Abstract
Background
Alcohol dependence is extremely common in patients with bipolar disorder and is associated with unfavorable outcomes including treatment non-adherence, violence, increased hospitalization and decreased quality of life. While naltrexone is a standard treatment for alcohol dependence, no controlled trials have examined its use in patients with co-morbid bipolar disorder and alcohol dependence. In this pilot study, the efficacy of naltrexone in reducing alcohol use and on mood symptoms was assessed in bipolar disorder and alcohol dependence.
Methods
Fifty adult outpatients with bipolar I or II disorders and current alcohol dependence with active alcohol use were randomized to 12 weeks of naltrexone (50 mg/day) add-on therapy or placebo. Both groups received manual-driven cognitive behavioral therapy designed for patients with bipolar disorder and substance-use disorders. Drinking days and heavy drinking days, alcohol craving, liver enzymes, and manic and depressed mood symptoms were assessed.
Results
The two groups were similar in baseline and demographic characteristics. Naltrexone showed trends (p < .10) toward a greater decrease in drinking days (binary outcome), alcohol craving, and some liver enzyme levels than placebo. Side effects were similar in the two groups. Response to naltrexone was significantly related to medication adherence.
Conclusions
Results suggest the potential value and acceptable tolerability of naltrexone for alcohol dependence in bipolar disorder patients. A larger trial is needed to establish efficacy.
Keywords: naltrexone, bipolar disorder, depression, mania, alcohol
INTRODUCTION
Lifetime rates of substance use disorders of approximately 61% are reported in bipolar I disorder and 48% in bipolar II disorder (Regier et al., 1990). When present in persons with bipolar disorder, substance use disorders are associated with decreased quality of life (Singh et al., 2005), increased hospitalization (Sonne et al., 1994), increased violence (Scott et al., 1998) and treatment non-adherence (Aagaard and Vestergaard, 1990). Alcohol is the most commonly abused substance in patients with bipolar disorder with a lifetime prevalence of 46% (Regier et al., 1990). Thus, the treatment of patients with bipolar and alcohol use disorders is a major public health concern.
One approach is to treat the substance use disorder by ameliorating the bipolar disorder symptoms. Two randomized, placebo-controlled trials used medications for symptoms of bipolar disorder and examined the impact on alcohol use (Brown et al., 2008; Salloum et al., 2005). Salloum et al reported that 24 weeks of valproate therapy was associated with a significant reduction in heavy drinking days in 59 patients with bipolar disorder I and alcohol dependence. Brown et. al. reported a reduction in depressive symptoms, but no significant between-group differences in alcohol use, in 115 outpatients with bipolar disorder I or II disorder and alcohol abuse or dependence given 12 weeks of quetiapine (Brown et al., 2008).
Another approach is to use medication to directly decrease substance use. One of the most efficacious medications for the treatment of alcohol dependence is the opioid antagonist naltrexone. This FDA-approved treatment of alcohol dependence has been shown to increase the time to first drink, the time to heavy drinking, days of drinking, alcohol craving and prevent relapse in patients with alcohol dependence in most (Anton et al., 1999; Chick et al., 2000; Heinala et al., 2001; O’Malley et al., 1992; Oslin et al., 1997; Volpicelli et al., 1992) but not all (Kranzler et al., 2000; Krystal et al., 2001) clinical trials.
To date, two studies have explored the use of naltrexone in alcohol-dependent patients with co-morbid bipolar disorder. Petrakis et. al. reported reduction in alcohol consumption in a group of 254 dual-diagnosis patients with a variety of psychiatric disorders, including 49 with bipolar disorder, treated with naltrexone, disulfiram or the combination (Petrakis et al., 2005). We previously reported a decrease in days of alcohol use, alcohol craving, and manic and depressive symptoms in 34 outpatients with bipolar disorder and alcohol dependence given open-label naltrexone for 16 weeks (Brown et al., 2006).
We now provide randomized, placebo-controlled data on naltrexone in patients with bipolar disorder and alcohol dependence. The primary aim of this pilot study was to determine if naltrexone add-on therapy was associated with a greater reduction in alcohol use as judged by percentage of drinking days (primary outcome measure), and heavy drinking days and serum γ-glutamyltransferase (GGT) levels (secondary outcome measures) than placebo therapy in outpatients with bipolar disorder and alcohol dependence. The secondary aim of the study was to determine if naltrexone add-on therapy is associated with a greater reduction in alcohol craving than placebo therapy in outpatients with bipolar disorder and alcohol dependence. Changes in mood and treatment adherence were also explored.
MATERIALS AND METHODS
A 12-week, randomized, double-blind, parallel-group, placebo-controlled trial of naltrexone was conducted in outpatients with bipolar disorder and alcohol dependence after providing UT Southwestern IRB approved written consent. Potential participants were identified through newspaper advertisements, physician referral and through flyers and brochures at clinics.
Inclusion criteria were outpatient English or Spanish-speaking men or women with a diagnosis of bipolar I or II disorder based on the Mini International Neuropsychiatric Inventory (MINI), a structured diagnostic interview based on DSM-IV criteria (Sheehan et al., 1998), current mood state of depressed or mixed (meeting criteria for both mania and depression) mood, current alcohol dependence, alcohol use of at least 5 drinks in the 7 days prior to intake, and age 18–70 years. Excluded were those with baseline Young Mania Rating Scale (YMRS) (Young et al., 1978) or 17-item Hamilton Rating Scale for Depression (HRSD17) (Hamilton, 1960) scores ≥ 30 to exclude those with very severe baseline mood symptoms, current dependence on substances other than alcohol (except nicotine), lifetime opiate abuse or dependence or current use by self report or urine drug screen, current therapy with acamprosate or disulfiram, mental retardation or other severe cognitive impairment, current incarceration, pregnant or nursing women or women of childbearing age who did not use acceptable methods of birth control during the study, prior therapy with naltrexone, high risk for suicide, intensive outpatient treatment (defined as ≥ 3 visits each week) for substance abuse (AA, NA meetings, or less intensive counseling at baseline were allowed), severe or life-threatening medical condition, aspartate aminotransferase (AST), alanine aminotransferase (ALT), or bilirubin > 3 times upper limit of normal, or a history of severe alcohol withdrawal.
Baseline assessments included the MINI, HRSD17, Inventory of Depressive Symptomatology–Self-Report 30-item version (IDS-SR30) (Rush et al., 1996), YMRS, Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999), Psychobiology of Recovery in Depression III - Somatic Symptom Scale (PRD-III) (Thase et al., 1996), Addiction Severity Index (McLellan et al., 1980) a liver panel, physical examination, and for women of childbearing potential a urine pregnancy test. Recent alcohol use (and if present other substance use) was quantified using the Timeline Follow Back (TLFB) method (Sobell and Sobell, 1992). Mood, alcohol use and craving and side effect assessments were repeated at each weekly appointment for 12 weeks. Naltrexone (50 mg/day) or identical appearing placebo was initiated in a blinded fashion at week 0 to participants with active alcohol use. All participants received manual-driven cognitive behavioral therapy (CBT) designed for patients with bipolar disorder and substance use (Schmitz et al., 2002). CBT was administered by trained and experienced therapists. The cognitive behavioral treatment was a 16 session manual based therapy designed specifically for treating patients with bipolar and co-morbid substance abuse disorders. Sessions included education about bipolar disorder and substance abuse, identification of symptoms of relapse, and coping skills training. Training techniques include daily monitoring of mood, drug use/craving and medication use, didactic presentations, practice exercises, and homework. Changes in concomitant medications were made, when necessary, using the Texas Medication Algorithm Project guidelines for bipolar depression and manic/mixed states (Crismon et al., 2007). Thus, concomitant medication doses could be adjusted or new medications added, if needed, to manage symptoms of bipolar disorder. Participants were paid $20/weekly for each assessment visit and received a $2 vouchers for food, and parking tokens or bus passes. The trial was registered at clinicaltrials.gov.
Statistical Analysis
Demographic and baseline clinical characteristics were compared between treatment groups using t-tests for continuous measures and chi squared tests for categorical measures. Percent of drinking days and heavy drinking days per week were analyzed using a declining-effects random-regression model (Kashner et al., 2003) where the treatment effect was divided into an initial period effect as measured by the change from the initial of treatment at baseline to the first visit (week 1) and a subsequent time trend treatment effect as measured by the change from week 1 to week 12. The initial treatment effect is estimated separately from the subsequent time trend treatment effect because the initial effect perhaps relates primarily to the non-specific effects of entering an alcohol treatment study and may be very different with regard to size and/or direction from the time trend effect which perhaps more accurately reflects the effects of the treatment. The division into two parts is done because treatment effect is frequently not constant over the course of the trial. If two distinct types of effects are present (initial effect and subsequent time trend effect), a model that allows for estimation of these effects will be more informative and better fitting than a model that does not address this feature of the data. Also, basing the initial effect on the change to the first measurement occasion means the maximum number of observations are available to estimate the subsequent time trend. Note that randomization does not have any affect on this non-constant nature of the treatment effect.
However, the validity of these random regression models are questionable because the substantial number of visits with zero percent of days (20.1% of visits had zero percent of drinking days and 44.9% had zero percent of heavy drinking days) violates the assumption of normality. Therefore, secondary analyses were conducted to examine whether or not the participant had any drinking days or heavy drinking days in a given week using a binary outcome of 0, for no days during the week and 1 for at least one day during the week. Binary data were analyzed in the same manner as the continuous outcome data except that we used a generalized linear mixed model (GLMM) (Wolfinger and O’Connell, 1993) which adapts the random-regression model for a binary outcome as implemented in the SAS Glimmix program. Effect size for binary and continuous outcomes was stated in terms of Cohen’s h and Cohen’s d, respectively (Cohen, 1998). Effect sizes were calculated using the adjusted model estimates evaluated at week 12. The effect sizes are not based on an analysis of completers at week 12 but include all of the available data. Number of drinks per drinking day and GGT levels were also analyzed using a declining effects random regression model; however, a log transformation of these outcomes was used to satisfy the assumption of normality necessary to use random regression. PACS, HRSD17, IDS-SR, and YMRS total scores were also analyzed using a declining effects random regression model. Because AST and ALT were measured only at baseline and week 12 they were assessed using analysis of covariance (ANCOVA) with the baseline level of the outcome measure as the covariate. All models included terms for time, treatment group, and treatment group by time interaction. The baseline value of the outcome measure was always used as a covariate. The following variables were considered as potential additional covariates: age, gender, race, education, family history of alcohol abuse, baseline HAMD, YMRS, PACS, ASI alcohol score, ASI drug score, GGT, AST, and ALT. Also considered were use (yes/no) at baseline of lithium, anticonvulsants (valproate), antipsychotics, antidepressants, sedatives, and total number of concomitant meds taken at baseline. No adjustment was made for multiple testing since it is preferable to err on the side of including unimportant covariates then to leave out important covariates. Covariates were selected if they improved the BIC (a measure of goodness of fit). We did not look at any p-values and covariates were selected without regard to whether they enhanced or diminished the significance of the group effect.
Models were checked for the presence of outliers and influential points. Log of week was used as the measure of time for all analyses except GGT because a straight-line relationship was obtained between the outcome measure and log of time whereas the relationship between outcome and time was not a straight line. Thus, time trends were reported as rate of change per log week since the rate of change is constant when time is measured in log units. Log weeks scale can be convereted into weeks as follows: 0 log weeks = 1 week, 1 log week = 2.7 weeks, 2 log weeks = 7.4 weeks, 2.4 log weeks = 12 weeks. Adherence was computed for the naltrexone group as the percent of pills taken per week (pills taken between visists/pills that should have been taken between visits). Adherence by week was included as a time-varying covariate in random regression models of change from baseline in percent of drinking days, percent of heavy drinking days, and drinks per drinking day using only the naltrexone group. All analyses used all available data (intent-to-treat sample). An assessment at baseline and at least one post-baseline were required for a patient to be evaluable.
RESULTS
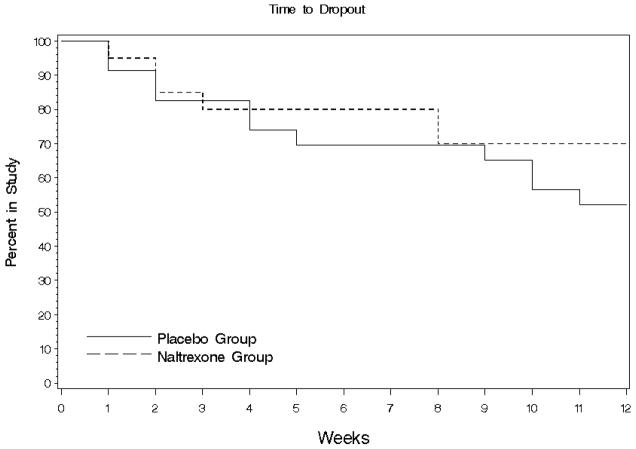
Of the 50 patients randomized (23 naltrexone, 27 placebo), 7 did not return after baseline (3 naltrexone, 4 placebo) leaving 43 evaluable patients. Of the 43 evaluable patients (20 naltrexone, 23 placebo) dropouts consisted of 3 patients after week 1 (1 naltrexone, 2 placebo), 4 patients after week 2 (2 naltrexone, 2 placebo), 1 naltrexone patient after week 3, 2 placebo patients after week 4, 1 placebo patient after week 5, 2 naltrexone patients after week 8, 1 placebo patient after week 9, 2 placebo patients after week 10, and 1 placebo patient after week 11. Thus, 26 patients completed (14 naltrexone, 12 placebo) the study. The average time in study was 6.8 weeks for the naltrexone group and 70.0% completed. For the placebo group the average time in study was 8.3 weeks and 52.2% completed. The difference between groups was not significant based on the log-rank test (chi-square=1.1, df=1, p=.2943). The average time in study was less in the naltrexone group even though the completion rate was higher because the patients who dropped out did so early in the study (all dropped out before week 8) (Figure 1). The two groups did not differ significantly on any baseline characteristic examined (Table 1).
Figure 1.
Survival analysis of naltrexone and placebo groups.
Table 1.
Demographic and baseline characteristics of the participants in the naltrexone and placebo groups (N=43)
| Treatment Group | Naltrexone (N=20) | Placebo (N=23) | All Patients (N=43) |
|---|---|---|---|
| Age, Mean±SD | 39.8±13.9 | 43.2±10.5 | 41.1±12.1 |
| High School Graduate, % | 95.0 | 95.7 | 95.3 |
| Female, % | 50.0 | 47.8 | 48.8 |
| White, % | 80.0 | 69.6 | 74.4 |
| Hispanic, % | 0.0 | 13.0 | 7.0 |
| Other, % | 5.0 | 4.3 | 4.7 |
| Family History of Alcoholism, % | 65.0 | 78.3 | 72.1 |
| Tobacco Use, % | 65.0 | 69.6 | 67.4 |
| Bipolar I, % | 70.0 | 73.9 | 72.1 |
| Bipolar II, % | 30.0 | 26.1 | 27.9 |
| Depressed, % | 85.0 | 82.6 | 83.7 |
| Mixed, % | 15.0 | 17.4 | 16.3 |
| Cannabis abuse, % | 25.0 | 17.4 | 20.9 |
| Cocaine abuse, % | 10.0 | 13.0 | 11.6 |
| Amphetamine abuse, % | 8.7 | 5.0 | 7.0 |
| Baseline Assessments | |||
| IDS-SR, Mean±SD | 41.3+10.9 | 45.5+10.1 | 43.5+10.5 |
| HRSD17, Mean±SD | 19.7+ 5.0 | 22.4+5.5 | 21.1+5.4 |
| YMRS, Mean±SD | 17.5+8.4 | 16.8+8.5 | 17.1+8.4 |
| PACS, Mean±SD | 22.3+5.3 | 22.5+4.1 | 22.4+4.7 |
| % of Drinking Days/Week, %±SD | 75.7+27.1 | 68.9+25.0 | 72.1+25.9 |
| % of Heavy Drinking Days/Week, %±SD | 54.3+31.6 | 54.0+27.2 | 54.2+29.0 |
| # of Drinks/Drinking Day, Mean±SD | 8.6+5.2 | 10. 0+7.4 | 9.4+6.4 |
| GGT, Mean±SD | 53.4+63.9 | 66.8+75.8 | 60.4+69.9 |
| AST, Mean±SD | 22.6+10.0 | 32.8+28.4 | 27.9+22.1 |
| ALT, Mean±SD | 23.8+19.2 | 34.6+34.9 | 29.5+28.7 |
| ASI Alcohol Score, Mean±SD | 0.5±0.3 | 0.6±0.2 | 0.6±0.3 |
| ASI Drug Score, Mean±SD | 0.0±0.0 | 0.0±0.1 | 0.0±0.1 |
| ASI Medical Score, Mean±SD | 0.3±0.3 | 0.3±0.3 | 0.3±0.3 |
| ASI Legal Score, Mean±SD | 0.0±0.0 | 0.1±0.2 | 0.0±0.1 |
| Concomitant Medications | |||
| Lithium, % | 5.0 | 13.0 | 9.3 |
| 13.0 | |||
| Total Anticonvulsants, % | 25.0 | 4.4 | 18.6 |
| Valproate, % | 20.0 | 8.7 | 11.6 |
| Lamotrigine, % | 0.0 | 0.0 | 4.7 |
| Oxcarbazepine, % | 5.0 | 2.3 | |
| Antipsychotics, % | 10.0 | 13.0 | 11.6 |
| Antidepressants, % | 40.0 | 34.8 | 37.2 |
| Sedative/Hypnotics, % | 20.0 | 13.0 | 16.3 |
Results of the random regression analysis are given in Table 2. Random regression analysis of percentage of drinking days per week (covariate: baseline percentage of drinking days) declined non-significantly more in the naltrexone group than the placebo group from baseline to week 1. The decline per log week from week 1 to week 12 was similar in each group (Table 2). Binary repeated measures analysis (covariates: baseline percent of drinking days and HRSD17 total score) showed that change in probability of having at least one drinking day between visits did not differ between groups from baseline to week 1 (f1,223=0.4, p=0.54) but a trend was observed toward greater decrease from week 1 to week 12 with naltrexone than placebo (f1,344 = 3.3, p=.07) (Figure 2). At week 12, 33.1% of naltrexone participants were estimated to have had zero drinking days that week compared to 7.3% of placebo participants (effect size = 0.68). Of note, anticonvusant use and specifically valproate use were examined as possible covariates but were rejected since the did not improve the fit of the model for this and other outcome measures. They also did not significantly change the results. However, a variable was also added to the dataset with the specific name of the anticonvulsant used. A 28% or greater improvement from baseline to exit in percent of drinking days was used for response since this roughly split the sample into equal parts. A 28% improvement is about 2 days fewer drinking days per week. Only 8 out of 43 (19%) of patients had a 50% or more improvement in percent drinking days. The following table shows the percent of patients receiving anticonvulsants each week by response status. Exit responders were more likely to have used valproate during the study than exit non-responders (38.9% vs. 12.5% at week 12). Thus, valproate use was likely not an important covariate because the effect is the same in both placebo and naltrexone groups.
Figure 2.
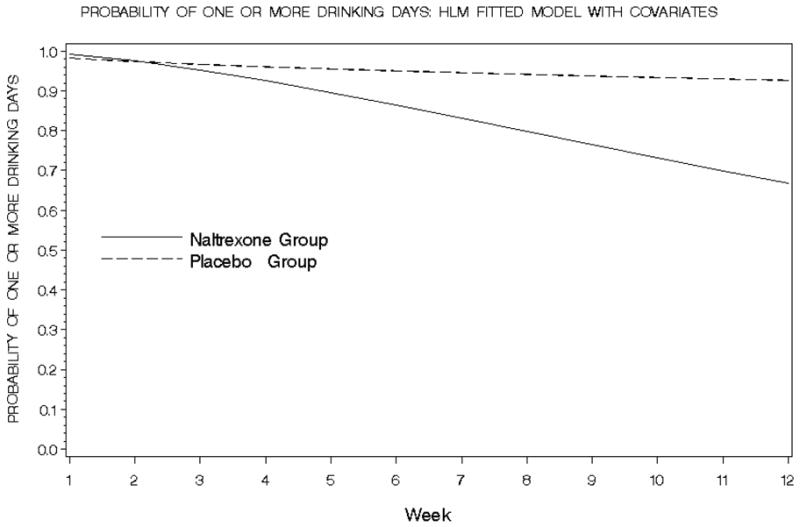
Probability of one or more drinking days in the naltrexone and placebo groups at each assessment.
Percentage of heavy drinking days per week (covariates: baseline percentage of heavy drinking days and ASI drug score) did not differ between groups either from baseline to week 1 or from week 1 to week 12 (Table 2). For the binary repeated measures analysis (covariates: baseline percentage of heavy drinking days, ALT, and PACS) change in probability of having at least one heavy drinking day between visits was not significantly different between groups from baseline to week 1 (f1,219=1.10, p=0.30) or from week 1 to week 12 (f1,267=0.30, p=0.86). An estimated 78.1% of the naltrexone group had zero heavy drinking days by week 12 compared with 54.4% of the placebo group (effect size = 0.51).
Analysis of the number of drinks per drinking day (covariates: number of drinks per drinking day) showed a non-significantly greater decline with naltrexone from baseline to week 1 and week 1 to week 12. At week 12 the number of drinks per drinking day was estimated to be reduced by 63.4% from baseline levels in the naltrexone group versus a 32.8% reduction in the placebo group (effect size=0.72).
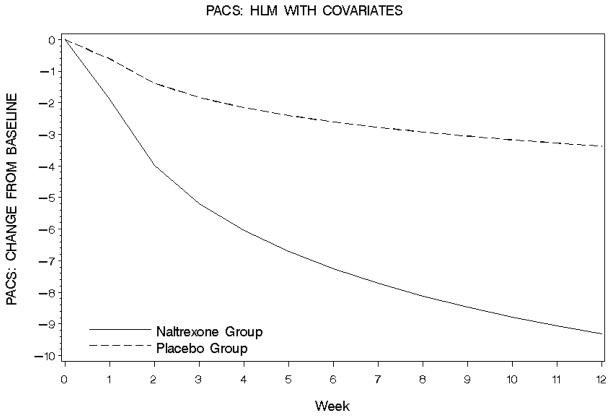
Decline in PACS (covariates: baseline PACS score, ASI alcohol and drug scores, YMRS score, level of education, use of lithium, antipsychotics, antidepressants or sedative/hyponotic/anxiolytics,) from baseline to week 1 was numerically larger for naltrexone participants than placebo while decline in PACS from week 1 to week 12 showed a trend toward significance favoring naltrexone (Table 2; Figure 3). At week 12, the naltrexone group had an estimated decline in PACS total scores of 9.3 points compared with 3.4 points for the placebo group (effect size=1.0).
Figure 3.
Change in alcohol craving as assessed by the PACS in the naltrexone and placebo groups.
Liver function measures showed greater numerical improvement in the naltrexone group than placebo group. GGT levels (n=33) assessed at weeks 0, 4, 8 and 12 (covariates: baseline GGT score, ASI drug scores, and level of education) showed no significant between-group differences in change from baseline to week 4 or from week 4 to week 12 (Table 2). From baseline to week 12 reduction in GGT was estimated to be 15.8% for naltrexone and 3.7% for placebo (effect size=0.42). AST (n=25) was assessed at week 0 and week 12 (covariates: baseline AST and lithium use). At week 12 the adjusted AST score showed a trend toward significantly lower AST in the naltrexone group (naltrexone=21.7 versus placebo=31.6, f1,21=4.20, p=.05). ALT (n=25) also assessed at week 0 and week 12 (covariates: baseline ALT and lithium use) and showed adjusted scores of 23.3 and 31.8 in the naltrexone and placebo groups, respectively, with a trend toward lower values in the naltrexone group (f1,21=3.00, p=.10).
The random regression model for HRSD17 (covariates: baseline HRSD17 score and level of education as covariates, and including a time squared term) showed no significant differences either from baseline to week 1 or from week 1 to week 12 (Table 2). Decline from baseline to week 12 was numerically larger in the naltrexone group than the placebo group (−8.3 points versus −6.4 points) (effect size=0.56). Analysis of the IDS-SR (covariates: baseline IDS-scores, gender, level of education, family history of alcoholism, sedative/hypnotic/anxiolytic use, and ASI alcohol and drug scores) showed a trend toward a significant between-group difference from baseline to week 1 but not from week 1 to week 12. At week 12, IDS-SR scores had declined by an estimated 21.9 points for naltrexone participants and 10.8 points for placebo participants (effect size=3.0) (Table 2). For the YMRS (covariates: baseline YMRS score, gender, lithium, antiseizure, antipsychotic or sedative/hypnotic/anxiolytic use, and ASI drug and alcohol scores) no significant between-group differences were observed from baseline to week 1 or from week 1 to week 12. At week 12, the decline from baseline in YMRS scores measured 9.3 points in the naltrexone group and 7.0 points in the placebo group (effect size=0.62).
The mean number of CBT sessions attended in the naltrexone and placebo groups (6.2±4.4 vs. 5.4±4.1) was similar (t48=0.7, p=.50). Outside care was uncommon and included weekly psychotherapy by a psychiatry resident and outpatient treatment at a substance abuse treatment facility (n=1 for each and both receiving placebo). Changes in concomitant medications consisted of addition of lithium (n=2 naltrexone, n=7 placebo), discontinuation of lithium (n=0 naltrexone, n=1 placebo), addition of an anticonvulsant (n=5 naltrexone, n=4 placebo), discontinuation of an anticonvulsant (n=1 naltrexone, n=0 placebo), addition of an antipsychotic (n=5 naltrexone, n=4 placebo), discontinuation of an antipsychotic n=0 naltrexone, n=3 placebo), addition of a sedative/hypnotic/anxiolytic (n=2 naltrexone, n=1 placebo) and discontinuation of a sedative/hypnotic/anxiolytic ((n=2 naltrexone, n=1 placebo). A total of 14 naltrexone patients had a medication addition and 3 a discontinuation as compared to 14 with additions and 5 with discontinuations in the placebo group. Chi squares comparisons of individual medication classes and overall additions and discontinuations showed no significant between-group differences (all p>0.05). Change in overall side-effect burden, as assessed by the PRD-III, was not significantly different between groups at week 6 (f1,26=0.02, p=.88) or week 12 (f1,25=1.10, p=.30).
Mean adherence, as measured by pill counts, for naltrexone-treated participants was 94.4% (SD=6.1), range 77.6% – 100%) and 95.3% (SD=7.4, range 69.0% – 100%) for placebo-treated participants. Including weekly adherence as a time-varying covariate in the random regression model using only the naltrexone participants produced a significantly large negative adherence coefficient for outcomes change from baseline in percent drinking days (f1,141=11.5, p=.0009), percent heavy drinking days (f1,127=11.6, p=.0009), but not drinks per drinking day (f1,103=1.1, p=.2998). For the placebo group, the random regression analyses using weekly adherence (pill counts) as a time-varying covariate produced small non-significant adherence coefficients for change from baseline in percent drinking days (f1,131<0.01, p=.9631), and percent heavy drinking days (f1,129=0.5, p=.4712), but a significant negative coefficient was found for drinks per drinking day (f 1,112=9.4, p=.0027).
DISCUSSION
To our knowledge, this is the first placebo-controlled trial to assess the efficacy of naltrexone specifically in patients with bipolar disorder and alcohol dependence. While alcohol consumption generally decreased numerically more with naltrexone than placebo, significant between-group differences on the primary outcome of drinking days were not found. The binary outcome of any drinking day each week showed a trend toward statistical significance, and naltrexone-treated patients were over four times more likely to have no drinking days at week 12. The PACS showed a trend toward a greater reduction with naltrexone suggesting that naltrexone reduced craving for alcohol in our sample.
GGT levels did not show significant between-group differences. However, AST and ALT levels showed trends toward lower levels at week 12 with naltrexone than placebo. These findings suggest that reductions in alcohol use with naltrexone were associated with changes in some, but not all, liver enzymes associated with alcohol dependence. It is surprising that we observed changes in liver enzymes that are generally less sensitive to change than the GGT. However, the finding is of clinical importance since AST and ALT elevations can be markers of alcohol-related hepatitis.
Overall, these data suggest that naltrexone was associated with a reduction in alcohol use compared to placebo even though the findings did not reach statistical significance. Our findings suggest that naltrexone was generally associated with a moderate effect size on alcohol use outcomes and a large effect size on craving in patients with bipolar disorder and alcohol dependence. Thus, naltrexone appears to be reasonably effective in reducing alcohol consumption and craving in this population.
Changes in the clinician-rated HRSD17 did not differ between groups, although a trend toward greater reduction with naltrexone than placebo was observed from baseline to week 1 on the self-rated IDS-SR. No differences in manic symptoms were found. Our open-label trial of naltrexone in patients with bipolar disorder and alcohol dependence found a significant baseline to exit with-group reduction in both manic and depressive symptoms (Brown et al., 2006). However, the changes were modest in magnitude.
Prior research suggests that adherence is related to reduction in drinking with naltrexone (Chick et al., 2000; Pettinati et al., 2000; Volpicelli et al., 1997). The current study had similar findings, with adherence, based on pill counts, producing a strong and significant negative coefficient on alcohol use measures in the naltrexone group. The negative coefficient for adherence suggests that higher adherence was associated with a larger decrease from baseline in alcohol consumption measures. Medication adherence can be poor in dual diagnosis patients (Brown et al., 2001) although in the current trial adherence, as judged by pill counts was relatively high. Therefore, interventions that might further improve adherence, such as pill containers and long-acting depot formulations of naltrexone might result in greater medication effect sizes in this population.
Strengths of the pilot study include the randomized, placebo-controlled design and examination of a complicated clinical population that has not been extensively studied in clinical trials. Small sample size is a limitation since this study may have been underpowered to detect significant differences despite large numerical differences and effect sizes. Use of concomitant psychotropic medications is a potential confound. However, these medications were similar in the two groups and were controlled for in the data analysis. The use of CBT as a psychosocial platform in both groups could have resulted in a reduction in alcohol use that limited our ability to detect medication effects. However, prior research suggests that the specific CBT used in this study increases retention in treatment without significantly reducing substance use (Schmitz et al., 2002). The COMBINE study found that either naltrexone or a combined behavioral intervention that integrated aspects of CBT was superior to medication management alone on drinking outcomes (Anton et al., 2006). However, the combination of combined behavioral intervention and naltrexone was not superior to either intervention alone. In addition, some data suggest that CBT may even increase naltrexone vs. placebo differences in clinical trials (Anton et al., 2005; Oslin et al., 2008). Naltrexone dose was fixed at 50 mg/day. We might have observed a greater reduction in alcohol use with a higher dose or a flexible dosing schedule (McCaul et al., 2000). It would have been useful to have genotyped the participants given emerging data on genotype as a predictor of response to naltrexone (Anton et al., 2008; Ray and Hutchison, 2007). Although participants in each group were similar at baseline, the modest sample size and relatively high attrition rate could have introduced bias in the study sample. A small study is also vulnerable to effects from outliers. However, the data analysis did not identify any outliers in the study. The participants were primarily Caucasian and recruited from a single geographic area, and many were not taking common bipolar disorders medications such as valproate or lithium at baseline potentially limiting the generalizability of the findings.
In summary, this study suggests that naltrexone was associated with improvement in some, but not all, alcohol-related outcome measures in patients with bipolar disorder and alcohol dependence. Reduction in alcohol use with naltrexone was related to medication adherence. Larger naltrexone trials in patients with bipolar disorder and alcohol dependence seem warranted.
Acknowledgments
This study was funded by NIH grant AA015389. The authors would like to thank Katie Gowen and Brandi Johnson for assistance with the assessment of study participants, and Drs. Maria Oroza and Alyson Nakamura for providing CBT to both treatment groups.
References
- Aagaard J, Vestergaard P. Predictors of outcome in prophylactic lithium treatment: a 2-year prospective study. J Affect Disord. 1990;18(4):259–66. doi: 10.1016/0165-0327(90)90077-l. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P, Waid LR, Myrick H, Voronin K, Thevos A, Wang W, Woolson R. Naltrexone combined with either cognitive behavioral or motivational enhancement therapy for alcohol dependence. J Clin Psychopharmacol. 2005;25(4):349–57. doi: 10.1097/01.jcp.0000172071.81258.04. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Waid LR, Latham PK, Malcolm RJ, Dias JK. Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. Am J Psychiatry. 1999;156(11):1758–64. doi: 10.1176/ajp.156.11.1758. [DOI] [PubMed] [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–17. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65(2):135–44. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ES, Beard L, Dobbs L, Rush AJ. Naltrexone in patients with bipolar disorder and alcohol dependence. Depress Anxiety. 2006;23(8):492–5. doi: 10.1002/da.20213. [DOI] [PubMed] [Google Scholar]
- Brown ES, Garza M, Carmody TJ. A randomized, double-blind, placebo-controlled add-on trial of quetiapine in outpatients with bipolar disorder and alcohol use disorders. J Clin Psychiatry. 2008;69(5):701–5. doi: 10.4088/jcp.v69n0502. [DOI] [PubMed] [Google Scholar]
- Brown ES, Suppes T, Adinoff B, Rajan Thomas N. Drug abuse and bipolar disorder: comorbidity or misdiagnosis? J Affect Disord. 2001;65(2):105–15. doi: 10.1016/s0165-0327(00)00169-5. [DOI] [PubMed] [Google Scholar]
- Chick J, Anton R, Checinski K, Croop R, Drummond DC, Farmer R, Labriola D, Marshall J, Moncrieff J, Morgan MY, Peters T, Ritson B. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35(6):587–93. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Lawrence Erlbaum Associates; Hillsdale, NJ: 1998. [Google Scholar]
- Crismon ML, Argo TR, Bendele SD, Suppes TS. Texas Medication Algorithm Procedural Manual: Bipolar Disorder Algorithms. Texas Department of State Health Services; 2007. Available at: http://www.dshs.state.tx.us/mhprograms/pdf/TIMABDman2007.pdf. [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21(3):287–92. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Kashner TM, Carmody TJ, Suppes T, Rush AJ, Crismon ML, Miller AL, Toprac M, Trivedi M. Catching up on health outcomes: the Texas Medication Algorithm Project. Health Serv Res. 2003;38(1 Pt 1):311–31. doi: 10.1111/1475-6773.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Modesto-Lowe V, Van Kirk J. Naltrexone vs. nefazodone for treatment of alcohol dependence. A placebo-controlled trial. Neuropsychopharmacology. 2000;22(5):493–503. doi: 10.1016/S0893-133X(99)00135-9. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345(24):1734–9. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ. Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology. 2000;22(5):480–92. doi: 10.1016/S0893-133X(99)00147-5. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49(11):881–7. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- Oslin D, Liberto JG, O’Brien J, Krois S, Norbeck J. Naltrexone as an adjunctive treatment for older patients with alcohol dependence. Am J Geriatr Psychiatry. 1997;5(4):324–32. doi: 10.1097/00019442-199700540-00007. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Lynch KG, Pettinati HM, Kampman KM, Gariti P, Gelfand L, Ten Have T, Wortman S, Dundon W, Dackis C, Volpicelli JR, O’Brien CP. A placebo-controlled randomized clinical trial of naltrexone in the context of different levels of psychosocial intervention. Alcohol Clin Exp Res. 2008;32(7):1299–308. doi: 10.1111/j.1530-0277.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57(10):1128–37. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Pierce JD, Jr, O’Brien CP. Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis. 2000;19(1):71–83. doi: 10.1300/J069v19n01_06. [DOI] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64(9):1069–77. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. JAMA. 1990;264(19):2511–8. [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477–86. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- Salloum IM, Cornelius JR, Daley DC, Kirisci L, Himmelhoch JM, Thase ME. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2005;62(1):37–45. doi: 10.1001/archpsyc.62.1.37. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Sayre S, McCleary P, Moeller FG, Swann A. Cognitive-behavioral treatment of bipolar disorder and substance abuse: a preliminary randomized study. Addic Disord Treat. 2002;1:17–24. [Google Scholar]
- Scott H, Johnson S, Menezes P, Thornicroft G, Marshall J, Bindman J, Bebbington P, Kuipers E. Substance misuse and risk of aggression and offending among the severely mentally ill. Br J Psychiatry. 1998;172:345–50. doi: 10.1192/bjp.172.4.345. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Singh J, Mattoo SK, Sharan P, Basu D. Quality of life and its correlates in patients with dual diagnosis of bipolar affective disorder and substance dependence. Bipolar Disord. 2005;7(2):187–91. doi: 10.1111/j.1399-5618.2004.00173.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption. Psychosocial and Biological Methods. 1992. pp. 41–72. [Google Scholar]
- Sonne SC, Brady KT, Morton WA. Substance abuse and bipolar affective disorder. J Nerv Ment Dis. 1994;182(6):349–52. doi: 10.1097/00005053-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Thase ME, Fava M, Halbreich U, Kocsis JH, Koran L, Davidson J, Rosenbaum J, Harrison W. A placebo-controlled, randomized clinical trial comparing sertraline and imipramine for the treatment of dysthymia. Arch Gen Psychiatry. 1996;53(9):777–84. doi: 10.1001/archpsyc.1996.01830090023004. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49(11):876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, Alterman AI, O’Brien CP. Naltrexone and alcohol dependence. Role of subject compliance. Arch Gen Psychiatry. 1997;54(8):737–42. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Wolfinger R, O’Connell M. Generalized linear models: a pseudo-likelihood approach. J Statist Comput Simul. 1993;48:233–43. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]