Abstract
Objective
Individual differences in subjective responses to alcohol are believed to have a genetic basis and have been associated with increased risk of alcohol-related problems. There are, however, conflicting results from past studies, perhaps owing to differences in subjective alcohol effects by limb of the blood alcohol curve and the passage of time. The current pilot study evaluated relations among serotonin transporter (SERT) genotype, subjective alcohol responses, and drinking behavior across both the ascending the descending limbs of the blood alcohol curve.
Method
Participants (N = 222; 68% male) were administered alcohol (target blood alcohol concentration of .06%) with a subsample (n = 86) providing genetic data. Following a social stressor, participants were provided the opportunity to engage in ad libitum alcohol consumption.
Results
SERT transporter was not significantly associated with ad lib drinking or subjective alcohol effects at individual time points, although a trend toward a SERT by blood alcohol concentration limb interaction was observed for ad lib drinking. In addition, SERT genotype predicted acute tolerance to alochol effects, with participants homozygous for the long SERT allele developing acute tolerance more rapidly than other genotypes.
Conclusions
Although SERT genotype was not reliably associated with ad lib drinking behavior, the results suggest that individuals with the long-long (LL) genotype may develop acute tolerance to alcohol effects more rapidly than heterozygotes or individuals homozygous for the short SERT allele.
GENETIC LINKAGE ANALYSES and sophisticated genotyping technology now confirm a clear and substantial genetic component to alcohol abuse and dependence as defined by the Diagnostic and Statistical Manual for Mental Disorders, Third Edition, Revised (DSM-III-R; American Psychiatric Association, 1987), and Fourth Edition (DSM-IV; American Psychiatric Association, 1994). A family history of alcoholism confers increased risk of personal alcohol-related problems, especially for male offspring of alcoholic fathers (Conway et al., 2003; Schuckit and Smith, 2000). Twin studies support this genetic risk, providing estimates of 40%-60% of the variance explained by genetic background (Heath et al., 1997; Kaprio et al., 1987). Nevertheless, the mechanisms through which genetic factors transmit risk are only now beginning to be uncovered.
Schuckit et al. (1996) demonstrated that young men with a family history of alcoholism showed a lower level of response to alcohol as assessed by subjective reports, body sway, and hormonal levels including cortisol, adrenocorticotropin, and prolactin (Schuckit, 1984a,b; 1985; 1998; Schuckit and Gold, 1988; Schuckit et al., 1987). Moreover, those with a low subjective response to alcohol during an alcohol challenge were at increased risk for development of alcoholism 15 years later. Similarly, Heath et al. (1999) found that a low subjective response to alcohol was associated with increased risk of alcohol dependence, although subjective response was only weakly related to family history status. A meta-analysis on subjective responses to alcohol among sons of alcoholics reached a similar conclusion, stating that “it may be appropriate to apprise the biological sons of male alcoholics that they may experience less subjective sensitivity to alcohol than other individuals” (Pollock, 1992).
Despite these very interesting findings, a consensus has not been reached about the prognostic value of subjective intoxication (SI) for alcohol-use disorders. Researchers from a variety of labs have failed to find a low-subjective response among those with a family history of alcoholism, instead finding that they are equally or more sensitive to alcohol's effects as measured by subjective reports (King et al., 1997; Nagoshi and Wilson, 1987), heart rate (Conrod et al., 1997; de Wit and McCracken, 1990), and hormonal levels (Gianoulakis et al., 1996).
One possible explanation for the inconsistency in past studies is that group differences in subjective effects vary as a function of the time elapsed following the consumption of alcohol. For example, those with a family history of alcoholism may experience alcohol effects more strongly initially but adapt to the effects more quickly. This more rapid adaptation to alcohol effects when blood alcohol concentrations are held constant or accounted for statistically is often referred to as acute tolerance. Acute tolerance generally refers to adaptation within the first several hours after alcohol is consumed.
Support for group differences in acute tolerance was demonstrated in a recent study in which blood alcohol levels were held constant and family history-positive individuals were found to have stronger initial intoxication after alcohol but more rapid adaptation to these effects over time (Morzorati et al., 2002). This study used an intravenous breath alcohol concentration clamp to keep breath alcohol concentrations at a steady state of 60 mg%. In addition to controlling for the effects of breath alcohol concentration, this approach eliminates effects that may be due to differential time course between individuals. At the same time, this approach is limited in external validity as steady-state breath alcohol levels are not typically maintained in real drinking contexts. Further, this approach does not allow assessment of differences in alcohol effects by limb of the blood alcohol curve, which may be important in understanding the motivational basis for alcohol consumption.
In their Differentiator Model, Newlin and Thomson (1990) also suggested that high-risk individuals are more sensitive to the initial effects of alcohol but more rapidly develop acute tolerance to these effects. They further suggest that, because the stimulating effects of alcohol typically occur as blood alcohol levels rise and sedative effects occur as they descend, these individuals experience greater stimulation and less sedation. Consistent with this model, stronger stimulant effects on the ascending limb and weaker sedative effects on the descending limb have been observed among individuals with a familial risk of alcoholism (Earleywine, 1994; Holdstock et al., 2000). This model provides a conceptually plausible and testable hypothesis regarding increased risk of alcoholism, whereby individuals who experience more positive and fewer negative effects are likely to drink more. Despite the appeal of this integrative model, additional studies are necessary to establish the potential genetic basis for this pattern of subjective effects.
Several candidate genes have been identified in the search for the genetic basis of individual differences in subjective response to alcohol. For example, Schuckit et al. (1999) found that genetic polymorphisms on the gamma-aminobutyric acid (GABA) α 6 receptor and the serotonin transporter (SERT) were associated with a low subjective response to alcohol. Individuals who were homozygous for the long allele in the promoter region of the SERT reported the lowest subjective response to alcohol and were at increased risk for developing alcoholism. Newlin and Thomson (1990) suggest that the subjective effects assessed initially in these young men in their early 20s (e.g., Schuckit and Gold, 1988) were primarily sedative effects, such that the inverse relation between subjective response and alcoholism risk is consistent with the differentiator model. At the same time, simple acute tolerance cannot be ruled out as a possible explanation, as past studies have not assessed the relations among SERT genotype, changes in subjective effects across time, and alcohol-related outcomes.
A better understanding of the mechanism through which the long allele of the SERT gene may relate to alcoholism risk is essential given the difficulty of other researchers to replicate Schuckit and colleagues’ findings (1999). Other studies have found either no relation between SERT and alcoholism or an increased risk of alcoholism among individuals with one or more copies of the short allele. The most comprehensive study to date is the Collaborative Study on the Genetics of Alcoholism, which found no association between SERT and alcoholism (Edenberg et al., 1998). A number of additional studies have reached a similar conclusion (Gorwood et al., 2000; Hill et al., 2002; Kranzler et al., 2002).
The functional properties of the SERT polymorphism (SLC6A4) appear to be as complex as the relations between this genetic marker and behavior. The short (S) allele appears to be associated with a low serotonin turnover rate and individuals homozygous for the long (L) allele have increased density and functional capacity of the SERT (Lesch et al., 1996). These results suggest that individuals with the L allele should demonstrate greater SERT protein availability, resulting in lower circulating levels of serotonin (Johnson, 2000). However, findings have been inconsistent with studies showing both the Sand L alleles to be associated with decreased SERT protein availability (Heinz et al., 2000; van Dyck et al., 2004). Heinz et al. (2000) found that SERT availability was higher for long-long (LL) homozygotes in a sample of healthy volunteers but also demonstrated higher SERT availability for short-short (SS) homozygotes in an alcohol-dependent population. Based in part on these findings, Heinz et al. (2001) suggest that individuals with the LL genotype may be more susceptible to the “long-term, toxic effects of chronic alcohol intake” (p. 491).
The goal of the current study was to examine the relation among SERT genotype, subjective alcohol effects, and ad lib drinking behavior. Based on past research (Schuckit et al., 1999), the long allele of the SERT gene was expected to be associated with a lower subjective alcohol response and heavier ad lib alcohol consumption. To extend past research, subjective alcohol effects were assessed across time and limb of the blood alcohol curve. It was hypothesized that the L allele would be associated with more rapid development of acute tolerance to subjective alcohol effects. In addition, based on the Differentiator Model (Newlin and Thomson, 1990), an interaction between SERT genotype and limb of the blood alcohol curve was anticipated such that the SERT L allele would be associated with greater subjective “high” on the ascending limb and lower subjective intoxication on the descending limb.
Method
Participants
Analyses for the present study were based on data collected at the pretest assessment for an alcohol prevention study. As part of the prevention study, participants older than age 21 completed pre- and postintervention alcohol challenge assessments in a simulated-bar laboratory on The University of Texas at Austin (UT) campus. The study protocol also included assessment of ad lib alcohol consumption following the introduction of a social stressor. The inclusion of a social stressor related to hypotheses that were not the focus of the current study and have been reported elsewhere (Kim, 2002). Thus, the current manuscript represents a pilot study of the relation between the SERT and ad lib consumption as part of a larger prevention study.
Through a subsequent collaboration with scientists in The Waggoner Center for Alcohol and Addiction Research (also al UT), participants were recontacted approximately 2 years after their initial bar lab assessment and asked to provide genetic samples. Of the 222 participants who completed the pretest assessment, researchers attempted to recontact a total of 183 participants in an effort to obtain genetic samples. The remaining 39 participants were not recontacted because their blood alcohol concentrations following the initial drink administration were outside of the target range (<.04% or >.08%) or because they were missing data on the predictor variables. Of those contacted, 86 provided genetic samples for analyses. The low response rate (47%) was based primarily on the inability to locate many of the participants from the initial study. The follow-up was not planned as part of the initial study. Thus, contact information was not updated regularly between the end of the initial study and the beginning of the follow-up. The majority of participants who were reachable by project staff agreed to participate in the follow-up.
Of those who completed the assessment, valid Polymerase Chain Reaction (PCR) data for the SERT was available for 83 participants. Within this subsample, the mean (SD) age was 22.2 (1.4), and men were disproportionately represented (69.9%). The sample was primarily white (56.6%), with 18.1% Hispanic participants. The primary racial minority groups represented were Asian American (15.7%) and black (3.6%), with the remaining 6.0% indicating that they belonged to another racial group.
Measures
Demographic information
Age, ethnicity, gender, socioeconomic status, and year in school were assessed using standard assessment forms.
Alcohol use
The Daily Drinking Questionnaire (DDQ; Collins et al., 1985) was used to assess both typical and heaviest weekly alcohol use. Scoring produced three continuous measures for typical number of drinking days (frequency), number of drinks per drinking day (quantity per drinking occasion), and total drinks per week (Frequency × Quantity). The DDQ has demonstrated good internal reliability and construct validity.
Subjective alcohol effects
Visual Analog Scales (VAS; Johanson and Uhlenhuth, 1980) were used to assess the degree to which individuals experienced SI and subjective high (SH). Participants were asked to rate the extent to which they felt these effects by marking 100-mm lines ranging from “not at all” to “extremely.” Past studies have successfully used this method as a measure of SI (Drug Effects Questionnaire [DEQ]; Doty and de Wit, 1995; Holdstock and de Wit, 2001; Holdstock et al., 2000; Kirk and de Wit, 1998). Participants also provided subjective evaluations of the number of standard drinks they consumed and their current blood alcohol concentration (BAC).
State affect
A revised version of the Profile of Mood States (POMS; Gabrielli et al., 1991) was used to assess any changes in affect associated wilh the social stressor. The POMS is a widely used measure of state affect with demonstrated internal reliability and construct validity. The tension reduction subscale was used to assess change in anxiety due to the introduction of the social stressor. The POMS was administered prior to beverage administration and three times following with internal reliabilities for the tension reduction subscale ranging from .58 to .71.
Procedures
Participants were scheduled early in the evening on a weekday for data collection. Reminder calls were conducted prior to the scheduled time and participants were instructed to refrain from use of alcohol or other drugs for 24 hours prior to their appointment and to refrain from eating after 12:00 pm on the day of their appointment. On arrival at the lab, research staff checked identification to ensure that participants were over 21 prior to them providing informed consent. Participants then completed a baseline breath test to ensure a zero BAC, were weighed for calculation of alcohol dose, and completed a series of questionnaires. All participants were then told that they would be served three alcoholic drinks to be consumed over the next 30 minutes. Participants received a total of 0.80 grams per kilogram of alcohol targeting a BAC of .06%. Each drink contained a 3:1 ratio of margarita mix to 80-proof tequila. After a 20-minute absorption period, BAC was assessed and participants provided ratings of subjective alcohol effects and state affect. After completing several cognitive tasks, BAC and subjective experiences were reassessed, and a social stressor was introduced. Graduate student supervisors collected all BAC data, as research assistants responsible for administering experimental tasks were blind to beverage condition. Mean BAC levels and subjective effect ratings for the sample at 20 minutes and 60 minutes after beverage administration are presented in Table 1 by racial and ethnic group.
Table 1.
Mean (SD) subjective effects and ad lib consumption, by ethnic group
| Measure | Asian American (n = 13) % or mean (SD) | Hispanic (n = 14) % or mean (SD) | White (n = 46) % or mean (SD) |
|---|---|---|---|
| SERT genotype | |||
| Homozygous short |

|

|

|
| Heterozygous | |||
| Homozygous long | |||
| Typical weekly consumption | 7.54 (3.71)a | 13.43 (5.32)b | 12.22 (9.40)b |
| BAC %, 20 min. | .059 (.011) | .057 (.009) | .057 (.010) |
| BAC %, 45-60 min. | .055 (.007) | .059 (.008)a | .054 (.008)b |
| BAC % change | .004 (.008) | -.002 (.010) | .003 (.009) |
| Subjective intoxication, 20 min. | 47.31 (30.46) | 41.07 (12.43) | 39.13 (17.99) |
| Subjective intoxication, 45-60 min. | 42.69 (28.98)a | 30.36 (14.47)b | 27.18 (14.74)b |
| Change in intoxication, T1-T2 | 4.61 (17.26) | 10.71 (16.16) | 11.95 (16.45) |
| Subjective high, 20 min. | 44.23 (25.32) | 44.64 (22.31) | 37.61 (18.42) |
| Subjective high, 45-60 min. | 35.38 (20.56) | 30.36 (20.04) | 24.67 (18.99) |
| Change in high | 8.85 (15.70) | 14.29 (21.29) | 12.93 (15.85) |
| Ad lib standard drinks, 0-3 | 0.80 (1.10) | 1.10 (0.83) | 1.39 (1.27) |
Notes: The sample presented here (n = 73) does not include black (n = 5) or “other” ethnicity (n = 3) groups. In addition, one white participant with missing data is not represented. Different superscripts indicate a statistically significant group difference. SERT = serotonin transporter; BAC = blood alcohol concentration; min. = minute; T1 = Time 1 (20 min.); T2 = Time 2 (45-60 min.).
Participants were then told that they would be giving a 5-minute speech that would be videotaped and later rated by opposite-gender peers (Levenson and Gottman, 1983). Participants were told that they would have 20 minutes to prepare for their speeches and that free beverages would be available during this time. They could order drinks from a menu that included beer, wine, mixed drinks, shots, and nonalcoholic beverages. Research assistants offered a drink every 5 minutes during the ad lib period and participants were free to spontaneously make drink orders. One of the research assistants carefully monitored the amount consumed to ensure that no participant's BAC exceeded .12%. If serving another drink would result in exceeding this limit, participants were told that they could not be served another beverage at that time.
After completing the research protocol, participants were given a partial debriefing regarding the purpose of the study. Because the first assessment provided baseline data for testing subsequent intervention effects, participants were not fully debriefed until the conclusion of the project (at the follow-up assessment). After debriefing, participants remained in the lab until their BAC reached .02%, at which time they were paid and provided transportation home. These procedures are in accord with National Institute on Alcohol Abuse and Alcoholism Guidelines for Ethyl Alcohol Administration in Human Experimentation.
Those who agreed to participate in the follow-up study returned to the lab to complete a brief series of questionnaires and provide genetic samples. Participants were given instructions for collecting two buccal epithelial (check cell) samples using cotton swabs. The samples were collected by research assistants who allowed them to air dry for 24 hours prior to sealing them in test tubes. Genetic samples were identified only by assigned study identification numbers for transport to the department of Molecular Biology. Under the supervision of a study co-author (S.E.B.), PCR analyses were conducted to determine genotype for the SERT gene-linked polymorphic region.
Serotonin transporter (SERT) genotyping
Identification of the L and S alleles of the SERT gene, SLC6A4 (Heils et al, 1996), were performed on DNA samples isolated from cheek cells using the Epicentre BuccalAmp DNA Extraction Kit according to manufacturer instructions (Epicentre Biotechnologies, Madison, WI). PCR amplification was carried out in a 50-μL final volume consisting of 1 U of Tfl DNA polymerase (Epicentre), 1 X PCR buffer, 1.5 mM MgCl2, 200 μM dNTPs, and 0.6 μM of each primer. The PCR conditions were as follows: 94°C, 2 minutes; (45 cycles of 94°C, 30 seconds; 70°C, 30 seconds; 72°C, 30 seconds); and a final extension of 72°C for 7 minutes. Each sample underwent four determinations in a minimum of two runs with two different oligos. Three of the 86 samples either yielded no valid PCR data or did not yield reproducible results.
The 5-HTTLPR polymorphism was amplified using two primers: 5'-CGT TGC CGC TCT GAA TGC CAG-3' and 5'-GGA TTC TGG TGC CAC CTA GAC GCC-3'. The L allele produces a fragment of 464 bp, whereas the S allele is 420 bp in length, representing a portion of the promoter region of the SLC6A4 gene (-1415 to -951). DNA of known genotype and deionized water were included as positive and negative controls, respectively.
Results
Data analytic plan
Analyses were conducted using Multivariate Analysis of Variance (MANOVA) and Analysis of Covariance (ANCOVA) and were restricted to the subsample of participants with complete genetic data. First, MANOVA was used to assess any difference between those who did (n = 83) and those who did not (n = 100) have usable genetic samples on the indicator and outcome vuriables. In addition, because of the heterogeneous sample, the authors were concerned about ethnic group differences accounting for associations between SERT genotype and outcome measures. Thus, preliminary MANOVA analysis was conducted to evaluate ethnic group differences on the indicator and outcome variables. Differences in BAC level by SERT genotype were also examined prior to the primary outcome analyses to rule out the possibility that differences in subjective alcohol effects might be due to differences in alcohol pharmacokinetics.
Primary outcome analyses included typical weekly drinking and BAC as covariates in ANCOVA. The BAC measure taken at the same time of the subjective response assessment (20 or 45-60 minutes) was used in all cases. For analyses of change in subjective response, change in BAC from 20 to 45-60 minutes was used as a covariate. Gender was also entered as a covariate rather than a fixed variable owing to the limited number of women in the smaller sample. Including interactions between gender and genotype would have resulted in cell sizes with as few as two participants. SERT genotype was included in all models as a fixed predictor of drinking-related outcomes. Data for SH was missing for one participant in the sample, resulting in one fewer case (n = 82) for all analyses involving this variable. All other analyses of primary outcomes were based on the complete sample with usable genetic data (n = 83).
Based on the sample size, power to detect a medium effect size in the proposed analyses was approximately .49, whereas power to detect a large effect size was approximately .90. These power estimates were derived using More Power Calculator, version 4.1 (Campbell and Thompson, 2002). Effect sizes were based on Cohen's D (Cohen, 1998), which was converted to percent variance accounted or η2 (.059 = medium effect; .138 = large effect). Thus, power to detect large effect sizes was adequate, whereas power to detect small to medium effects was rather low.
Statistical analyses
Completers versus noncompleters on indicator and outcomes measures
Chi-square analyses identified no significant differenccs in gender or BAC limb between those who provided genetic samples and those who did not. A MANOVA was conducted to assess differences between completers and noncompleters on typical weekly drinking, BAC measures (20 minute, 60 minute, change), subjective effect measures (20 minutes SI and SH, 60 minute SI and SH, and change in SI and SH), and ad lib consumption. The omnibus F value for all measures was not statistically significant (F = 1.25, 1/66 df, p = .26). Only two univariate comparisons were statistically significant (SI at 20 minutes, F = 4.82, 1/173 df, p < .05; change in SI, F = 5.03, 1/173 df, p < .05), with a trend toward significance for two other variables (typical weekly consumption and ad lib consumption). Those who provided genetic samples reported lower typical weekly consumption, stronger initial SI, greater change in SI across time, and less ad lib consumption.
Ethnic group differences on indicator and outcome measures
In these analyses, Asian American, white, and Hispanic groups were contrasted. The other ethnic groups, black (n = 3) and “others” (n = 5), included too few participants for meaningful statistical comparisons. First, a chi-square test was conducted to examine ethnic group differences in SERT genotype. Next, MANOVA compared mean values for typical weekly drinking, BAC measures (20 minute, 60 minute, change), subjective effect measures (20 minutes SI and SH, 60 minute SI and SH, and change in SI and SH), and ad lib consumption by ethnic group membership.
Results of the chi-square test were statistically significant (χ2 = 14.74, 4 df, p = .005), suggesting ethnic group differences in SERT genotype. Hispanic and Asian American samples did not differ from one another, but each differed significantly from the white sample (χ2 = 9.63. 2 df, p = .008; χ2 = 10.09, 2 df, p = .006, respectively). Relative to whites, both Hispanic and Asian American samples were more likely to be homozygous for the short SERT allele. Results of the MANOVA indicated that Asian American participants (n = 13) experienced stronger feelings of high and intoxication, less rapid adaptation to these effects, and lower typical and ad lib consumption relative to the other two groups, although the overall model was not statistically significant (F = 1.71, 8/64 df, p = .11). The only statistically significant univariate analysis was for SI (F = 3.78, 2/70 df, p < .05), with Asian Americans reporting less SI relative to both whites and Hispanics. Three two-group contrasts (two for weekly consumption and one for BAC at 45-60 minutes) were statistically significant, although the overall effect of group membership was not. See Table 1 for means, standard deviations, and all significant two-group contrasts. Although the overall model was not significant, the sample size was relatively small for detecting differences between ethnic groups.
Because of the observed ethnic group difference on SI and well-documented differences in alcohol metabolism among Asian American populations, analyses were conducted first with the entire sample and then in a restricted sample (not including Asian American participants) when results in the total sample were statistically significant. Analyses were additionally replicated in the white sample for comparison with past studies with this population and to provide further confidence in the consistency of the findings. Unfortunately, the small sample of Asian American participants did not permit analyses with sufficient power to detect difference by genotype.
BAC by SERT genotype
Potential differences in BAC by SERT genotype were assessed using MANOVA. BAC at each time point and change in BAC across time were included in the analysis. Neither the overall model (F = 0.47, 2/79 df, p = .63) nor any of the univariate analyses (all p's > .60) identified an effect of SERT genotype on BAC.
SERT genotype as a predictor of ad lib consumption
In the ANCOVA evaluating the relation between SERT genotype and ad lib consumption, the covariates of gender (F = 9.25, 1/73 df, p < .01) and typical weekly drinking (F = 10.00, 1/73 df, p < .01) were significantly assoc iated with ad lib consumption. Although no main effect was evident for SERT genotype, there was a trend toward a genotype by BAC limb interaction (F = 2.83, 2/73 df, p = .07). Although not statistically significant, the η2 value of .072 suggests a medium effect size (Cohen, 1998). Examination of the means (Table 2) indicates that the long allele was associated with heavier consumption for participants on the descending BAC limb, whereas the short allele was associated with heavier consumption for participants on the ascending BAC limb.
Table 2.
Mean (SD) ad lib consumption by SERT genotype and BAC limb
| Measure | SS (n = 25) Mean (SD) | SL (n = 39) Mean (SD) | LL (n = 17) Mean (SD) |
|---|---|---|---|
| Ascending BAC, no. standard drinks | 1.82 (1.21) | 1.25 (1.08) | 0.94 (1.02) |
| Descending BAC, no. standard drinks | 0.64 (0.85) | 1.43 (1.20) | 1.07 (1.49) |
Notes: SERT = serotonin transporter; BAC = blood alcohol concentration; SS = homozygous for the short SERT allele; SL = heterozygous; LL = homozygous for the long SERT allele. Means and standard deviations presented are not corrected for covariates that were included in the statistical model. One participant was missing data for the first BAC assessment and is therefore not represented in the analysis.
SERT and subjective alcohol effects (20 minutes after beverage administration)
In the ANCOVAs at 20 minutes following alcohol administration, the only covariate associated with subjective response was typical weekly consumption, which was significantly related to SI (F = 6.23, 1/76 df, p < .05). Greater typical consumption was associated with lower SI ratings. SERT genotype was not significantly associated with either SI or SH.
SERT and subjective alcohol effects (45-60 minutes after beverage administration)
Analyses at 45-60 minutes following alcohol administration included BAC limb as a fixed variable and assessed interactions between BAC limb and other predictor variables (SERT). The only covariate significantly associated with either subjective effect measure was typical weekly alcohol consumption. Those who reported heavier typical weekly alcohol consumption indicated significantly lower SI (F = 6.61, 1/73 df, p = .01) and a trend toward lower SH (F = 2.84, 1/72 df, p = .10). There were no main effects or interactions for SERT or BAC limb.
SERT and change in subjective alcohol effects across time
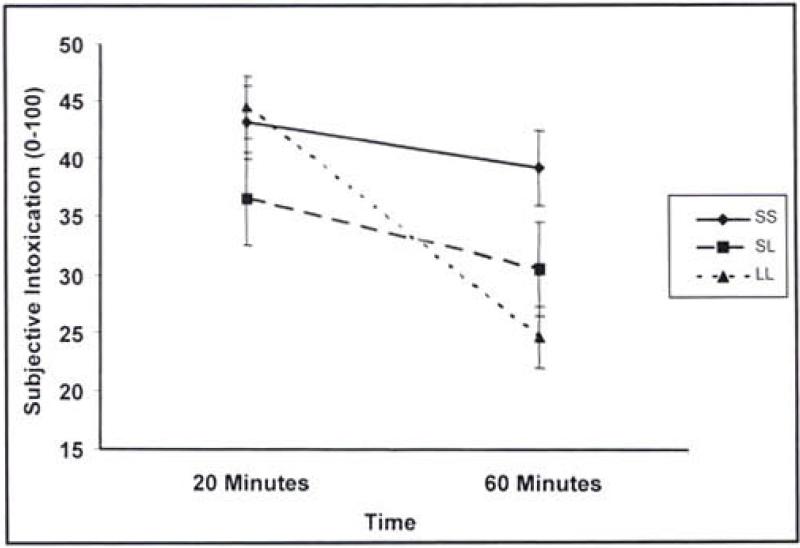
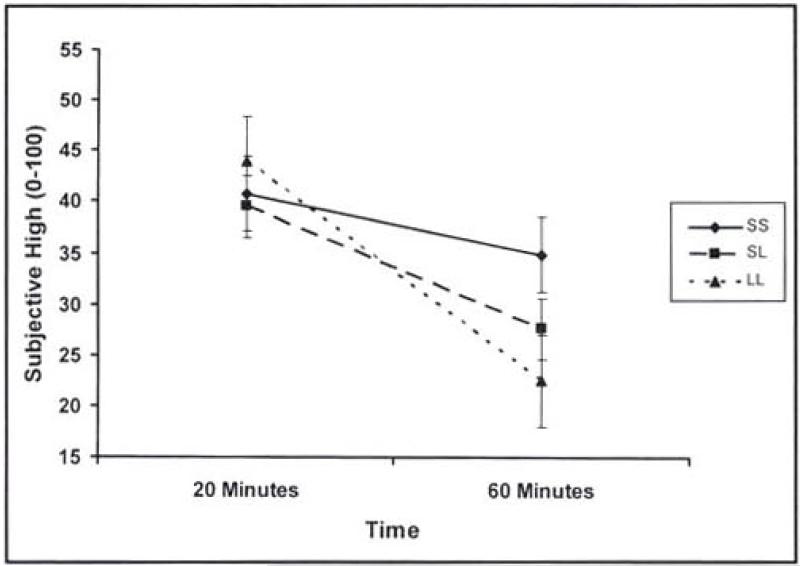
In the assessment of acute tolerance, analyses controlled for change in BAC. In the SI analysis, the covariate of BAC change was positively associated with change in SI from Time 1 to Time 2 though not quite statistically significant (F = 3.85, 1/76 df, p =.05). A significant main effect for SERT genotype also emerged (F = 3.84, 2/76 df, p = .03). Follow-up pairwise comparisons showed that individuals with the LL genotype reported greater acute tolerance to SI relative to both the SL (p = .02) and SS (p = .01) genotypes, which did not significantly differ from one another. The same general pattern of results emerged for SH (F = 4.63, 2/75 df, p = .01), although only the LL versus SS comparison was statistically significant (p < .01) with a trend toward significance for the LL versus SL comparison (p = .05). See Figures 1 and 2 for graphic representations of the relation between SERT genotype and subjective responses (SI and SH, respectively).
Figure 1.
Change in subjective intoxication over time by serotonin transporter (SERT) genotype
Figure 2.
Changes in subjective high over time by serotonin transporter (SERT) genotype
Identical analyses were conducted in the sample without Asian American participants with results very similar to those found in the total sample. The main effect of SERT in the SI model was nearly significant (F = 3.12, 2/63 df, p = .05), and the effect sizes were nearly identical in the full and restricted samples (partial η2 = .092 and .090, respectively). The SERT main effect in the SH model remained statistically significant (F = 3.88, 2/62 df, p = .03), and the effect sizes were again quite similar in the full and restricted samples (partial η2 = .110 and .111, respectively). In the sample of white participants, the results were again consistent with those found in the overall sample. The main effect of SERT in the SI model was nearly significant (F = 2.84, 2/40 df, p = .07), and the effect size in the white sample (.124) was actually larger than in the full sample (.092). Results for SH were similar, with a nearly significant main effect of SERT (F = 3.00, 2/40 df, p = .06), and a larger effect size (.130) than in the full sample (.111).
Discussion
The current study assessed relations among SERT genotype, alcohol effects (intoxication and high), limb of the blood alcohol curve, acute tolerance, and ad lib alcohol consumption. Results were neither entirely consistent nor inconsistent with past research on the SERT genotype and subjective responses to alcohol (Schuckit et al., 1999). Although lower subjective response was not found for individuals with the long allele of the SERT, the direction of the effect at 45-60 minutes was consistent with past studies and, with a larger sample size, would likely reach statistical significance. The assessment of subjective responses at multiple time points in the current study provided an opportunity to evaluate changes in subjective response across time and the impact of the SERT genotype on this index of acute tolerance. Greater acute tolerance was observed among individuals with the long allele of the SERT.
Differences in findings across previous studies therefore may be due to the time at which subjective effects were assessed. The current data suggest that the longer the time between consumption and assessment of alcohol effects, the greater the likelihood that individuals with the long allele will report lower subjective responses relative to individuals with the short allele. The second of the two assessments in the current study was between 45 and 60 minutes postadministration of alcohol, slightly shorter than the 60-minute assessment period typically used in past studies. Future studies should include multiple assessments after alcohol administration to help clarify the nature of the association between SERT genotype and subjective effects. This distinction may well be important, as different mechanisms of risk for alcoholism might be related to low response versus acute tolerance.
At least in the current study, individuals with the SERT long allele adapted more quickly to alcohol effects, with a trend toward greater alcohol consumption on the descending BAC limb. Thus, individuals with the long allele may be more likely to continue drinking when blood alcohol levels begin to descend whereas others would terminate drinking at this point. Such an interpretation should be made with caution given the relatively weak association between SERT genotype and ad lib drinking in this study. Even if SERT genotype is not reliably associated with drinking behavior, it may nonetheless be an important factor in the development of alcohol-related problems. It is possible that acute tolerance in individuals homozygous for the long allele represents a marker for increased risk of long-term alcohol toxicity (Heinz et al., 2001).
A number of limitations must be addressed in interpreting the results of the current study, including the use of single-item measures or subjectivc effects, lack of a placebo control, assessment of subjective effects at only two time points, and use of a small and heterogeneous genetic sample. Because of these issues, results must be interpreted with caution pending replication.
Subjcetive alcohol effects were assessed with single items for “high” and “intoxication.” Although similar measures have been used in past studies, they are certainly not ideal. The authors conceptualized “high” as being consistent with stimulant effects on the ascending limb, and “intoxication” as being consistent with impairing or sedating effects experienced on the descending limb. Although ratings of high were in fact higher on the ascending limb and ratings of intoxication were higher on the descending limb, all participants may not have interpreted these items similarly. Future studies with measures of stimulation and sedation (e.g., Biphasic Alcohol Effects Scale [BAES]; Martin et al., 1993) are necessary to confirm the interpretation of the results posited in the current study.
The lack of a placebo control makes it possible that subjective experiences reflected beliefs about alcohol effects rather than true alcohol effects. Although this possibility cannot be ruled out, additional data from our lab suggest that this is not likely the case. Using the BAES, participants administered alcohol reported stronger experiences of both stimulation and sedation than participants in a placebo group. Further, subjective effects were predictive of ad lib consumption in the alcohol condition, but not in the placebo condition (Roth et al., 2002). Thus, subjective experiences after alcohol appear to reflect “true” alcohol effects that relate to ad lib consumption in a meaningful way.
Subjective alcohol effects were assessed only twice prior to the ad lib consumption period, making it impossible to precisely match BAC levels on the ascending and descending limb for each participant. In assessing acute tolerance, matching BACs on each limb would have been preferable, but it would have resulted in ad lib access on only the descending limb for all participants. This would have prevented comparison of ad lib drinking by BAC limb, essential for assessing the differentiator model. Future studies of the association between acute tolerance and ad lib consumption should take advantage of recent technology that allows researchers to hold blood alcohol levels constant across time (Morzorati et al., 2002).
The size and ethnic diversity of the subsample that provided genetic data are a serious concern for the genetic analyses. Population stratification can lead to the identification of relations between genes and behavior when no true relationship exists. The fact that the results for ad lib consumption and SI were replicated when the sample was restricted to non-Asian American participants lends some confidence to the findings. The small sample size is also of concern owing to limited power to detect small to medium effect sizes, which are rather common in behavioral genetic research. Given the difficulty of collecting data on subjective alcohol responses from a large sample, preliminary analyses like the ones presented in the current study seem appropriate pending larger-scale analyses. The results must simply be considered suggestive of a relation until more comprehensive genetic studies can be completed.
The ad lib drinking period in the current study followed introduction of a social stressor, an aspect of the study designed to test hypotheses unrelated to the genetics of subjective alcohol responses. The inclusion of a social stressor may have impacted the degree to which individuals experienced intoxication versus high after alcohol consumption. Although this possibility cannot be ruled out, consistent results for both measures with respect to acute tolerance provide some confidence in the reliability of this effect. In addition, analyses were replicated controlling for changes in anxiety levels following the introduction of the social stressor. When controlling for anxiety as measured by the POMS, the results for analyses of ad lib consumption and subjective alcohol effects did not substantively change. Nonetheless, future studies of ad lib consumption under different social and psychological conditions are necessary to confirm the results of this study.
Despite the limitations outlined above, the results of the current study provide a basis for testing of more refined hypotheses. First, it is essential to assess subjective responses at multiple time points to determine if SERT is associated more strongly with subjective effects at specific time points or with changes in subjective responses across time. In addition, assessment of a range of alcohol effects (stimulant and sedative) is crucial. The current study suggests that SERT genotype is associated with more rapid adaptation to effects that are experienced both positively and negatively. If this is the case, it is not surprising that SERT genotype does not reliably predict drinking behavior, as the loss of both positive and negative effects may offset one another. Moreover, a lack of association between SERT genotype and drinking behavior does not rule out SERT as an important contributor to alcohol-related problems. It does, however, suggest that other mechanisms of action must be considered.
Acknowledgments
The authors would like to acknowledge the support of the Waggoner Center for Alcohol and Addictions Research (WCAAR), under the direction of Dr. R. Adron Harris, which provided the funding for the genetic analyses performed in this study.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism grants RO1-AA11683 and 5T32-AA07471.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R) Washington, DC: 1987. [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Washington, DC: 1994. [Google Scholar]
- Campbell JID, Thompson VA. More power to you: Simple power calculations for treatment effects with one degree of freedom. Behav. Res. Meth. Instr. Comput. 2002;34:332–337. doi: 10.3758/bf03195460. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition Lawrence Erlbaum; Mahwah, NJ: 1998. [Google Scholar]
- Collins RL, Parks GA, Marlatt GA. Social determinants of alcohol consumption: The effects of social interaction and model status on the self-administration of alcohol. J. Cons. Clin. Psychol. 1985;53:189–200. doi: 10.1037//0022-006x.53.2.189. [DOI] [PubMed] [Google Scholar]
- Conrod PJ, Peterson JB, Phil RO, Mankowski S. Biphasic effects of alcohol on heart rate are influenced by alcoholic family history and rate of alcohol ingestion. Alcsm Clin. Exp. Res. 1997;21:140–149. [PubMed] [Google Scholar]
- Conway KP, Swendsen JD, Merikangas KR. Alcohol expectancies, alcohol consumption, and problem drinking: The moderating role of family history. Addict. Behav. 2003;28:823–836. doi: 10.1016/s0306-4603(02)00265-4. [DOI] [PubMed] [Google Scholar]
- de Wit H, McCracken SG. Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcsm Clin. Exp. Res. 1990;14:63–70. doi: 10.1111/j.1530-0277.1990.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Doty P, de Wit H. Effect of setting on the reinforcing and subjective effects of ethanol in social drinkers. Psychopharmacology. 1995;118:19–27. doi: 10.1007/BF02245245. [DOI] [PubMed] [Google Scholar]
- Earleywine M. Anticipated biphasic effects of alcohol vary with risk for alcoholism: A preliminary report. Alcsm Clin. Exp. Res. 1994;18:711–714. doi: 10.1111/j.1530-0277.1994.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Reynolds J, Koller DL, Begletter H, Bucholz KK, Conneally PM, Crowe R, Goate A, Hesselbrock V, Li T-K, Nurnberger JI, Jr., Porjesz B, Reich T, Rice JP, Schuckit M, Tischfield JA, Foroud T. A family-based analysis of whether the functional promoter alleles of the serotonin transporter gene HTT affect the risk for alcohol dependence. Alcsm Clin. Exp. Res. 1998;22:1080–1085. [PubMed] [Google Scholar]
- Gabrielli WF, Nagoshi CT, Rhea SA, Wilson JR. Anticipated and subjective sensitivities to alcohol. J. Stud. Alcohol. 1991;52:205–214. doi: 10.15288/jsa.1991.52.205. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary beta-endorphin to ethanol in subjects at high risk of alcoholism. Arch. Gen. Psychiat. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Gorwood P, Batel P, Ades J, Hamon M, Boni C. Serotonin transporter gene polymorphisms, alcoholism, and suicidal behavior. Biol. Psychiat. 2000;48:259–264. doi: 10.1016/s0006-3223(00)00840-4. [DOI] [PubMed] [Google Scholar]
- Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic and environmental contributions to alcohol dependence risk in a national twin sample: Consistency of findings in women and men. Psychol. Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- Heath AC, Madden PAF, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol. Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J. Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Heinz A, Jones DW, Mazzanti C, Goldman D, Ragan P, Hommer D, Linnoila M, Weinberger DR. A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity. Biol. Psychiat. 2000;47:643–649. doi: 10.1016/s0006-3223(99)00171-7. [DOI] [PubMed] [Google Scholar]
- Heinz A, Mann K, Weinberger DR, Goldman D. Serotonergic dysfunction, negative mood states, and response to alcohol. Alcsm Clin. Exp. Res. 2001;25:487–495. [PubMed] [Google Scholar]
- Hill EM, Stoltenberg SF, Bullard KH, Li S, Zucker RA, Burmeister M. Antisocial alcoholism and serotonin-related polymorphisms: Association tests. Psychiat. Genet. 2002;12:143–153. doi: 10.1097/00041444-200209000-00005. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in responses to ethanol and d-amphetamine: A within-subject study. Alcsm Clin. Exp. Res. 2001;25:540–548. [PubMed] [Google Scholar]
- Holdstock L, King AC, de Wit H. Subjective and objective responses to ethanol in moderate/heavy and light social drinkers. Alcsm Clin. Exp. Res. 2000;24:789–794. [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: Diazepam. Psychopharmacology. 1980;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N. Neuropharmacological treatments for alcoholism: Scientific basis and clinical findings. Psychopharmacology. 2000;149:327–344. doi: 10.1007/s002130000371. [DOI] [PubMed] [Google Scholar]
- Kaprio J, Koskenvuo M, Langinvainio H, Romanov K, Sarna S, Rose RJ. Genetic influences on use and abuse of alcohol: A study of 5,638 adult Finnish twin brothers. Alcsm Clin. Exp. Res. 1987;11:349–356. doi: 10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. Group Dynamics of Social Drinking in College Students. University of Texas at Austin; Austin, TX: 2002. unpublished masters thesis. [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O'Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Individual differences in the priming effect of ethanol in social drinkers. J. Stud. Alcohol. 1998;61:64–71. doi: 10.15288/jsa.2000.61.64. [DOI] [PubMed] [Google Scholar]
- Kranzler H, Lappalainen J, Nellissery M, Gelernter J. Association study of alcoholism subtypes with a functional promoter polymorphism in the serotonin transporter protein gene. Alcsm Clin. Exp. Res. 2002;26:1330–1335. doi: 10.1097/01.ALC.0000030840.48315.40. [DOI] [PubMed] [Google Scholar]
- Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. J. Pers. Social Psychol. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the biphasic alcohol effects scale. Alcsm Clin. Exp. Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Morzorati SL, Ramchandani VA, Flury L, Li T-K, O'Connor S. Self-reported subjective perception of intoxication reflects family history of alcoholism when breath alcohol levels are constant. Alcsm Clin. Exp. Res. 2002;26:1299–1306. doi: 10.1097/01.ALC.0000025886.41927.83. [DOI] [PubMed] [Google Scholar]
- Nagoshi CT, Wilson JR. Influence of family alcoholism history on alcohol metabolism, sensitivity, and tolerance. Alcsm Clin. Exp. Res. 1987;11:392–398. doi: 10.1111/j.1530-0277.1987.tb01330.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychol. Bull. 1990;108:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Pollock VE. Meta-analysis of subjective sensitivity to alcohol in sons of alcoholics. Amer. J. Psychiat. 1992;149:1534–1538. doi: 10.1176/ajp.149.11.1534. [DOI] [PubMed] [Google Scholar]
- Roth J, Corbin WR, Fromme K. Alcohol-induced stimulation and sedation as predictors of further consumption.. Paper presented at the 24th annual meeting of the Research Society on Alcoholism; San Francisco, CA. June 2002. [Google Scholar]
- Schuckit MA. Relationship between the course of primary alcoholism in men and family history. J. Stud. Alcohol. 1984a;45:334–338. doi: 10.15288/jsa.1984.45.334. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective responses to alcohol in sons of alcoholics and control subjects. Arch. Gen. Psychiat. 1984b;41:879–884. doi: 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch. Gen. Psychiat. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Biological, psychological and environmental predictors of the alcoholism risk: A longitudinal study. J. Stud. Alcohol. 1998;59:485–494. doi: 10.15288/jsa.1998.59.485. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Arch. Gen. Psychiat. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold E, Risch SC. Serum prolactin levels in sons of alcoholics and control subjects. Amer. J. Psychiat. 1987;144:854–859. doi: 10.1176/ajp.144.7.854. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Umbereen A, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABAα6 receptors and the serotonin transporter in the level of response to alcohol: A pilot study. Biol. Psychiat. 1999;45:647–651. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. The relationships of a family history of alcohol dependence, a low level of response to alcohol and six domains of life functioning to the development of alcohol use disorders. J. Stud. Alcohol. 2000;61:827–835. doi: 10.15288/jsa.2000.61.827. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr. Alcohol challenges in young men from alcoholic pedigrees and control families: A report from the COGA project. J. Stud. Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Staley JK, Jacobsen LK, Seibyl JP, Laruelle M, Baldwin RM, Innis RB, Gelernter J. Central serotonin transporter availability measured with [1231]beta-CIT SPECT in relation to serotonin transporter genotype. Amer. J. Psychiat. 2004;161:525–531. doi: 10.1176/appi.ajp.161.3.525. [DOI] [PubMed] [Google Scholar]