Summary
In species as varied as humans and flies, humoral/central nervous system barrier structures are a major obstacle to the passive penetration of small molecules including endogenous compounds, environmental toxins and drugs. In vivo measurement of blood brain physiologic function in vertebrate animal models is difficult and current ex vivo models for more rapid experimentation using, for example, cultured brain epithelial cells, only partially reconstitute the anatomy and physiology of a fully intact BBB. To address these problems, we and others continue to develop in vivo assays for studying the complex physiologic function of CNS barriers using the fruit fly Drosophila melanogaster (Dm). These methods involve the introduction of small molecule reporters of BBB physiology into the fly humoral compartment by direct injection. Since these reporters must cross the Dm BBB in order to be visible in the eye, we can directly assess genetic or chemical modulators of BBB function by monitoring retinal fluorescence. This assay has the advantage of utilizing a physiologically intact BBB in a model organism that is economical and highly amenable to genetic manipulation. In combination with other approaches outlined here, such as brain dissection and behavioral assessment, one can produce a fuller picture of BBB biology and physiology. In this chapter, we will provide detailed methods for examining BBB biology in the fly, including a Dm visual assay to screen for novel modulators of the BBB.
Keywords: subperineural glia, blood brain barrier, xenobiotic, abc transport, chemoprotection, live assays, drug partition, hemolymph, retinal chemo-exclusion, drosophila
1. Introduction
Considerable effort is being spent on the development and optimization of methods to allow drug transit across the BBB. However, because the BBB is such a complex and intricately regulated structure, producing a comprehensive in vitro facsimile of the barrier using cultured cells has, at least to date, been problematic. The BBB’s purpose is tied to central nervous system function. Robust responsiveness (i.e. preserving the state of readiness) is critical to neuronal performance, as free-living organisms must be ready to respond to constantly changing environmental circumstances. (e.g. predation or food sources) (1). All organisms must guard against the chemical world, since many naturally occurring toxins would cause rapid deterioration of CNS function if allowed to accumulate in neurons (2). This strong selective pressure favored the origination and elaboration of BBB-based chemoprotection in the majority of higher organisms (3). Unfortunately even though molecular targets, toxicologic sensitivities and complex nervous system functions are conserved from worms and flies through man, little is known about the evolutionary origin of chemoprotective physiologies of the CNS.
In vivo BBB animal models, primarily in rodents, have confirmed the importance of the two components mentioned above and are the leading systems for brain-specific drug partition studies in the pharmaceutical literature (4, 5). Nevertheless, the anatomically compact nature of the vertebrate BBB makes live pharmacokinetic assays technically challenging and cumbersome for forward chemical or genetic screens. Furthermore, analysis of the multiplicity of individual components involved in chemical protection physiology is not practical because vertebrate life spans are long and the number of testable individual gene reduction permutations greatly limited. To fill this void, a number of in vitro models using polarized cells are available for testing the transport efficiency of compounds across barriers, but each system has substantial limitations (6). MDCK cells and composite glia/endothelium double cell layers are amenable to gene knockdowns and high throughput screens, but neither system fully recapitulates the elaborate physiologic constraints of in vivo BBB function. These systems also lack CNS or humoral inputs that might be essential for establishment and maintenance of barrier physiology. Hence there is a pressing need for a system that can combine molecular genetic, genomic, chemical biology and integrative physiology tools to probe how CNS-specific chemoprotective physiologies and their control paradigms are made manifest. For this we turned to Drosophila melanogaster and asked what aspects of BBB physiology can be modeled in an invertebrate.
While substantial physiologic differences exist between flies and vertebrates in the content their humoral space and the cell types and structure of the Dm CNS barrier we will refer to this structure as the Dm BBB for simplicity. Dm has an open circulatory system in which hemolymph is pumped around the body and bathes organs externally. Here the CNS is surrounded and separated from the humoral space by a thin layer of glially-derived epithelial cells (7-9), making the Dm humoral/CNS interface topologically much simpler than the vertebrate BBB. However on a cellular level, the vertebrate and insect BBBs share many common features. In particular, one specific cell layer of the Dm BBB, the sub-perineural glia (SPG), possesses elaborate laterally-localized homotypic junctional complexes, or pleated septate junctions, that create a tight barrier to paracellular diffusion (10, 11). The Dm proteins that make up the pleated septate junctions are nearly identical to the vertebrate proteins that compose the tight junctions (12, 13). Furthermore, disruption of the pleated septate junctions leads to defects in Dm BBB function (9, 14). Furthermore, Dm possess a full array of xenobiotic transporters from both the ATP-binding cassette (ABC) and solute carrier (SLC) families that participate in active drug flux between biologic compartments (15-17). Together with tight paracellular borders, these transport mechanisms provide two means of protecting the CNS from xenobiotic and other threats (Fig A).
Figure A.
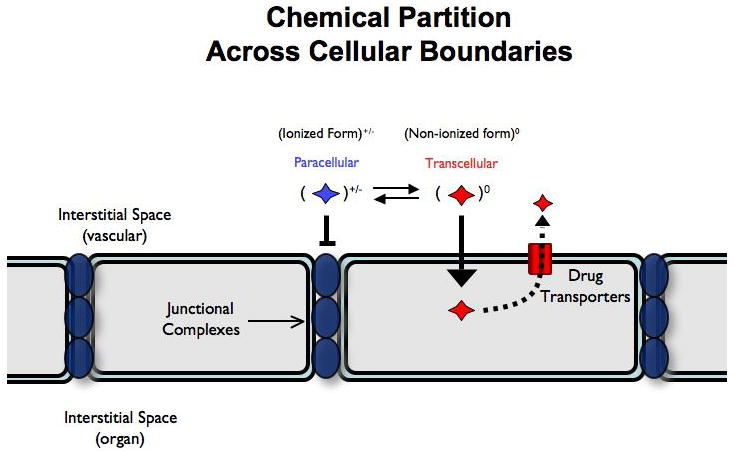
To form a barrier against xenobiotics, the BBB employs two main modes of exclusion: a) tight paracellular junctions that exclude charged molecules (blue) and b) a wide array of drug transporters that efflux charge neutral, lipophilic compounds back into the vacular space (red). A charge neutral dye is not excluded from W.T. or mutant animals as it can take a transcellular route to entry across the BBB.
We have developed several assays for movement of xenobiotics across the BBB using the fruit fly Drosophila melanogaster (Dm). These include real-time live imaging of the fly retina to assess blood retinal barrier (BRB) integrity in two modes (paracellular and transcellular routes of entry), quantitative measure of dye penetration into the brain parenchyma, confocal imaging of barrier layers and finally, quantitative measures of hemolymph drug concentration through hemolymph extraction. The retinal assay in particular takes advantage of using a barrier in which normal cell-cell interactions and regulatory systems are entirely intact. And by co-injecting a fluorescent effluxable substrate (such as Rho123) with a non-effluxable molecule (such as 10 kDaTexas Red-dextrans) we can simultaneously assess both modes of exclusion at the barrier (Fig B).
Figure B.
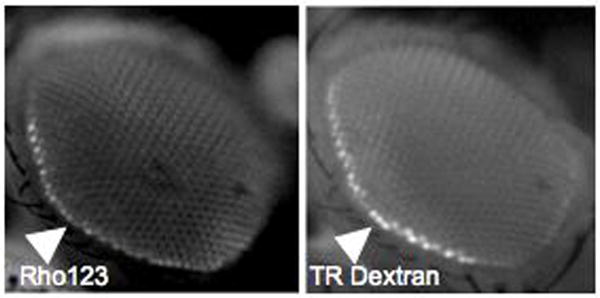
Simultaneous assessment of transport and diffusion barriers by hemplymph co-injection of Rhodamine 123 (Rho123) and 10 kDa Texas Red Dextran (TR). Images are taken live under CO2 anesthesia 15 minutes post injection using green and red filter sets respectively. Hemolymph exclusion from the CNS is seen as a bright line around the retina (white triangles). We call this the hemolymph exclusion line (HEL).
Taken as a whole, Dm is a highly tractable to chemical and genetic manipulations. The ability to 1) screen for mutants defective in specific aspects of drug transport function, 2) express genes involved in drug transport in specific cell types in vivo, and 3) perform live assays of BBB function make Dm an ideal system to elucidate the physiology of the BBB in a robust and evolutionarily conserved model animal.
2. Materials
2.1 Intrahemolymph Dye Dosing and Retinal Image Acquisition
White minus (w-) Drosophila melanogaster flies
Cornmeal and molasses agar media and culture vials and bottles 3.
3 kDa FITC dextran (Invitrogen D-3306)
Rhodamine B (RhoB) flourecent dye (Sigma R6626)
dissecting instruments including sharpened, fine point forceps
1 mL pipette with feather affixed to the tip
0.1% SDS
1x PBS
Dissection dish
Dissecting light microscope (Zeiss)
Dissecting fluorescent microscope with two color/channel camera (Zeiss)
Costar Special Optics 96-well plates (glass bottom, black-walled; Corning, NY)
2.2 Brain dissection and quantitative measure of brain specific chemical retention
The above reagents plus:
Tecan SpectraFluor Plus Reader (Männedorf, Switzerland)
2.3 Assessing BBB physiology in situ
Cyclosporine A dissolved in H2O (Sigma, C1832)
Paraformaldehyde (Thermo Scientific 28906)
Fluorescein sodium salt flourecent dye (Sigma, F6377)
DakoCytomation Fluorescent Mounting medium (Dako, Denmark).
10kDa tetramethyl-rhodamine dextran (TMRD) (Invitrogen D-1817)
10kDa Texas Red dextran (TRD) (Invitrogen D-1863)
Zeiss LSM-510 confocal microscope
Glass coverslips (Corning 286518)
Glass slides (Fisher Scientific 12-550-33)
Nail polish
2.4 Drug Pharmacokinetics in Dm
Same reagents as for 2.1 and 2.2 above plus:
Fine point needle (26G 3/8inch)
0.5 mL microfuge tubes (perforated at the very bottom)
1.5 mL microfuge tubes
3. Methods
3.1 Intrahemolymph Dye Dosing and Retinal Image Acquisition
Live imaging is a useful and highly reproducible (see Note 1) means of probing the functional properties of the BBB. The goal of this method is to assess the integrity of the BBB in flies without invasive procedures. Various dyes are chosen for their size or charge and injected into a remote site of the fly. The presence or absence of a hemolymph exclusion line (HEL, see Note 2) is a real-time measure of BBB chemical permeability. This approach is remarkably handy for screening through various genetic backgrounds to find changes in barrier permeability to test dyes and assessing the chemical nature of small molecules, putative chemical modulators and genetic contributors to BBB function.
3.1.a Intrahemolymph injection
Prepare a micromanipulator fitted with a thin borosilicate needle that has been pre-loaded with the dye(s) of choice (FITC-D and/or RhoB, see Note 3).
Using the dissecting microscope, break the very end of the injection needle to induce flow of dye to tip of the needle.
Using a permeable pad (see Note 5), CO2 anesthetize a sufficient number of flies for the experiment (5-10 flies per experimental condition).
To immobilize the flies for accurate and reproducible injection, suction is applied through a small hole in a parafin-covered vacuum box and the fly is held with forceps at the base of the wings.
Insert the microinjection needle between the posterior abdominal wall body segments of CO2 anesthetized animals under a dissecting microscope (Fig. C, bright line seen in 0.5 sec. image).
Apply positive pressure to the needle under direct visualization over 1-2 seconds (just until the abdominal segments begin to separate) to deliver an average volume of 100 nL dye per injection s.d. +/- 25 (range 70-130 nl).
Allow animals to recover from injection in food vials at 25°C.
Figure C.
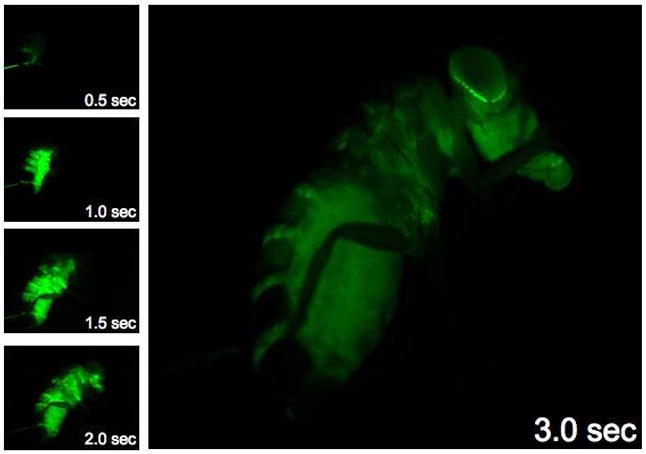
Live whole animal dye injection and chemical partition in the hemolymph compartment. A series of images taken on a fluorescent dissecting microscope during a live injection of 3kDa FITC dextran. Note the rapid delivery of hemolymph around the entire animal and specific hemolymph partition at the retina/cuticle interface.
3.1.b. Visual assessment of drug partition
Two (2) hours following dye injection, flies are CO2 anesthetized and placed on a CO2 pad at low flow to maintain narcosis.
Using the feather probe, gently orient the flies so that they are on their sides with the compound eye facing up towards the objective of the flourescent dissecting scope.
Adjust the excitation and emission of the scope in accord with the dye that is being appreciated (For Texas Red or RhoB: ex: 535/em: 595; For Rho123 or FITC: ex: 485/em: 535).
The retinal dye penetration phenotype is then scored qualitatively by assessment of sharpness and intensity of the HEL (see Fig. D for mutant extreme mutant phenotype).
Figure D.
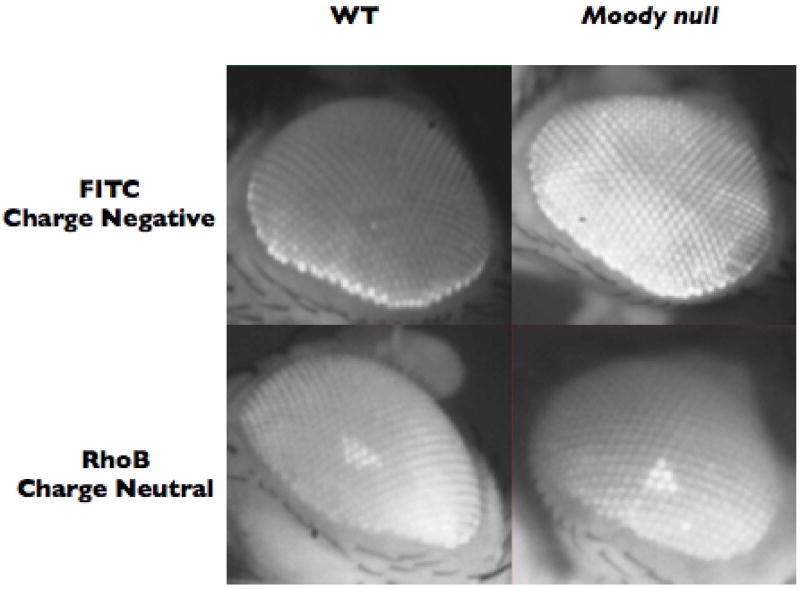
Small molecule fluors have different properties in different mutant backgrounds. In Dm FITC demonstrates exclusion from the retina of a white minus (W-) wild type (WT) animal (note the distinct hemolymph exclusion line and lack of dye in the center of the retina, upper left). A moody null animal allows dye into the retina and demonstrates loss of the hemolymph exclusion line (upper right). Visual-retinal leakiness of the moody null BBB to small molecule dyes is corroborated by quantitative brain dissection assays (see section 3.2). A charge neutral dye like RhoB has equal access to WT and mutant animals as it takes a transcellular route into the CNS by diffusing across lipid bilayers. Thus choosing the appropriate fluorescent reporter is essential for they type of transport physiology to be tested
3.2 Brain dissection and quantitative measure of brain specific chemical retention
Once differences in retinal phenotypes have been observed, we can then look at how much or how little dye actually transits the BBB into the brain parenchyma. This quantitative fluorimetry approach affords this opportunity (see Note 3).
Rhodamine B (see Note 4) and 3kDa FITC dextran are dissolved in H2O at 1.25 mg/mL and 25 mg/mL respectively, and brought to neutral pH.
Sibling animals of flies used for retinal assay are decapitated, and brains rapidly dissected (< 90 seconds) from the cuticle, trachea and fat body in 1X PBS (see Note 6).
With single forceps tip, brains are washed once in 1X PBS and placed in the fluorimeter plate well (1 brain/well) containing 50 uL 0.1% SDS.
Incubate in SDS for 5-10 minutes at room temperature to allow the dye to dissociate from the brain tissue.
Dye released from brain samples is measured using a Tecan Genios fluorimeter in both the red (RhoB) and green channels (FITC)) 535 and 595 (Rho B) 485 and 535 (FITC).
Average values of dye retained in CNS tissue are determined after subtracting the fluor counts or units obtained from brains of non-injected flies (or SDS alone) from brains of injected flies. (see Note 7).
3.3 Assessing BBB physiology in situ
Overview
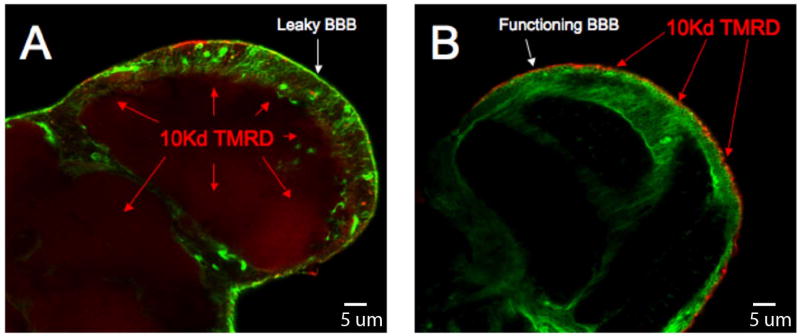
To directly visualize the amount of dye leaking into the brain parenchyma, brains are dissected from the fly head and examined through confocal microscopy methods. Using a fluorescent dye such as tetramethyl rhodamine dextran (TMRD) that will only pass through the barrier if it is compromised, one can, in combination with the above methods, produce a full picture of the integrity of the Dm BBB.
Animals to be stained with C219 antibody are injected with 200 uM cyclosporine A in H2O (see Note 8) and 25 mg/ml 3kDa Texas Red dextran and allowed to recover overnight.
The next day flies are anesthetized with CO2, decapitated, and the proboscis removed with forceps.
Whole heads are placed in fixative (3.7% paraformaldehyde in 1X PBS) for 15 minutes at room temperature.
Central brains are removed from the cuticle in 1X PBS, carefully preserving brain surface structures, and washed with 1X PBS. (see Note 6)
Isolated brains are incubated in blocking buffer for 1 hour (1X PBS, 5% goat serum, 4% Tween 20) then probed in primary antibody overnight at 4°C.
Brains are washed 3 X 30’ in 1X PBS and probed with appropriate fluorescently labeled secondary antibodies for 45’ at room temperature (1:200, Invitrogen).
Brains are washed 3 X 45’ in 1X PBS and mounted on glass slides using mountain medium.
Brains are mounted on glass slides with ~40 um posts (see Note 9) then covered with a coverslip and sealed with nail polish.
Laser and detector gain settings for fluorescent background noise are defined using brains with no primary antibody exposure.
At the coverslip interface, the brain is slightly pressed against the glass providing a flat brain interface with widths of 10-20 um. This preparation allows for proper anatomic identification of dorsal-ventral brain orientation, and overall quality of the brain preparation.
Since the BBB is a continuous surface around the Dm CNS, the depth of confocality is changed to find a cross sectional image.
To observe a tangential section, follow the edge of the brain to its greatest extent laterally. This provides the highest resolution of the apical-basal polarization of the BBB epithelia (see Fig. E).
Figure E.
Confocal microscopy of the physiologic barrier to drug transport. A cross sectional confocal image of a Dm brain at the lobular plate hemolymph injected with 10 kDa tetramethyl-rhodamine dextran (TMRD). Both brain are marked with a pan glial driver (REPO-GAL4) crossed to a transgene expressing GFP (green channel) using the UAS/GAL4 system of Brand and Perrimon (23). On left the moody mutant shows strong dextran signal (red) penetrating the brain. On the right is the moody mutant rescued with Moody-GFP showing wild type BBB function. Here the dextran is fixed to surface tissue outside the brain (red).
3.4 Drug Pharmacokinetics in Dm
3.4.a Overview
To assess dye whole animal pharmacokinetics (half-life), we collect hemolymph injected whole flies at 0, 1, 2, 4, 8 and 16 hours post injection.
-
1
All animals are injected with a mixture of 1.25 mg/mL RhoB and 25 mg/mL 3 kDa FITC dextran.
-
1
Put the 20 flies in a 1.5ml centrifuge tube and add 200 uL of 0.1% SDS.
-
2
Crush the flies by hand for 1min using a plastic micropestle.
-
3
Grind mechanically for another 1 min using a pestle motor and inspect the slurry to be sure flies have been completely homogenized.
-
4
Vortex for 30 seconds
-
5
Centrifuge for 5 min at maximum speed (13.5 rpm)
-
6
Repeat crush and spin times one.
-
7
Samples are diluted 10X in 0.1% SDS, vortexed, centrifuged and 100 uL placed in a 96 well fluorimeter plate wells.
-
8
Fluorescent units are determined using a TECAN fluorimeter for FITC (ex/em:485/535) or RhoB (ex/em:535/595). All samples are measured in the linear range of a standard curve for each fluor.
3.4.b Hemolymph drug/xenobiotic concentration: Hemolymph extraction methods
This method is employed to compare transbarrier partition efficiency by assessing chemical content of the humoral compartment. Hemolymph can be recovered post injection from flies and drug half-life can be established for the humoral space. From this humoral/CNS partition, ratios can be established similar in principle to Lung and Wolfner (18).
Inject 25 flies with ~100 nL of a specific fluorescent dye (such as RhoB).
Select 20 of the injected flies and, using a fine needle, perforate the dorsal aspect of the fly at mid-thorax.
Collect these flies in porous filtered 0.5ml tubes placed inside a larger 1.5ml solid micro-centrifuge tube.
Centrifuge this at 5.6 g for 15 min. Typically recovery is ~1-2 uL of hemolymph from the 20 flies.
Add 0.5 uL of the collected hemolymph in 50 uL 0.1% SDS and measure the fluorescence in a fluorimeter with excitation and emission settings for RhoB of 535 and 595, respectively.
-
To calculate the hemolymph dye content or concentration:
Where (Flh) is the hemolymph fluor concentration, Flh is the measured fluor units and Vm is the volume of hemolymph measured.
Acknowledgments
This work was done with the support of the NIH (GM081863) and the UCSF Department of Anesthesia and Perioperative Care, San Francisco, CA.
Footnotes
Fly hemolymph volume is estimated to be between 150 and 250 nL. With practice, very reproducible injection quantities can be introduced into the humoral compartment without significant injury to the animal.
The distinct hemolymph exclusion line (HEL), combined with a lack of dye in the center of the retina, demonstrates that the BBB is physiologically intact. BBB vulnerability is demonstrated through observing a moody null animal, which has increased drug transport into the retina and loss of the hemolymph exclusion line. The moody null lacks the GPCR Moody, a protein that has been demonstrated to affect the full integrity of the Dm BBB (19).
Brain dissection of Dm is relatively easy and quite reproducible. Since the flies are grown under standard conditions and are genetically identical, it can be assumed that they possess nearly identical brain size. Fluorimetric analysis of chemical content of the brain is easily correlated with live imaging described above. However this method could be applied as easily to radiolabelled drugs using a scintillation counter for quantitation. Furthermore very small quantities of experimental compounds can be used, thus allowing chemical library’s to be tested as BBB modulators in a whole animal. Finally individual dye chemistry can determine the transport pathway into and out of the brain and thus different physiologic properties of the BBB can be tested by choosing specific chemical properties of your reporter. RhoB, a charge neutral dye, will penetrate the brain and retina quickly and efflux slowly and is best for quantitative measures of efflux. Other dyes like FITC salt penetrate poorly in the WT animals and are only found in the brain because of genetic or chemical perturbations and are thus best for live functional assessment of chemical partition.
FITC, a classic small molecule substrate for assessing paracellular barrier transport in vertebrates, is highly charge negative and shows exclusion from the retina of a white minus (W-) W.T. animal and is not a substrate for efflux transport in vertebrates. (20). RhoB, another BBB effluxable dye, was used instead of Rho123, as RhoB produces better fluorescent signal in a quantitative assay. Dextrans are stable over very long periods of time in hemolymph and poorly penetrate the brain of W.T. animals (19).
The CO2 pad is constructed from a 5 inch by 3 inch plastic box, the top of which is covered with a porous polyethylene sheet. One end of the box has nozzle which is connected to a CO2 regulator that will allow low but free flow of gas to flies placed on the surface of the pad.
For a very helpful demonstration on how to dissect a brain from Drosophila, you can direct your web browser to: http://jfly.iam.u-tokyo.ac.jp/html/movie/
Isolated brains from fluor uninjected animals contain insignificant intrinsic fluorescent signal.
Cyclosporine A holds ABC B1 in an open conformation and improves C219 antibody staining in situ (21, 22).
In order to allow sufficient space between the slide and coverslip so as to not crush the delicate brain, 4 posts are fashioned on the slide from drops of common nail polish, one for each corner of the coverslip.
References
- 1.Davis GW. Homeostatic Control of Neural Activity: From Phenomenology to Molecular Design. Annu Rev Neurosci. 2006;29:307–23. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- 2.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 3.Abbott NJ. Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol. 2005;25:5–23. doi: 10.1007/s10571-004-1374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schinkel AH, Mayer U, Wagenaar E, Mol CA, van Deemter L, Smit JJ, van der Valk MA, Voordouw AC, Spits H, van Tellingen O, Zijlmans JM, Fibbe WE, Borst P. Normal viability and altered pharmacokinetics in mice lacking mdr1-type (drug-transporting) P-glycoproteins. Proc Natl Acad Sci U S A. 1997;94:4028–33. doi: 10.1073/pnas.94.8.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–60. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garberg P, Ball M, Borg N, Cecchelli R, Fenart L, Hurst RD, Lindmark T, Mabondzo A, Nilsson JE, Raub TJ, Stanimirovic D, Terasaki T, Oberg JO, Osterberg T. In vitro models for the blood-brain barrier. Toxicol In Vitro. 2005;19:299–334. doi: 10.1016/j.tiv.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Treherne JE, Pinchon Y. In: Advances in insect physiology. Treherne JE, Berridge MJ, Wigglesworth VB, editors. London: Academic; 1972. [Google Scholar]; Kerkut GA, Gilbert LI, editors. Biochemistry, and Pharmacology. Vol. 5. London: Pergamon; pp. 115–37. [Google Scholar]
- 8.Carlson SD, Juang JL, Hilgers SL, Garment MB. Blood barriers of the insect. Annu Rev Entomol. 2000;45:151–74. doi: 10.1146/annurev.ento.45.1.151. [DOI] [PubMed] [Google Scholar]
- 9.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–97. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards JS, Swales LS, Bate M. The differentiation between neuroglia and connective tissue sheath in insect ganglia revisited: the neural lamella and perineurial sheath cells are absent in a mesodermless mutant of Drosophila. J Comp Neurol. 1993;333:301–8. doi: 10.1002/cne.903330214. [DOI] [PubMed] [Google Scholar]
- 11.Tepass U, Hartenstein V. The development of cellular junctions in the Drosophila embryo. Dev Biol. 1994;161:563–96. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- 13.Wu VM, Beitel GJ. A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr Opin Cell Biol. 2004;16:493–9. doi: 10.1016/j.ceb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U. GPCR signaling is required for blood-brain barrier formation in drosophila. Cell. 2005;123:133–44. doi: 10.1016/j.cell.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ. Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. Journal of Neuroscience. 2009 Mar 18;29(11):3538–50. doi: 10.1523/JNEUROSCI.5564-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dean M, Annilo T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu Rev Genomics Hum Genet. 2005;6:123–42. doi: 10.1146/annurev.genom.6.080604.162122. [DOI] [PubMed] [Google Scholar]
- 17.Torrie LS, Radford JC, Southall TD, Kean L, Dinsmore AJ, Davies SA, Dow JA. Resolution of the insect ouabain paradox. Proc Natl Acad Sci U S A. 2004;101:13689–93. doi: 10.1073/pnas.0403087101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lung O, Wolfner MF. Drosophila seminal fluid proteins enter the circulatory system of the mated female fly by crossing the posterior vaginal wall. Insect Biochem Mol Biol. 1999;29:1043–52. doi: 10.1016/s0965-1748(99)00078-8. [DOI] [PubMed] [Google Scholar]
- 19.Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U. moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell. 2005;123:145–56. doi: 10.1016/j.cell.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 20.Nag S. The Blood-Brain Barrier. Humana Press; Totowa, NJ: 2003. [Google Scholar]
- 21.van Den Elsen JM, Kuntz DA, Hoedemaeker FJ, Rose DR. Antibody C219 recognizes an alpha-helical epitope on P-glycoprotein. Proc Natl Acad Sci U S A. 1999;96:13679–84. doi: 10.1073/pnas.96.24.13679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Demeule M, Vachon V, Delisle MC, Beaulieu E, Averill-Bates D, Murphy GF, Beliveau R. Molecular study of P-glycoprotein in multidrug resistance using surface plasmon resonance. Anal Biochem. 1995;230:239–47. doi: 10.1006/abio.1995.1469. [DOI] [PubMed] [Google Scholar]
- 23.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]