might have responded to the combined EGFR and SFK inhibition. Ongoing studies of dasatinib in NSCLC (eg, NCT000459342) are important to detect responses to this single agent as well as potential biomarkers of response.
A number of strategies can be considered for optimizing the use of dasatinib or other SFK inhibitors in cancer patients. There is now good evidence that genomic alterations in tyrosine kinases can, in some instances, be predictive of response to tyrosine kinase inhibitors (ie, EGFR mutations in lung cancer). In the absence of genomic alterations in dasatinib targets that lead to oncogene addiction, tumor regressions with dasatinib alone would be unlikely. Although activation mutations in SFK members are not found in lung cancer, gene copy number increases have been identified in c-Src, EPHA3, and ABL in lung cancer cells that predict response to dasatinib.40 Thus, selection of patients based on these alterations could be fruitful. Alternatively, abnormalities in SFK-regulating proteins, such as CSK or CBL, may activate these critical kinases in the absence of mutation or amplification.41 It is important to note that dasatinib has a number of kinase targets beyond SFK,42 some of which are not well studied but may have important roles in lung cancer biology. It is possible that some targets exhibit yet-to-be-described activating mutations that render cells dependent on this particular kinase for growth and survival; their identification could lead to highly individualized therapy. Finally, genomic or even proteomic signatures could be useful in determining tumors that are likely to respond to EGFR and SFK inhibitors.43
Another strategy would be to identify tumors in which Src members collaborate with other kinases, such as EGFR, to drive tumor growth and/or survival.44 Dual EGFR and SFK inhibition may be an important strategy for tumors such as squamous cell carcinoma of the head and neck, glioblastoma, and colon cancer, where EGFR signaling is relevant and EGFR-targeting agents have antitumor effects in patients.18,45 Preclinical studies have demonstrated that cells with activating EGFR mutations are sensitive to SFK inhibitors, including dasatinib.25,46,47 This suggests that dual EGFR and SFK inhibition could be a rational strategy for this patient subset. Given the short half-life of dasatinib in plasma, one possibility is limited target inhibition in vivo. Duration and extent of kinase inhibition can be critical for killing cancer cells dependent on oncogenic tyrosine kinases.48 Because dasatinib has partial effects on EGFR and the duration of levels in plasma is brief, this could limit its ability to kill EGFR-mutant lung cancers in vivo. Dasatinib and other Src tyrosine kinase inhibitors may reduce the emergence of acquired resistance to EGFR through gatekeeper mutation or through amplified MET.49,50
SFKs affect tumor angiogenesis, and this could delay tumor growth. Our results suggest that levels of proangiogenic factors, such as VEGF, are reduced by erlotinib and dasatinib therapy, and this could indicate antiangiogenic effects of the treatment. Antimetastatic properties of SFK inhibitors are also a potentially important antitumor mechanism that is not recognizable by tumor size measurements. Finally, SFK signaling is important in osteoclast function during the process of bone metastasis.51,52 Metastasis-associated bone degradation is potently reduced by SFK inhibitors; assessment by imaging or quantitation of excreted osteolysis products is important for patients with metastatic bone disease.
In conclusion, the combination of erlotinib and dasatinib is tolerable and shows evidence of antitumor activity in previously treated patients with advanced NSCLC. Future trials should consider this regimen in patients dependent on EGFR for survival or growth of tumors. A better understanding of dasatinib action and other SFK tyrosine kinase inhibitors could also optimize therapy through improved patient selection using genomic or proteomic tools.
Supplementary Material
Acknowledgment
We thank Katie Weekly and Debbie Goodman for data management and analysis, Patricia Johnston for administrative assistance, the patients and families who participated in this study, and colleagues at Bristol-Myers Squibb (Lewis C. Strauss, MD, and Les Enterline, PA-C, CCRA) for assistance.
Appendix
Methods
Pharmacokinetics.
The area under the curve for the dosing interval (AUC0-T), maximum concentration (Cmax), time to maximum concentration (Tmax), and oral clearance were determined for erlotinib on both day 8 (single agent) and day 15 (in combination with dasatinib). These same parameters, in addition to half-life (t1/2), were estimated for dasatinib on day 15. Segmented AUC between samples was determined by the trapezoid rule, and Cmax and Tmax were observed values. The oral clearance was determined by dividing the dose by the AUC. The half-life of dasatinib was determined by dividing 0.693 by the terminal rate constant λz, which is determined from the log-linear fit of the terminal portion of the drug concentration-time curve.
Results
Adverse event comparison between once-daily and twice-daily dasatinib schedules.
We compared the incidence of adverse events between the twice-daily and once-daily dosing schedules for dasatinib. The most commonly seen toxicities were fairly evenly balanced across the treatment schedules, adjusting the once-daily counts by the ratio of the number of cycles on the twice-daily or once-daily schedules (ie, by 1.72 = 62/36). The most common toxicities on the twice-daily and once-daily (adjusted) arms were as follows: rash, n = 33 and n = 43.1; diarrhea, n = 33 and n = 27.6; fatigue, n = 26 and n = 24.1; nausea, n = 21 and n = 29.3; anemia, n = 27 and n = 22.4; lymphopenia, n = 23 and n = 25.8; anorexia, n = 21 and n = 25.8; and platelets, n = 12 and n = 20.7, respectively. There were six toxicities with at least eight occurrences in the twice-daily arm and a ratio greater than 2 compared with the once-daily arm; these six toxicities on the twice-daily and once-daily (adjusted) arms were as follows: hypoalbuminemia, n = 12 and n = 0; alopecia, n = 11 and n = 1.7; taste alteration, n = 9 and n = 1.7; dyspnea, n = 9 and n = 1.7; hyponatremia, n = 8 and n = 0; and hyperglycemia, n = 8 and n = 3.4, respectively. There were no toxicities with eight or more adjusted events in the once-daily arm that were twice as frequent as those in the twice-daily arm.
Reasons for discontinuation during cycle 1.
The reasons for discontinuing therapy during cycle 1 for patients in cohort 3 were as follows: sudden death (n = 1), withdrawal of consent on day 14 (grade 2 fatigue; n = 1), and rapid progression of disease (n = 1). The reasons for discontinuing therapy during cycle 1 for patients in cohort 4 were as follows: withdrawal of consent on day 9 (grade 2 noninfectious fever; n = 1), rapid progression of disease (n = 1), withdrawal of consent on day 11 (grade 2 diarrhea; n = 1), and death secondary to disease progression (n = 1).
Analogous geometric means for Figure 3 biomarker data.
The geometric means for the plasma biomarkers are as follows: vascular endothelial growth factor receptor, 56.2, 44.7, and 43.7 pg/mL on days 0, 15, and 29, respectively; interleukin-8, 26.9, 22.9, and 22.4 pg/mL on days 0, 15, and 29, respectively; and basic fibroblast growth factor, 11.5, 9.3, and 8.9 pg/mL on days 0, 15, and 29, respectively.
Footnotes
Supported in part by grants from the American Society of Clinical Oncology Advanced Clinical Research Award in Lung Cancer (E.B.H.), the Moffitt Cancer Center Specialized Programs of Research Excellence in Lung Cancer (Grant No. P50-CA119997), and by Bristol-Myers Squibb Oncology; also supported in part by the Clinical Pharmacology Core, Protocol Support and Management Core, and the Flow Cytometry Core at the H. Lee Moffitt Cancer Center and Research Institute (Tampa, FL).
Presented in part at 44th Annual Meeting of the American Society of Clinical Oncology Annual, May 30-June 3, 2008, Chicago, IL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00444015.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Alberto Chiappori, Genentech (C); Gerold Bepler, OSI Pharmaceuticals (C) Stock Ownership: None Honoraria: Alberto Chiappori, Genentech Research Funding: None Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Eric B. Haura, Gerold Bepler
Financial support: Eric B. Haura
Administrative support: Eric B. Haura, Gerold Bepler
Provision of study materials or patients: Eric B. Haura, Tawee Tanvetyanon, Alberto Chiappori, Charles Williams, George Simon, Jhanelle Gray, Sharon Litschauer, Gerold Bepler
Collection and assembly of data: Eric B. Haura, Leticia Tetteh, Anthony Neuger, Lanxi Song, Michael J. Schell
Data analysis and interpretation: Eric B. Haura, Scott Antonia, Anthony Neuger, Lanxi Song, Bhupendra Rawal, Michael J. Schell, Gerold Bepler
Manuscript writing: Eric B. Haura, Tawee Tanvetyanon, Anthony Neuger, Lanxi Song, Michael J. Schell, Gerold Bepler
Final approval of manuscript: Eric B. Haura, Tawee Tanvetyanon, Alberto Chiappori, Charles Williams, Scott Antonia, Jhanelle Gray, Sharon Litschauer, Leticia Tetteh, Anthony Neuger, Lanxi Song, Michael J. Schell, Gerold Bepler
REFERENCES
- 1.Parsons SJ, Parsons JT. Src family kinases, key regulators of signal transduction. Oncogene. 2004;23:7906–7909. doi: 10.1038/sj.onc.1208160. [DOI] [PubMed] [Google Scholar]
- 2.Ishizawar R, Parsons SJ. C-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Tice DA, Biscardi JS, Nickles AL, et al. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci U S A. 1999;96:1415–1420. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maa MC, Leu TH, McCarley DJ, et al. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: Implications for the etiology of multiple human cancers. Proc Natl Acad Sci U S A. 1995;92:6981–6985. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karni R, Jove R, Levitzki A. Inhibition of pp60c-Src reduces Bcl-XL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptors. Oncogene. 1999;18:4654–4662. doi: 10.1038/sj.onc.1202835. [DOI] [PubMed] [Google Scholar]
- 6.Biscardi JS, Maa MC, Tice DA, et al. C-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 7.Kloth MT, Laughlin KK, Biscardi JS, et al. STAT5b, a mediator of synergism between c-Src and the epidermal growth factor receptor. J Biol Chem. 2003;278:1671–1679. doi: 10.1074/jbc.M207289200. [DOI] [PubMed] [Google Scholar]
- 8.Sinibaldi D, Wharton W, Turkson J, et al. Induction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: Role of activated STAT3 signaling. Oncogene. 2000;19:5419–5427. doi: 10.1038/sj.onc.1203947. [DOI] [PubMed] [Google Scholar]
- 9.Morgan DO, Kaplan JM, Bishop JM, et al. Mitosis-specific phosphorylation of p60c-src by p34cdc2-associated protein kinase. Cell. 1989;57:775–786. doi: 10.1016/0092-8674(89)90792-7. [DOI] [PubMed] [Google Scholar]
- 10.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 11.Ellis LM, Staley CA, Liu W, et al. Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem. 1998;273:1052–1057. doi: 10.1074/jbc.273.2.1052. [DOI] [PubMed] [Google Scholar]
- 12.Mukhopadhyay D, Tsiokas L, Zhou XM, et al. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature. 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 13.Laird AD, Li G, Moss KG, et al. Src family kinase activity is required for signal transducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther. 2003;2:461–469. [PubMed] [Google Scholar]
- 14.Gray MJ, Zhang J, Ellis LM, et al. HIF-1alpha, STAT3, CBP/p300 and Ref-1/APE are components of a transcriptional complex that regulates Src-dependent hypoxia-induced expression of VEGF in pancreatic and prostate carcinomas. Oncogene. 2005;24:3110–3120. doi: 10.1038/sj.onc.1208513. [DOI] [PubMed] [Google Scholar]
- 15.Jiang BH, Agani F, Passaniti A, et al. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: Involvement of HIF-1 in tumor progression. Cancer Res. 1997;57:5328–5335. [PubMed] [Google Scholar]
- 16.Trevino JG, Summy JM, Gray MJ, et al. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: Implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 17.Park SI, Shah AN, Zhang J, et al. Regulation of angiogenesis and vascular permeability by Src family kinases: Opportunities for therapeutic treatment of solid tumors. Expert Opin Ther Targets. 2007;11:1207–1217. doi: 10.1517/14728222.11.9.1207. [DOI] [PubMed] [Google Scholar]
- 18.Koppikar P, Choi SH, Egloff AM, et al. Combined inhibition of c-Src and epidermal growth factor receptor abrogates growth and invasion of head and neck squamous cell carcinoma. Clin Cancer Res. 2008;14:4284–4291. doi: 10.1158/1078-0432.CCR-07-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Irby RB, Yeatman TJ. Role of src expression and activation in human cancer. Oncogene. 2000;19:5636–5642. doi: 10.1038/sj.onc.1203912. [DOI] [PubMed] [Google Scholar]
- 20.Masaki T, Igarashi K, Tokuda M, et al. Pp60c-src activation in lung adenocarcinoma. Eur J Cancer. 2003;39:1447–1455. doi: 10.1016/s0959-8049(03)00276-4. [DOI] [PubMed] [Google Scholar]
- 21.Leung EL, Tam IY, Tin VP, et al. SRC promotes survival and invasion of lung cancers with epidermal growth factor receptor abnormalities and is a potential candidate for molecular-targeted therapy. Mol Cancer Res. 2009;7:923–932. doi: 10.1158/1541-7786.MCR-09-0003. [DOI] [PubMed] [Google Scholar]
- 22.Song L, Turkson J, Karras JG, et al. Activation of Stat3 by receptor tyrosine kinases and cytokines regulates survival in human non-small cell carcinoma cells. Oncogene. 2003;22:4150–4165. doi: 10.1038/sj.onc.1206479. [DOI] [PubMed] [Google Scholar]
- 23.Wei L, Yang Y, Zhang X, et al. Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene. 2004;23:9052–9061. doi: 10.1038/sj.onc.1208091. [DOI] [PubMed] [Google Scholar]
- 24.Johnson FM, Saigal B, Talpaz M, et al. Dasatinib (BMS-354825) tyrosine kinase inhibitor suppresses invasion and induces cell cycle arrest and apoptosis of head and neck squamous cell carcinoma and non-small cell lung cancer cells. Clin Cancer Res. 2005;11:6924–6932. doi: 10.1158/1078-0432.CCR-05-0757. [DOI] [PubMed] [Google Scholar]
- 25.Song L, Morris M, Bagui T, et al. Dasatinib (BMS-354825) selectively induces apoptosis in lung cancer cells dependent on epidermal growth factor receptor signaling for survival. Cancer Res. 2006;66:5542–5548. doi: 10.1158/0008-5472.CAN-05-4620. [DOI] [PubMed] [Google Scholar]
- 26.Chung BM, Dimri M, George M, et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene. 2009;28:1821–1832. doi: 10.1038/onc.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 28.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 29.Lepper ER, Swain SM, Tan AR, et al. Liquid-chromatographic determination of erlotinib (OSI-774), an epidermal growth factor receptor tyrosine kinase inhibitor. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:181–188. doi: 10.1016/j.jchromb.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Zhao M, He P, Rudek MA, et al. Specific method for determination of OSI-774 and its metabolite OSI-420 in human plasma by using liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;793:413–420. doi: 10.1016/s1570-0232(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 31.Christopher LJ, Cui D, Li W, et al. Biotransformation of [14C]dasatinib: In vitro studies in rat, monkey, and human and disposition after administration to rats and monkeys. Drug Metab Dispos. 2008;36:1341–1356. doi: 10.1124/dmd.107.018234. [DOI] [PubMed] [Google Scholar]
- 32.Christopher LJ, Cui D, Wu C, et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008;36:1357–1364. doi: 10.1124/dmd.107.018267. [DOI] [PubMed] [Google Scholar]
- 33.Quintás-Cardama A, Kantarjian H, O'Brien S, et al. Pleural effusion in patients with chronic myelogenous leukemia treated with dasatinib after imatinib failure. J Clin Oncol. 2007;25:3908–3914. doi: 10.1200/JCO.2007.12.0329. [DOI] [PubMed] [Google Scholar]
- 34.de Lavallade H, Punnialingam S, Milojkovic D, et al. Pleural effusions in patients with chronic myeloid leukaemia treated with dasatinib may have an immune-mediated pathogenesis. Br J Haematol. 2008;141:745–747. doi: 10.1111/j.1365-2141.2008.07108.x. [DOI] [PubMed] [Google Scholar]
- 35.Bergeron A, Rea D, Levy V, et al. Lung abnormalities after dasatinib treatment for chronic myeloid leukemia: A case series. Am J Respir Crit Care Med. 2007;176:814–818. doi: 10.1164/rccm.200705-715CR. [DOI] [PubMed] [Google Scholar]
- 36.Pérez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non–small-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 37.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 38.Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 39.Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sos ML, Michel K, Zander T, et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J Clin Invest. 2009;119:1727–1740. doi: 10.1172/JCI37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oneyama C, Hikita T, Enya K, et al. The lipid raft-anchored adaptor protein Cbp controls the oncogenic potential of c-Src. Mol Cell. 2008;30:426–436. doi: 10.1016/j.molcel.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 42.Rix U, Hantschel O, Durnberger G, et al. Chemical proteomic profiles of the BCR-ABL inhibitors imatinib, nilotinib, and dasatinib reveal novel kinase and nonkinase targets. Blood. 2007;110:4055–4063. doi: 10.1182/blood-2007-07-102061. [DOI] [PubMed] [Google Scholar]
- 43.Bild AH, Yao G, Chang JT, et al. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439:353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 44.Cappuzzo F, Hirsch FR, Rossi E, et al. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643–655. doi: 10.1093/jnci/dji112. [DOI] [PubMed] [Google Scholar]
- 45.Lu KV, Zhu S, Cvrljevic A, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69:6889–6898. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McDermott U, Sharma SV, Dowell L, et al. Identification of genotype-correlated sensitivity to selective kinase inhibitors by using high-throughput tumor cell line profiling. Proc Natl Acad Sci U S A. 2007;104:19936–19941. doi: 10.1073/pnas.0707498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Kalyankrishna S, Wislez M, et al. SRC-family kinases are activated in non-small cell lung cancer and promote the survival of epidermal growth factor receptor-dependent cell lines. Am J Pathol. 2007;170:366–376. doi: 10.2353/ajpath.2007.060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shah NP, Kasap C, Weier C, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14:485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Fu YN, Yeh CL, Cheng HH, et al. EGFR mutants found in non-small cell lung cancer show different levels of sensitivity to suppression of Src: Implications in targeting therapy. Oncogene. 2008;27:957–965. doi: 10.1038/sj.onc.1210684. [DOI] [PubMed] [Google Scholar]
- 50.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 51.Araujo J, Logothetis C. Targeting Src signaling in metastatic bone disease. Int J Cancer. 2009;124:1–6. doi: 10.1002/ijc.23998. [DOI] [PubMed] [Google Scholar]
- 52.Boyce BF, Xing L, Yao Z, et al. SRC inhibitors in metastatic bone disease. Clin Cancer Res. 2006;12:6291s–6295s. doi: 10.1158/1078-0432.CCR-06-0991. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.