Abstract
Objective:
To investigate the combined effect of both pioglitazone and methotrexate on disease activity of rheumatoid arthritis in a biphasic study; experimental and clinical.
Methods:
Experimentally: 50 rats were divided into 5 equal groups; controls, experimental arthritis, methorexate treated (0.1 mg/Kg daily), pioglitazone-treated (10 mg/kg daily), and methotrexate and pioglitazone treated. Clinically: forty-nine diabetic rheumatoid arthritis patients were included. Patients group consisted of 28 patients and they received pioglitazone 30 mg orally beside their usual treatment. Control group consisted of 21 patients and they continued their usual treatment plus placebo. Disease activity was assessed using DAS28 score. Patients were followed up for 3 months.
Results:
Pioglitazone produced a significant improvement of serum oxidative stress parameters (P < 0.05), and inflammatory cytokines in the treated arthritic group (P < 0.05). Clinically, the pioglitazone treated group showed significant improvement in DAS28 (P = 0.001) and C-reactive protein (P < 0.0001) compared to placebo group.
Conclusion:
The concomitant use of the PPAR γ agonist pioglitazone and methotrexate appears to be promising therapeutic strategy for rheumatoid arthritis patients.
Keywords: pioglitazone, PPAR-γ agonist, anti-inflammatory, oxidative stress, rheumatoid arthritis, disease activity
Introduction
Rheumatoid arthritis (RA) is an autoimmune systemic inflammatory disease. Recent studies have found that there is increased prevalence of coronary artery atherosclerosis1,2 metabolic syndrome3 and insulin resistance4 among RA patients. Furthermore, hyperinsulinemia has been associated with disease activity,4 suggesting that inflammation and hyperinsulinemia somehow interact and facilitate one another.
Pioglitazone is a member of oral antidiabetic agents, the thiazolidinediones (TZDs). TZDs have been proven to improve insulin resistance, reduce circulating hyper-insulinemia and master the regulation of adipogenesis and glucose metabolism.5,6 They act as ligands for the peroxisome proliferator-activated receptor gamma (PPAR-γ), a member of the nuclear hormone receptor superfamily of transcription factors. PPAR-γ is expressed in a broad array of human tissues,5 Recently, PPAR-γ has been suggested to be an important immunomodulator; it suppresses the production of inflammatory cytokines7,8 matrix metalloproteinases,9 reactive oxygen species10 and inhibits pro-inflammatory gene expression.11 Furthermore, PPAR-γ ligands have been shown to induce apoptosis in T lymphocytes,12,13 and macrophages.14
Since the potential anti-inflammatory properties of PPAR-γ ligands on RA activity have been investigated in several experimental arthritis models, this study was conducted to evaluate the effect of PPAR-γ agonist pioglitazone combined with methotrexate (MTX) in RA either experimentally or clinically in RA diabetic patients.
Methods
Experimental study
Drugs used
Methotrexate (Ebewe Co, Austria, ampoule 50 mg/5 ml) and Pioglitazone hydrochloride (Amoun Co, Egypt, tab 30 mg dissolved in 0.5% sodium carboxy methyl cellulose for oral administration).
Animals used
This study was carried on 50 male Sprague-Dawley rats weighing 200–250 gm/rat. Animals had free access to food and water. They were exposed to the same housing conditions of heat and humidity. Animals were handled with the Guide for Care and Use of Laboratory Animals as adopted by the National Institutes of Health.
Animal grouping
Rats were divided into 5 equal groups (10 rats for each). Group 1 Control group: rats received 0.5% sodium carboxy methyl cellulose; Group 2 Control group: Non-treated arthritic group, arthritic rats received 0.5% sodium carboxy methyl cellulose; Group 3 Control group: MTX treated arthritic group, arthritic rats received MTX 0.1 mg/Kg/d;16 Group 4 Control group: Pioglitazone treated arthritic group, arthritic rats received pioglitazone 10 mg/kg/d;17 Group 5 Control group: MTX and Pioglitazone treated arthritic group, arthritic rats received MTX and Pioglitazone by the same previous doses. Animals were treated with drugs every 24 h, starting from day 28 orally via gastric tube for 16 days.
Induction of collagen- induced arthritis (CIA)
Collagen-induced arthritis was induced as previously described by Billingham.15 Bovine type II collagen (CII) was dissolved in 0.01 M acetic acid at a concentration of 2 mg ml–1 by stirring overnight at 4 °C. Dissolved CII was frozen at −70 °C until use. Complete Freund’s adjuvant (CFA) was prepared by the addition of Mycobacterium tuberculosis H37Ra at a concentration of 2 mg/ml (Difco, Laboratories, Detroit, Michigan). Before injection, CII was emulsified with an equal volume of CFA by centrifugation at 300 r.p.m. for 5 minutes (Virtis 23 homogenizer, Gardmer, New York). On day 1, rats were injected intradermally at the base of the tail with 100 μl of the emulsion (containing 100 μg of CII). On day 21, a second injection of CII in CFA was administered. Animals were received drugs every 24 h, starting from day 28, orally via gastric tube for 16 days.
Arthritic score of CIA
Arthritis was monitored every 4 days by using a macroscopic scoring system ranging from 0 to 4 for each limb, as previously described by Bakharevski et al,18 as follows. 0, no arthritis; 1, swelling and/or redness of one to two interphalangeal (IP) joints; 2, involvement of three to four IP joints or one larger joint; 3, more than four joints red/swollen; 4, severe arthritis of an entire paw, yielding a score of 0 to 16 per animal.
Biochemical Analyses
After the end of treatment, all rats were sacrified by an overdose of anesthesia. Trunk blood of each rat was collected and allowed to clot and centrifuged at 7000 r.p.m. for 15 minutes. The serum samples were separated carefully and stored at −30 °C until the biochemical assay.
Quantitative determination of serum IL-1β using ELISA kit (Biosource International, Inc, USA).19 Serum TNF-α was performed by TNF-α ELISA kit (Ray Biotech, Inc, USA).20 Serum thiobarbituric acid reactive substance (TBARS) was performed using Malondialdehyde Assay kit Northwest, Life Science Specialities, (NWLSS, Canada).21 Serum total glutathione (GSH) was performed using ELISA kit (Cayman Chemical Company, USA).22 Serum superoxide dismutase activity (SOD) was performed using an ELISA kit (Cayman Chemical Company, USA).23
The absorbance of each sample was read on plate ELISA reader.
Clinical Study
Patients and methods
Inclusion criteria
Forty-nine rheumatoid arthritis (RA) patients with diabetes mellitus type II, attending the Rheumatology and Immunology outpatient clinic in Mansoura University Hospital, Egypt between January 2009 and March 2010 were included in the study. The diagnosis of RA was confirmed by a rheumatologist according to the American College of Rheumatology (ACR) 1987 criteria for RA.24 Disease activity was assessed by calculating the disease activity for 28 joint indices (DAS28). Online calculator is available at www.das-score.nl.
Exclusion criteria
Pregnant and lactating subjects and patients with history of heart failure, renal failure, active liver disease or elevated aminotransferases greater than twice the upper limit of normal or with known contraindication to pioglitazone were excluded from the study. Patients aged <18 years was not eligible for the current study.
Study design
The present study is a prospective, single blind, therapeutic clinical trial. Eligible participants were RA and diabetic patients. They were subdivided into two groups. The patients group, consisting of the clinically more active (having 2 of the following: >6 swollen joints, >6 tender joints, morning stiffness >one hour) RA patients (n = 28). They received pioglitazone 30 mg orally, once daily for 12 weeks in addition to their treatment. The control group consisted of the remaining 21 patients who continued their treatment plus placebo orally, once daily for 12 weeks. The treatment of rheumatoid arthritis should be stable for at least three months before study entry and should include methotrexate orally 15 mg/ week plus non steroidal anti-inflammatory and/or oral corticosteroids ≤7.5 mg/day orally.
All participants underwent two study visits at 0 and 12 weeks for assessment of disease activity, blood sampling, urine pregnancy test and response to therapy. Fasting blood samples were drawn for complete blood count (CBC), erythrocyte sedimentation rate (ESR), C- reactive protein (CRP), liver aminotransferases, and serum creatinine. All subjects also had non study visits at 4, and 8 week for regular follow up and occurrence of side effects.
All subjects gave their informed consent and the study was approved by the Ethical Committee of Mansoura School of Medicine.
Statistical analysis
Data were analyzed using the statistical package SPSS version 17.0. Experimental data were expressed as means ± standard error (SE). Group differences were compared by using ANOVA followed by Tukey test. While clinical data were expressed as means ± standard deviation (SD) and group differences were compared by using t-test. Probability levels <0.05 were considered significant.
Results
Results of the experimental study characteristics of CIA in rats
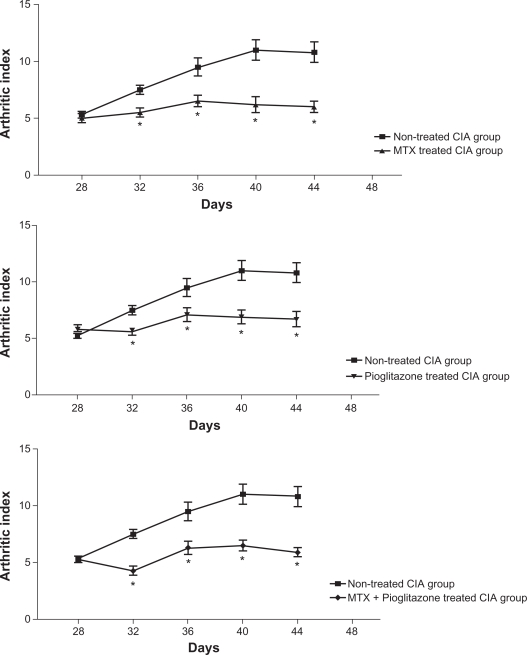
There was no macroscopic evidence of either paw erythema or edema in the normal control rats. As shown in Figure 1, animals subjected to CIA showed that the paw erythema and swelling increased in frequency and severity in a time-dependent mode with maximum arthritis indices of approximately 11 observed between 40 to 44 days post-CII immunization. Either MTX, pioglitazone or their combination exerted a significant suppression (P1 < 0.001) of the arthritis index between days 32 and 44 post-CII immunization versus that of non-treated arthritic rats. There were non-significant changes between the combination-treated and the single drug-treated arthritic groups.
Figure 1.
Effect of methotrexate (0.1 mg/kg) and pioglitazone (30 mg/kg) combination on the arthritic index of CIA rats.
Notes: Values are expressed as mean ± SEM. *P < 0.001 versus non-tretaed CIA group; Rats number = 10.
Serum oxidative stress parameters
The serum level of oxidative stress parameters were compared to the non-arthritic group. The lipid peroxidation product TRBS was significantly increased (19.14 ± 0.87 versus 8.17 ± 0.41 μ mol/L, P1 = 0.001). Meanwhile the antioxidant markers were significantly decreased; serum GSH (6.58 ± 0.33 versus 11.32 ± 0.13 μ mol, P = 0.001) and serum SOD (0.138 ± 0.01 versus 0.376 ± 0.02 U/ml, P = 0.001).
Either MTX or pioglitazone treatment produced significant decrease of serum TBARS (14.22 ± 1.2 and 14.95 ± 0.97 μ mol/L, P = 0.001) and significant increase of both serum GSH (8.90 ± 0.68, P = 0.008 and 7.20 ± 0.59 μ mol, P = 0.001) and serum SOD (0.256 ± 0.01, P = 0.001 and 0.227 ± 0.02 U/ml, P = 0.012) as compared to the non-treated arthritic group.
MTX and pioglitazone combination produced significant decrease of serum TBARS (9.23 ± 0.62 μ mol/L, P < 0.05 and significant increase of both serum GSH (10.97 ± 0.76 μ mol, P < 0.05) and serum SOD (0.396 ± 0.02 U/ml, P < 0.05) as compared to the non-treated, and/or pioglitazone treated arthritic groups (Table 1).
Table 1.
Effect of methotrexate (0.1 mg/kg) and pioglitazone (30 mg/kg) combination on the serum oxidative stress parameters; TBARS, GSH, SOD, in CIA rats.
| TBARS μ mol/L | GSH μ mol | SOD U/mL | |
|---|---|---|---|
| Non-arthritic group | 8.17 ± 0.41 | 11.32 ± 0.13 | 0.376 ± 0.02 |
| Non-treated CIA group | 19.14 ± 0.87 | 6.58 ± 0.33 | 0.138 ± 0.01 |
| P1 = 0.001 | P1 = 0.001 | P1 = 0.001 | |
| MTX treated CIA group | 14.22 ± 1.20 | 8.90 ± 0.68 | 0.256 ± 0.01 |
| P1 = 0.001 | P1 = 0.001 | P1 = 0.001 | |
| P2 = 0.001 | P2 = 0.008 | P2 = 0.001 | |
| Pioglitazone treated CIA group | 14.95 ± 0.97 | 7.20 ± 0.59 | 0.227 ± 0.02 |
| P1 = 0.001 | P1 = 0.03 | P1 = 0.001 | |
| P2 = 0.001 | P2 = 0.001 | P2 = 0.012 | |
| MTX + Pioglitazone treated CIA group | 9.23 ± 0.62 | 10.97 ± 0.76 | 0.396 ± 0.02 |
| P1 = 0.04 | P1 = NS | P1 = NS | |
| P2 = 0.001 | P2 = 0.001 | P2 = 0.001 | |
| P3 = 0.001 | P3 = 0.45 | P3 = 0.001 | |
| P4 = 0.001 | P4 = 0.001 | P4 = 0.001 |
Notes: Values are expressed as mean ± SEM. P < 0.05 was significant. Rats number = 10; P1 versus non-arthritic group; P2 versus Non-treated CIA group; P3 versus MTX treated CIA group; P4 versus Pioglitazone treated CIA group.
Abbreviations: TBARS, thiobarbituric acid reactive substance; GSH, glutathione; SOD, superoxide dismutase activity; CIA, collagen induced arthritis; MTX, methotrexate.
Serum inflammatory mediators
The serum level of the inflammatory mediator, TNF-α was significantly increased in the non treated CIA rats as compared to the non-arthritic group (178.77 ± 14.79 versus 21.71 ± 1.90 pg/ml, P = 0.001). Also, IL-1β, was significantly increased in CIA rats versus the non-arthritic group (24.21 ± 2.39 versus 6.46 ± 0.58 pg/ml, P = 0.001).
Either MTX or pioglitazone treatment resulted in significant reduction of serum level of TNF-α (45.64 ± 4.05 and 92.79 ± 8.06 pg/ml respectively, P = 0.001) and IL1β (12.33 ± 1.42 and 15.43 ± 1.39 pg/ml respectively, P = 0.001) in comparison to that of the non-treated arthritic group. It was also noticed that MTX caused significant decrease in both TNF-α (P = 0.001) and IL1β (P = 0.04) as compared to pioglitazone treatment of the arthritic group.
Further, MTX and pioglitazone combination produced significant improvement of both serum level of TNF-α (36.44 ± 2.53 pg/ml, P < 0.05) and IL1β (10.00 ± 0.90 pg/ml, P < 0.05) in comparison to the non-treated and pioglitazone treated arthritic group (Table 2). There was a non-significant change between the combination and MTX treated arthritic groups.
Table 2.
Effect of methotrexate (0.1 mg/kg) and pioglitazone (30 mg/kg) combination on the serum TNF-α, IL-1β in CIA rats.
| TNF-α pg/mL | IL-1β pg/mL | |
|---|---|---|
| Non-arthritic group | 21.71 ± 1.90 | 6.46 ± 0.58 |
| Non-treated CIA group | 178.77 ± 14.79 | 24.21 ± 2.39 |
| P1 = 0.001 | P1 = 0.001 | |
| MTX treated CIA group | 45.64 ± 4.05 | 12.33 ± 1.42 |
| P1 = 0.001 | P1 = 0.001 | |
| P2 = 0.001 | P2 = 0.001 | |
| Pioglitazone treated group | 92.79 ± 8.06 | 15.43 ± 1.39 |
| P1 = 0.001 | P1 = 0.001 | |
| P2 = 0.001 | P2 = 0.001 | |
| P3 = 0.001 | P3 = 0.04 | |
| MTX + Pioglitazone treated CIA group | 36.44 ± 2.53 | 10.00 ± 0.90 |
| P1 = 0.018 | P1 = 0.015 | |
| P2 = 0.001 | P2 = 0.001 | |
| P4 = 0.001 | P4 = 0.001 |
Notes: Values are expressed as mean ± SEM. P < 0.05 was significant. Rats number = 10; P1 versus non-arthritic group; P2 versus Non-treated CIA group; P3 versus MTX treated CIA group; P4 versus Pioglitazone treated CIA group.
Abbreviations: CIA, collagen induced arthritis; MTX, methotrexate; TNF, tumour necrosis factor; IL, interleukin.
Results of the Clinical Study
The pioglitazone treated group of patient showed marked improvement as regard the swollen joint count (SJC), tender joint count (TJC), ESR, CRP, DAS (P < 0.0001). This was also associated with significant reduction of blood glucose levels. (Table 3).
Table 3.
Clinical and laboratorial characteristics of pioglitazone and methotrexate treated group of patients before and after treatment by pioglitazone (n = 28).
| Before | After | P | |
|---|---|---|---|
| SJC | 4.7 ± 1 | 2.7 ± 0.7 | <0.0001 |
| TJC | 6 ± 1.2 | 3.1 ± 1.7 | <0.0001 |
| ESR (mm/h) | 49.9 ± 11.9 | 31.4 ± 7.4 | <0.0001 |
| DAS28 | 5.2 ± 0.5 | 3.8 ± 0.4 | <0.0001 |
| CRP (μg/mL) | 20.4 ± 4.7 | 8.1 ± 3.1 | <0.0001 |
| Fasting serum glucose (mg/dL) | 219.6 ± 57 | 163.1 ± 28.1 | <0.0001 |
| Post prandial serum glucose (mg/dL) | 297.1 ± 120.3 | 217 ± 59.8 | 0.003 |
Note: P significant at level <0.05.
Abbreviations: SJC, swollen joint count; TJC, tender joint count; ESR, erythrocyte sedimentation rate; DAS28, disease activity score for 28 joint indices; CRP, C- reactive protein.
On the other hand, the placebo treated (pioglitazone non treated) group of patients showed significant improvement of DAS, TJC, ESR, CRP. However, there were no significant improvement of SJC nor the blood glucose levels (Table 4).
Table 4.
Clinical and laboratorial characteristics of placebo and methotrexate treated group of patients before and after treatment (n = 21).
| Before | After | P | |
|---|---|---|---|
| SJC | 4.1 ± 2.5 | 3.1 ± 1.9 | ns |
| TJC | 5.6 ± 2.2 | 3.6 ± 1.3 | 0.001 |
| ESR (mm/h) | 32.1 ± 8.4 | 21.7 ± 3.5 | <0.0001 |
| DAS28 | 4.6 ± 0.5 | 4.2 ± 0.5 | 0.025 |
| CRP (μg/mL) | 18.7 ± 5.2 | 13.6 ± 3.8 | 0.001 |
| Fasting serum glucose (mg/dL) | 174.4 ± 36.4 | 163.6 ± 28.8 | ns |
| Post prandial serum glucose (mg/dL) | 276.7 ± 113.3 | 245.7 ± 48.1 | ns |
Note: P significant at level <0.05.
Abbreviations: ns, non significant; SJC, swollen joint count; TJC, tender joint count; ESR, erythrocyte sedimentation rate; DAS28, disease activity score for 28 joint indices; CRP, C- reactive protein.
When comparing the pioglitazone treated group of patients with the placebo treated group before treatment, it was found that there were significant differences as regard ESR, DAS28 score indicating that pioglitazone treated patients had more active disease (Table 5).
Table 5.
Clinical and laboratorial characteristics of pioglitazone treated and non treated groups of patients* before treatment by pioglitazone and placebo.
| Pioglitazone treated group | Pioglitazone non treated group | P | |
|---|---|---|---|
| Age | 43.4 ± 6.8 | 44.1 ± 7.1 | ns |
| SJC | 4.7 ± 1 | 4.1 ± 2.5 | ns |
| TJC | 6 ± 1.2 | 5.6 ± 2.2 | ns |
| ESR (mm/h) | 49.9 ± 11.9 | 32.1 ± 8.4 | <0.0001 |
| DAS28 | 5.2 ± 0.5 | 4.6 ± 0.5 | <0.0001 |
| CRP (μg/mL) | 20.4 ± 4.7 | 18.7 ± 5.2 | ns |
| Fasting serum glucose (mg/dL) | 219.6 ± 57 | 174.4 ± 36.4 | 003 |
| Post prandial serum glucose (mg/dL) | 297.1 ± 120.3 | 276.7 ± 113.3 | ns |
Note: P significant at level <0.05.
all patients received 15 mg methotrexate orally once weekly.
Abbreviations: ns, non significant; SJC, swollen joint count; TJC, tender joint count; ESR, erythrocyte sedimentation rate; DAS28, disease activity score for 28 joint indices; CRP, C- reactive protein.
After 3 months, there were significant reductions of DAS28 and CRP in patients assigned for pioglitazone treatment compared to the other group of patients (P = 0.001 and P < 0.0001 respectively). Notably, there were no significant differences in blood glucose levels between the two groups (Table 6).
Table 6.
Clinical and laboratorial characteristics of pioglitazone treated and non treated groups of patients* after treatment by pioglitazone and placebo.
| Pioglitazone treated group | Pioglitazone non treated group | P | |
|---|---|---|---|
| SJC | 2.7 ± 0.7 | 3.1 ± 1.9 | ns |
| TJC | 3.1 ± 1.7 | 3.6 ± 1.3 | ns |
| ESR (mm/h) | 31.4 ± 7.4 | 21.7 ± 3.5 | <0.0001 |
| DAS28 | 3.8 ± 0.4 | 4.2 ± 0.5 | 0.001 |
| CRP (μg/mL) | 8.1 ± 3.1 | 13.6 ± 3.8 | <0.0001 |
| Fasting serum glucose (mg/dL) | 163.1 ± 28.1 | 163.6 ± 28.8 | ns |
| Post prandial serum glucose (mg/dL) | 217.1 ± 59.8 | 245.7 ± 48.1 | ns |
Note: P significant at level <0.05.
all patients received 15 mg methotrexate orally once weekly.
Abbreviations: ns, non significant; SJC, swollen joint count; TJC, tender joint count; ESR, erythrocyte sedimentation rate; DAS28, disease activity score for 28 joint indices; CRP, C- reactive protein.
Seven (25%) out of the 28 patients who received pioglitazone in the current study reported weight gain. The increase in weight ranged from 2–4 kg. No other adverse effects were observed.
Discussion
RA is a chronic, systemic inflammatory disease that is characterized by progressive joint destruction. Treatment of rheumatoid arthritis is a major health problem. The available disease modifying antirheumatic drugs (DMARDs) and the recent biologics have promising protecting effects progressive joint destruction.25,26 The clinical use of these therapies has been limited however due to several issues including safety and cost treatment.
In this study the lipid peroxidation products, TBARS was significantly elevated in the serum of CIA rats. Identification of TBARS in the serum has been recognized as indirect evidence of the effect of free radicals as mediators of tissue damage and inflammatory arthropathy in the pathogenesis of RA.27 ROS generated by phagocytes in the inflamed rheumatoid joint are not efficiently scavenged resulting in increased levels of lipid peroxidation products both in the synovial fluid and blood as a result of transportation by the circulatory system.28
The excessive state of oxidative stress in CIA rats, as evidenced by increased TBARS was associated with impaired antioxidant status components (GSH level and SOD activity) as compared to the control group. This is in agreement with Hassan et al29 who showed that RA was associated with significant depletion in GSH levels supporting a hypothesis that defense mechanisms against ROS are impaired in RA. In inflammatory arthritis, polymorphonuclear leukocytes and macrophages are responsible for this impaired antioxidant status.30,31
The present results showed that MTX treatment caused significant improvement of the arthritis index as well as modulation of the alterated parameters produced in CIA rats with respect to serum lipid peroxidation, anti-oxidants levels, serum TNF-α and IL1β as compared to the CIA group. This can be attributed to its established antiinflammatory, anti-proliferative, immunosuppressive effects on activated T lymphocytes, increasing the rate of apoptosis of T cells, increasing endogenous adenosine release, altering the expression of cellular adhesion molecules, influencing production of cytokines, humoral responses and bone formation.4,32,33
In this study, it has been demonstrated that pioglitazone produced a significant improvement of the arthritis index that was associated with a significant reduction of the oxidative stress markers and the serum cytokines (TNF-α and IL1β) as compared to CIA group.
MTX and pioglitazone combination also exerted a significant improvement of the arthritis index and significant reduction of the oxidative stress markers and the serum cytokines (TNF-α and IL1β) as compared to the non-treated CIA group.
Tomita and associates34 observed that oral administration of PPAR-γ agonist resulted in reduced concentrations of proinflammatory cytokines at both the local and systemic levels. PPAR-γ agonist also decreased the expression of IL-1β and TNF-α in arthritic synovium in joints of mice with established collagen-induced arthritis. In support of this proposal the finding of Koufany et al35 that the TZDs (rosiglitazone 10 mg/kg/day or pioglitazone 30 mg/ kg/day) decreased the expression of IL-1β and TNF-α in inflamed synovium, which is a primary source for systemic inflammatory cytokines. This could be due to the ability of PPAR-γ agonists to inhibit IL-1β induced nitric oxide synthase expression and synthesis in chondrocytes from patients with osteoarthritis. PPAR-γ ligands also inhibited IL-1β induced metalloproteinase-13 (MMP-13) expression and production in chondrocytes. In addition to IL-1β, other cytokines produced in arthritic joint tissues, such as IL-17 and TNF-α, may contribute to joint destruction through production of nitric oxide (NO) and MMP-13. As expected, PPAR-γ activators inhibited the production of NO and MMP-13 in response to TNF-α, IL-17, suggesting that PPAR-γ activators may target common pathways leading to NO and MMP-13 production.8 Further, PPAR-γ activator inhibits IL-1β induced COX-2 expression and PGE2 production by human chondrocytes and synovial fibroblasts.36 A high level of COX-2 induced prostaglandins is known to be associated with the oxidative stress as COX-2 over expression induces the generation of free radicals and lipid peroxide formations.37 Consequently, modulation of COX-2 by PPAR-γ agonist is another mechanism of the anti-inflammatory, antioxidative effect of pioglitazone. Moreover, the anti-arthritic potency of PPAR-γ agonists may be due to their ability to inhibit the NF κB pathway in arthritic tissues. 38 Also, TZDs decreased the expression of basic fibroblast growth factor (bFGF), which is a powerful mitogen for both synovial fibroblasts and endothelial cells whose neutralization was reported to attenuate arthritis severity.39
To the best of our knowledge, this is the first clinical study evaluating the effects of a PPAR-γ agonist (pioglitazone) combined with MTX as therapy for rheumatoid arthritis. In the present study, the group of patients treated with pioglitazone exhibited significant improvement either clinically or in the laboratory. Also, there were significant reductions in the ESR, CRP and DAS28 compared to the placebo treated group of patients. Of note, there was no difference in the blood glucose level between the two groups at the end of the study, indicating that the improvement in disease activity indices and markers is not due to the antibiabetic effect of pioglitazone. These results could be explained by the suggested anti-inflammatory effects of PPAR-γ ligands.40 Recent studies have indicated that many of the cells involved with RA, namely mononuclear leukocytes,41 articular cartilage and chondrocytes42 express PPAR-γ receptors.
In the experimental phase of the study, we have highlighted the participation of cytokines in the inflammation, even in the early phases of induced arthritis. It was reported that PPAR-γ agonists can inhibit the expression of adhesion molecules,43 the release of TNFα from the cultered RA synovial membrane cells and spleen cells.34,44
Thus, there are several potential mechanisms by which PPAR-γ agonists could exert their anti-inflammatory effect.
Lately, the PPAR-γ agonists are emerging potential therapies for inflammatory cardiovascular diseases,11 psoriatic arthritis,45 multiple sclerosis,40 chronic obstructive pulmonary disease46 and glomerulosclerosis. 47 The findings in the present study suggest that PPAR-γ agonist (pioglitazone) could be a potential therapeutic agent for RA.
Conclusion
In conclusion, this study demonstrated that the concomitant use of MTX and pioglitazone may cause a synergistic effect in rheumatoid arthritis. We speculated that the observed beneficial effects of both drugs may be dependent upon a combination of the following pharmacological actions; 1) They inhibit inflammatory cytokines TNF-α, IL-1β. 2) They appear to prevent the activation of ROS, ultimately the degree of tissue injury. The antioxidant and the anti-inflammatory effects of pioglitazone and MTX support the possibility that patients with RA may benefit from therapy with both of the above in combination.
Abbreviations
- RA
Rheumatoid arthritis;
- TZDs
thiazolidinediones;
- PPAR-γ
the peroxisome proliferator-activated receptor gamma;
- MTX
methotrexate;
- CIA
collagen II induced arthritis;
- DAS28
disease activity for 28 joint indices;
- CBC
complete blood count;
- ESR
erythrocyte sedimentation rate;
- CRP
C- reactive protein;
- TBARS
thiobarbituric acid reactive substance;
- GSH
glutathione;
- SOD
superoxide dismutase activity;
- TNF
tumour necrosis factor;
- IL
interleukin;
- SJC
swollen joint count;
- TJC
tender joint count.
Footnotes
Authors Contributions
All the authors shared study design, data collection and literature research. DS designed, performed and wrote the initial version of the clinical study and critically revised the final version of the manuscript and she was responsible for the statistical analysis of the clinical results. EET designed, conducted and wrote the initial version of the study. HAM and VB designed and performed the experimental study and were responsible for the statistical analysis of the experimental part and writing the initial version of the manuscript. DS and AZE were responsible for performing the biochemical analyses and helped in writing the initial version of the manuscript.
Disclosure
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Chung CP, Avalos I, Raggi P, Stein CM. Atherosclerosis and inflammation: insights from rheumatoid arthritis. Clin Rheumatology. 2007;26:1228–33. doi: 10.1007/s10067-007-0548-7. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum. 2005;35:8–17. doi: 10.1016/j.semarthrit.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Karvounaris SA, Sidiropoulos PI, Papadakis JA, et al. Metabolic syndrome is common among middle-to-older aged Mediterranean patients with rheumatoid arthritis and correlates with disease activity: a retrospective, cross-sectional, controlled, study. Ann Rheum Dis. 2007;66(1):28–33. doi: 10.1136/ard.2006.053488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shahin D, Eltoraby E, Mesbah A, Houssen M. Insulin resistance in early untreated rheumatoid arthritis patients. Clinical Biochemistry. 2010;43:661–5. doi: 10.1016/j.clinbiochem.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Larsen TM, Toubro S, Astrup A. PPAR gamma agonists in the treatment of type II diabetes: is increased fatness commensurate with long term efficacy? International Journal of Obesity. 2003;27:147–61. doi: 10.1038/sj.ijo.802223. [DOI] [PubMed] [Google Scholar]
- 6.Rosen ED, Speigelman BM. PPARγ: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Che. 2001;276:37731–34. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- 7.Jiang C, Ting AT, Seed B. PPAR γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 8.Fahmi H, Di Battista JA, Pelletier JP, et al. Peroxisome proliferator-activated receptor gamma activators inhibit interleukin-1 beta-induced nitric oxide, matrix metalloproteinase 13 production in human chondrocytes. Arthritis Rheum. 2001;44:595–607. doi: 10.1002/1529-0131(200103)44:3<595::AID-ANR108>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Mix KS, Coon CI, Rosen ED, Suh N, Sporn MB, Brinckerhoff CE. Perixisome proliferator- activated receptor-γ- independent repression of collagenase gene expression by 2-cyano-3,12- dioxooleana-1,9-dien-28-oic acid and prostaglandin15-deoxy-Δ(12,14) J2: a role for smad signaling. Mol Pharmacol. 2004;65:309–18. doi: 10.1124/mol.65.2.309. [DOI] [PubMed] [Google Scholar]
- 10.Hwang J, Kleinhenz DJ, Lassègue B, Griendling KK, Dikalov S, Hart CM. Peroxisome proliferator-activated receptor {gamma} ligands regulate endothelial membrane superoxide production. Am J Physiol Cell Physiol. 2005;288:C899–905. doi: 10.1152/ajpcell.00474.2004. [DOI] [PubMed] [Google Scholar]
- 11.Verrier E, Wang L, Wadham C, et al. PPARγ agonists ameliorate endothelial cell activation via inhibition of diacylglycerol- protein kinase C signaling pathway. Role of diacylglycerol Kinase. Circ Res. 2004;94:1515–22. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- 12.Clarke RB, Bishop-Bailey D, Estrada-Hernandez T, Hla T, Puddington L, Padula S. The nuclear receptor PPARγ and immunoregulation: PPARγ mediates inhibition of helper T cell responses. J Immunol. 2000;164:1346–71. doi: 10.4049/jimmunol.164.3.1364. [DOI] [PubMed] [Google Scholar]
- 13.Harris SG, Phipps RP. The nuclear receptor PPARγ is expressed by mouse T lymphocytes and PPAR γ agonists induce apoptosis. Eur J Immunol. 2001;31:1098–105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Chinetti G, Griglio S, Antonucci M, et al. Activation of proliferator-activated receptors induces apoptosis of human monocyte-derived macrophages. J Biol Chem. 1998;273(40):25573–80. doi: 10.1074/jbc.273.40.25573. [DOI] [PubMed] [Google Scholar]
- 15.Billingham MEJ. Models of arthritis, the search for anti-arthritic drugs. Pharmacol Ther. 1983;21:389–428. doi: 10.1016/0163-7258(83)90062-1. [DOI] [PubMed] [Google Scholar]
- 16.Bendele AM, McComb J, Gould T, et al. Animal models of arthritis: relevance to human disease. Toxicologic Pathol. 1999;27:134–42. doi: 10.1177/019262339902700125. [DOI] [PubMed] [Google Scholar]
- 17.Majithiya JB, Paramar AN, Balaraman R. Pioglitazone, a PPAR agonist, restores endothelial function in aorta of streptozotocin-induced diabetic rats. Cardiovascular Research. 2005;66(1):150–61. doi: 10.1016/j.cardiores.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Bakharevski O, Stein-Oakley AN, Thomson NM, Ryan PF. Collagen induced arthritis in rats: Contrasting effect of subcutaneous versus intradermal inoculation of type II collagen. Rheumatol. 1998;25:1945–52. [PubMed] [Google Scholar]
- 19.Wang CX, Olschowka JA, Wrathall JR. Increase of interleukin-1β mRNA, protein in the spinal cord following experimental traumatic injury in the rat. Brain research. 1997;759(2):190–6. doi: 10.1016/s0006-8993(97)00254-0. [DOI] [PubMed] [Google Scholar]
- 20.Guo L, Ye C, Chen W, et al. Anti-inflammatory, analgesic potency of carboxyamidotriazole, a tumorostatic agent. J Pharmacol Exp Ther. 2008;325(1):10–6. doi: 10.1124/jpet.107.131888. [DOI] [PubMed] [Google Scholar]
- 21.Botsoglou NA. Rapid, Sensitive, specific thiobarbituric acid method for measuring lipid peroxidation in animal tissue, food, feedstuff samples. J Agric Food Chem. 1994;42(9):1931–7. [Google Scholar]
- 22.Baillie TA, Slatter JG. Glutathione: a vehicle for the transport of chemically reactive metabolites in vivo. Acc. Chem Res. 1991;24(9):264–70. [Google Scholar]
- 23.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, ugly. Am J Physio Cell Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 24.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Hurd E, Cush J, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46:614–24. doi: 10.1002/art.10141. [DOI] [PubMed] [Google Scholar]
- 26.Suryaprasad AG, Prindiville T. The biology of TNF blockade. Autoimmun Rev. 2003;2:346–57. doi: 10.1016/s1568-9972(03)00048-x. [DOI] [PubMed] [Google Scholar]
- 27.Ostrakhovitch E, Afanasev I. Oxidative stress in rheumatoid arthritis. Leukocytes suppression by rutin, other antioxidants, chelators. Biochem Pharmacol. 2001;62(6):743–6. doi: 10.1016/s0006-2952(01)00707-9. (15) [DOI] [PubMed] [Google Scholar]
- 28.Baskol G, Demir H, Baskol M, Kilic E, Ates F, Kocer D, et al. Assessment of paraoxonase 1 activity, malondialdehyde levels in patients with rheumatoid arthritis. Clin Biochem. 2005;38:951–5. doi: 10.1016/j.clinbiochem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Hassan M, Hadi R, Al-Rawi Z, Padron V, Stohs S. The glutathione defense system in the pathogenesis of rheumatoid arthritis. J Appl Toxicol. 2001;21(1):69–73. doi: 10.1002/jat.736. [DOI] [PubMed] [Google Scholar]
- 30.Jaswal S, Mehta HC, Sood AK, Kaur J. Antioxidant status in rheumatoid arthritis role of antioxidant therapy. Clin Chim Acta. 2003;338(1–2):123–9. doi: 10.1016/j.cccn.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Kamanli A, Naziroğlu M, Aydilek N, Hacievliyagil C. Plasma lipid peroxidation, antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct. 2004;22(1):53–7. doi: 10.1002/cbf.1055. [DOI] [PubMed] [Google Scholar]
- 32.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers, patients with rheumatoid arthritis. Rheumatology. 2003;42:1189–96. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 33.Huang CC, Hsu PC, Hung YC, et al. Ornithine decarboxylase prevents methotrexate-induced apoptosis by reducing intracellular reactive oxygen species production. Apoptosis. 2005;10:895–907. doi: 10.1007/s10495-005-2947-z. [DOI] [PubMed] [Google Scholar]
- 34.Tomita T, Kakiuchi Y, Tsao PS. THR0921, a novelperoxisome proliferator-activated receptor γ agonist, reduces the severity of collagen-induced arthritis. Arthritis Res Ther. 2006;8:R7. doi: 10.1186/ar1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koufany M, Moulin D, Bianchi A, et al. Anti-inflammatory effect of antidiabetic thiazolidinediones prevents bone resorption rather than cartilage changes in experimental polyarthritis. Arthritis Res Ther. 2008;10(1):R6. doi: 10.1186/ar2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPAR. J Biol Chem. 2000;275:28028–32. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- 37.Fahmi H, Pelletier JP, Martel-Pelletier J. PPAR gamma ligands as modulators of inflammatory, catabolic responses in arthritis. An overview. J Rheumatol. 2002;29(1):3–14. [PubMed] [Google Scholar]
- 38.Shiojiri T, Wada K, Nakajima A, et al. PPAR γ ligands inhibit nitrotyrosine formation, inflammatory mediator expressions in adjuvant-induced rheumatoid arthritis mice. Eur J Pharmacol. 2002;448:231–8. doi: 10.1016/s0014-2999(02)01946-5. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita A, Yonemitsu Y, Okano S, et al. Fibroblast growth factor-2 determines severity of joint disease in adjuvant-induced arthritis in rats. J Immunol. 2002;168:450–7. doi: 10.4049/jimmunol.168.1.450. [DOI] [PubMed] [Google Scholar]
- 40.Racke MK, Gocke AR, Muir Mark, et al. Nuclear receptors and autoimmune disease. The potential of PPAR agonists to treat multiple sclerosis. J Nutr. 2006;136:700–3. doi: 10.1093/jn/136.3.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 42.Sabatini M, Bardiot A, Lesur C, et al. Effects of agonists of peroxisome proliferator-activated receptor gamma on proteoglycan degradation and matrix metalloproteinase production in rat cartilage in vitro. Osteoarthritis Cartilage. 2002;10:673–9. doi: 10.1053/joca.2002.0827. [DOI] [PubMed] [Google Scholar]
- 43.Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor gamma activators. Circulation. 2002;101:235–8. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- 44.Sumariwalla PF, Palmer CD, Pickford LB, Feldmann M, Foxwell BMJ, Brennan FM. Suppression of tumour necrosis factor production from mononuclear cells by a novel synthetic compound, CLX-090717. Rheumatology. 2009;48:32–8. doi: 10.1093/rheumatology/ken398. [DOI] [PubMed] [Google Scholar]
- 45.Bongartz T, Coras B, Vogt T, Schölmerich J, Müller-Ladner U. Treatment of active psoriatic arthritis with the PPARγ ligand pioglitazone: an open-label pilot study. Rheumatology. 2005;44(1):126–9. doi: 10.1093/rheumatology/keh423. [DOI] [PubMed] [Google Scholar]
- 46.Patel HJ, Belvisi MG, Bishop-Bailey D, Yacoub MH, Mitchell JA. Activation of peroxisome proliferator-activated receptors in human airway smooth muscle cells has a superior anti-inflammatory profile to corticosteroids: relevance for chronic obstructive pulmonary disease therapy. J Immunol. 2003;170:2663–9. doi: 10.4049/jimmunol.170.5.2663. [DOI] [PubMed] [Google Scholar]
- 47.Zou R, Xu G, Liu X, et al. PPARγ agonists inhibit TGF-β-PKA signaling in glomerulosclerosis. Acta Pharmacologica Sinica. 2010;31:43–50. doi: 10.1038/aps.2009.174. [DOI] [PMC free article] [PubMed] [Google Scholar]