Abstract
CTCF plays diverse roles in the regulation of eukaryotic genes. A new study by Lefevre et al., 2008 reveals a novel mechanism in which non-coding RNA transcription and nucleosome repositioning evicts CTCF from a regulatory element, to facilitate induction of a nearby gene.
Keywords: CTCF, insulator, transcription, chromatin
The Zinc finger protein CTCF binds directly to specific DNA sequences and plays multiple roles in the regulation of eukaryotic genes, including regulation of enhancer-promoter interactions, imprinting, inhibition of nucleolar transcription, and co-activation of unlinked genes (Wallace and Felsenfeld, 2007). These diverse functions are in part mediated by the ability of CTCF to stabilize long-range chromatin contacts and organize chromosomes into higher order complexes through interactions with distinct nuclear partners. While site-specific phosphorylation and poly-ADP-ribosylation are two known post-translation modifications crucial to CTCF functions (El-Kady and Klenova, 2005; Yu et al., 2004), the mechanisms that modulate CTCF binding to its target sequence are not completely understood.
Bacterial lipopolysaccharides (LPS) rapidly induce expression of the chicken lysozyme gene via well-characterized upstream enhancer and silencer elements. LPS initiates transcription through stepwise recruitment of transcription factors (NF1, Fli-1 and CREB binding protein) followed by concomitant alterations in chromatin structure within the upstream cis-elements (Lefevre et al., 2005). A recent study by Bonifer and coworkers on the induction of the lysozyme gene by LPS explores how CTCF/cohesin-mediated repression of an enhancer element can be overcome through abrogation of CTCF binding to DNA, prior to gene activation (Lefevre et al., 2008).
Consistent with earlier studies, the authors observe recruitment of C/EBPβ, Fos/AP1 and RNA Polymerase II (RNAPII) to a hormone response element (HRE) upstream of the lysozyme gene within 20 minutes of LPS stimulation (Figure 1B). Induction of transcription is accompanied by alterations in the chromatin landscape characterized by the rapid induction of DNase I hypersensitivity (DHS) at the HRE and at the upstream −2.7 kb C/EBP sites; however, micrococcal nuclease (MNase) hypersensitivity gradually declines at the −2.4 kb CTCF occupancy site. This finding suggests that LPS induces the displacement of nucleosomes into the CTCF occupancy site while simultaneously exposing the upstream C/EBP enhancer element and HRE. It has been previously reported that repositioning of nucleosomes in the H19 Imprinting Control Region attenuates CTCF-target site interaction and results in the loss of CTCF insulator function (Kanduri et al., 2002). Consistent with the notion that CTCF cannot bind nucleosomal DNA, the authors observe specific depletion of CTCF from its occupancy site by chromatin immunoprecipitation after LPS stimulation. Moreover, RNAi-mediated depletion of CTCF leads to earlier onset of LPS-induced lysozyme gene expression. Taken together, these results suggest that the upregulation of lysozyme expression is mediated in part by the removal of CTCF repression and by LPS-induced recruitment of transcription factors to the upstream enhancer.
Figure 1. LPS-induced chromatin reorganization within −3 kb cis-element of the lysozyme gene.
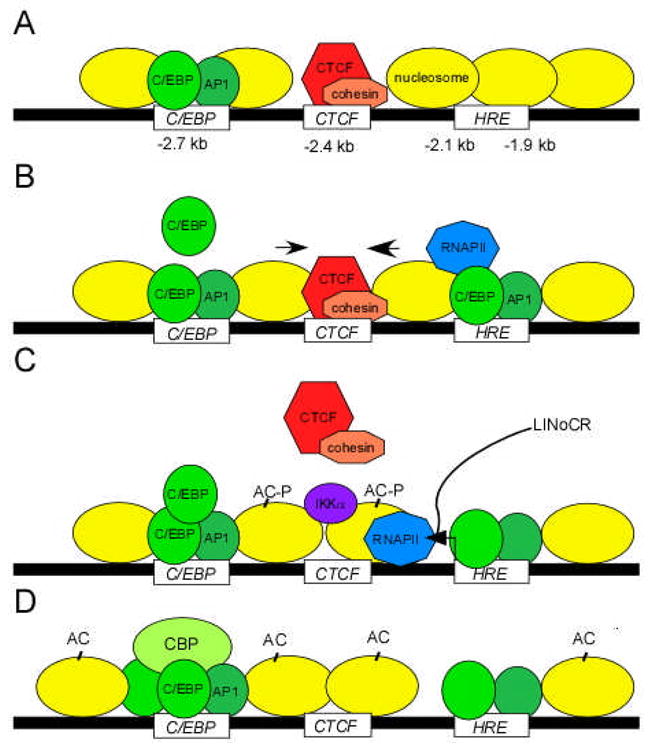
(A) In unstimulated monocytes, CTCF and cohesin form an insulator complex at the −2.4 kb silencer element. (B) Short LPS stimulation induces binding of C/EBPβ, AP1 and RNAPII to the exposed HRE, and the displacement of nucleosomes towards the CTCF occupancy site (black arrows). (C) At the one hour time-point, LINoCR transcription, IKKα recruitment, specific H3 phosphoacetylation (AC-P), and repositioning of a nucleosome over the CTCF site culminate in the eviction of the CTCF/cohesin complex. (D) Prolonged LPS stimulation prevents binding of CTCF/cohesin due to re-positioning of the nucleosome over its site. Recruitment of additional C/EBP and CBP to the −2.7 kb enhancer increases H3 acetylation (-AC) and maintains the lysozyme gene in an active state.
What are the implications of chromatin changes and the enrichment of RNAPII at the −1.9 kb to −2.7 kb upstream regulatory regions upon LPS stimulation? Recent findings indicating that many intergenic regions are transcribed into non-coding RNAs (ncRNA, reviewed in Prasanth and Spector, 2007) led the authors to search for possible transcripts within the lysozyme cis-regulatory region. A novel antisense transcript LINoCR (LPS Inducible Non Coding RNA) that overlaps the −2.4 kb/−2.7 kb region was identified and found to be induced by LPS in a manner similar to the lysozyme gene. The authors go on to provide compelling evidence that the HRE acts as an LPS-responsive promoter to activate LINoCR transcription (Figure 1C).
Consistent with reports associating histone H3 phosphoacetylation with the activation of inducible genes (Clayton et al., 2000), the authors observe IKKα recruitment and a similar phosphoacetylation pattern over the LINoCR locus upon LPS induction. In addition, short-term treatment with the inhibitor of transcriptional elongation, 5,6-dichloro-1-β-D-ribofuranosyl-benzimidazole, permitted transcription of many immediate early genes, but abrogated LPS-induced LINoCR expression, IKKα recruitment, histone H3 phosphoacetylation, and specific CTCF/cohesin eviction. These data demonstrate that ncRNA transcription is necessary for the changes in chromatin features within the −3kb region and for the consequential eviction of CTCF from its occupancy site.
In summary, evidence presented by Lefevre et al. supports a model in which transcription-dependent chromatin remodeling leads to physical dislodgement of CTCF prior to gene activation. CTCF and cohesin form an insulator complex at position −2.4 kb (Figure 1A). LPS stimulation triggers destabilization of nucleosomes and exposure of the two flanking enhancer elements, which in turn allows recruitment of additional C/EBPβ proteins to the −2.7 kb element and the initiation of ncRNA synthesis from the HRE (Figure 1B). Transient transcription of LINoCR and the concomitant passage of the RNAPII complex through the −2.4 kb element are correlated with IKKα recruitment, H3 phosphoacetylation and repositioning of a nucleosome over the CTCF occupancy site, a sequence of events leading to the eventual eviction of the CTCF/cohesin insulator complex (Figure 1C & D). The results suggest an alternative mechanism, other than covalent modification of the CTCF protein, to regulate the activity of the CTCF insulator.
Recent studies indicate that 98 % of the transcriptional output of the human genome consists of ncRNAs that perform diverse regulatory roles through distinct mechanisms, including dosage compensation, imprinting, gene silencing, modulation of transcription and translation (Prasanth and Spector, 2007). Furthermore, ncRNAs have been reported to regulate epigenetic states by facilitating occupancy of chromatin-binding proteins. Characterization of the four human HOX loci has led to the identification of 231 HOX ncRNAs whose expression patterns demarcate broad chromosomal domains of differential histone methylation and RNA polymerase accessibility (Rinn et al., 2007). For example, the HOTAIR ncRNA residing in the HOXC locus represses transcription of the HOXD locus in trans through recruitment of the Polycomb Repressive Complex 2 (PRC2) that is required for subsequent histone H3 lysine-27 trimethylation of the HOXD locus (Rinn et al., 2007). In addition, Ohta and coworkers have shown that RNAPII transcription of ncRNAs is required for chromatin remodeling at the fbp1+ locus in Schizosaccharomyces pombe during transcriptional activation (Hirota et al., 2008). These observations are conceptually similar to the mechanism by which LINoCR regulates CTCF binding. Given the importance of CTCF in maintaining chromosome organization and of PRC2 in gene silencing in different cellular contexts, these findings raise the possibility that many ncRNAs regulate the interaction between chromatin proteins and their DNA targets, and that this function might be an important epigenetic regulatory strategy during diverse developmental processes.
References
- Clayton AL, Rose S, Barratt MJ, Mahadevan LC. EMBO J. 2000;19(14):3714–26. doi: 10.1093/emboj/19.14.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Kady A, Klenova E. FEBS Lett. 2005;579(6):1424–34. doi: 10.1016/j.febslet.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Hirota K, Miyoshi T, Kugou K, Hoffman CS, Shibata T, Ohta K. Nature. 2008 doi: 10.1038/nature07348. in press. [DOI] [PubMed] [Google Scholar]
- Kanduri M, Kanduri C, Mariano P, Vostrov AA, Quitschke W, Lobanenkov V, Ohlsson R. Mol Cell Biol. 2002;22(10):3339–44. doi: 10.1128/MCB.22.10.3339-3344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre P, Witham J, Lacroix CE, Cockerill PN, Bonifer C. Mol Cell. 2008 doi: 10.1016/j.molcel.2008.07.023. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre P, Lacroix C, Tagoh H, Hoogenkamp M, Melnik S, Ingram R, Bonifer C. J Biol Chem. 2005;280(30):27552–60. doi: 10.1074/jbc.M502422200. [DOI] [PubMed] [Google Scholar]
- Prasanth KV, Spector DL. Genes & Dev. 2007;21(1):11–42. doi: 10.1101/gad.1484207. [DOI] [PubMed] [Google Scholar]
- Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, Goodnough LH, Helms JA, Farnham PJ, Segal E, Chang HY. Cell. 2007;129(7):1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JA, Felsenfeld G. Curr Opin Genet Dev. 2007;17(5):400–7. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Ginjala V, Pant V, Chernukhin I, Whitehead J, Docquier F, Farrar D, Tavoosidana G, Mukhopadhyay R, Kanduri C, Oshimura M, Feinberg AP, Lobanenkov V, Klenova E, Ohlsson R. Nat Genet. 2004;36(10):1105–10. doi: 10.1038/ng1426. [DOI] [PubMed] [Google Scholar]


