Abstract
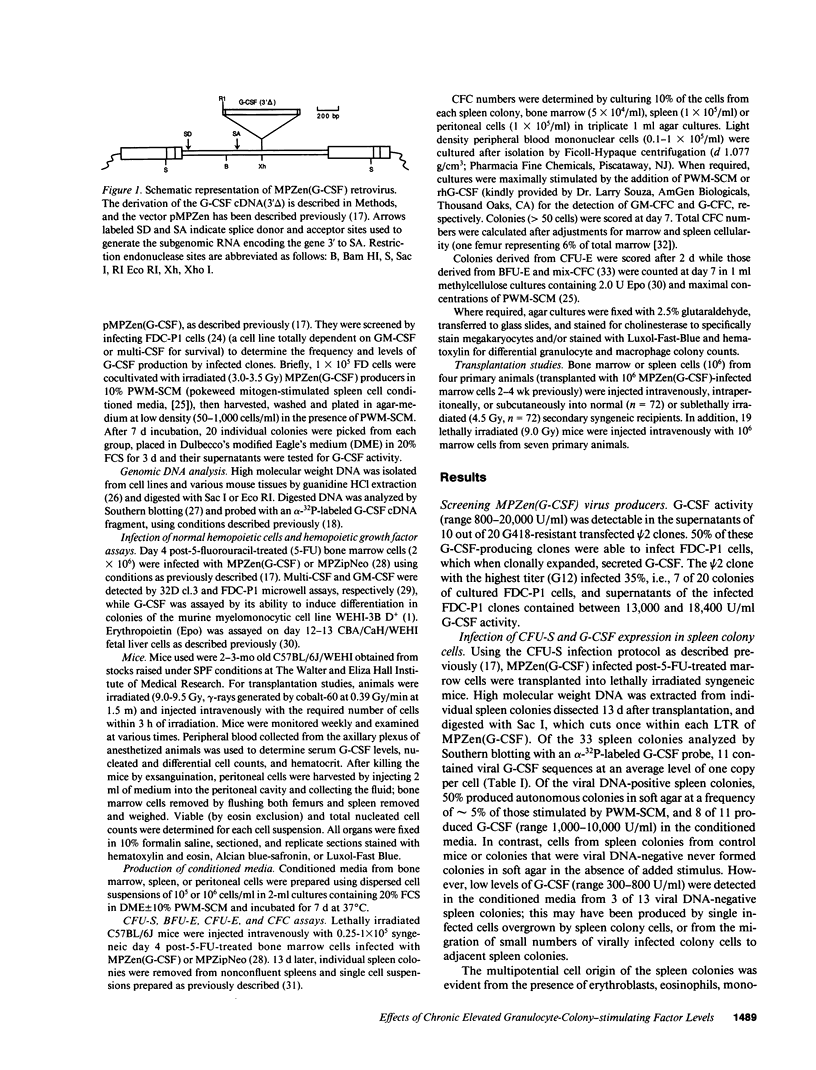
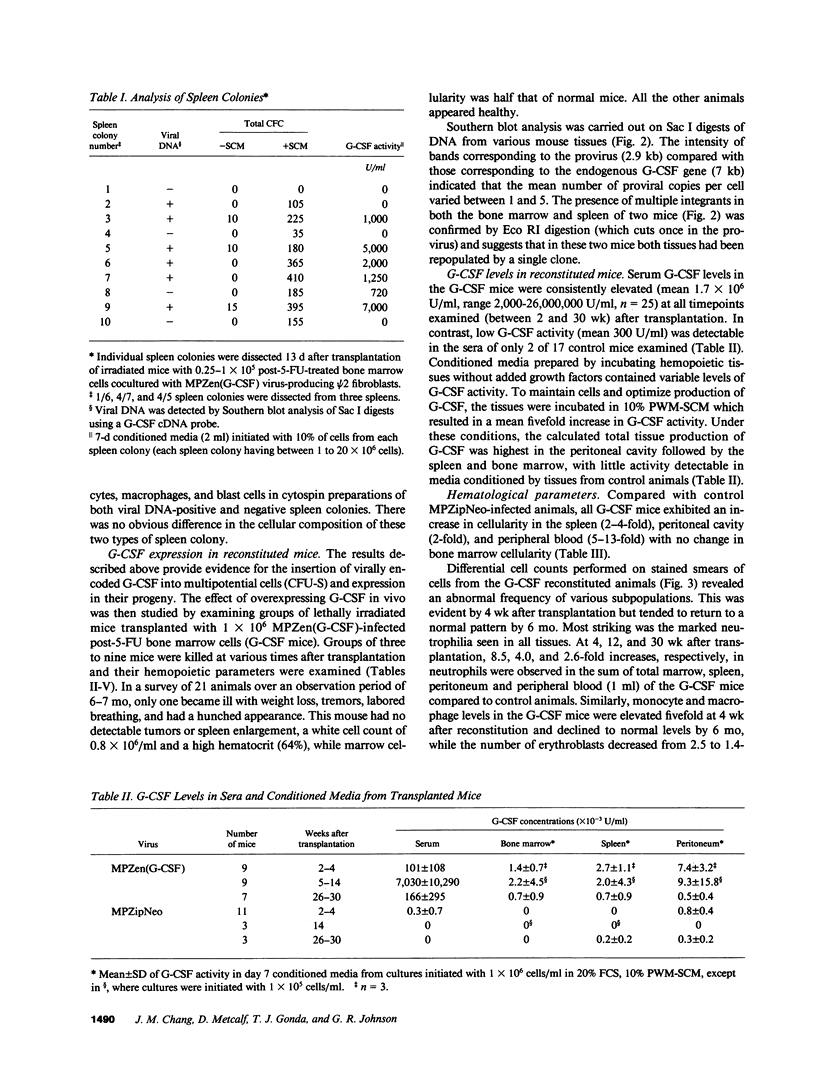
Murine marrow cells infected with a retroviral vector (MPZen) bearing a granulocyte-colony-stimulating factor (G-CSF) cDNA insert were transplanted into lethally irradiated recipients to study the effects of autocrine production of G-CSF in normal hemopoietic cells. Most animals remained healthy with no evidence of tissue damage throughout the observation period (4-30 wk) despite high circulating G-CSF levels (range 2,000-26,000,000 U/ml). A dramatic neutrophilic granulocytosis was observed in all hemopoietic tissues with neutrophilic infiltration occurring in the lung and liver. Spleen, peritoneal, and peripheral blood cellularity increased approximately three-, two-, and eightfold, respectively, but total bone marrow cell counts remained unchanged. Progenitor cell numbers granulocyte-macrophage colony-forming cell (GM-CFC), granulocyte colony-forming cell (G-CFC), burst-forming unit-erythroid (BFU-E), colony-forming unit-erythroid (CFU-E) and mixed colony-forming cells (Mix-CFC) were elevated between 10-100-fold in the spleen, peritoneal cavity, and peripheral blood, but were unaffected or slightly depressed in the marrow. No tumors developed in syngeneic recipients transplanted with bone marrow or spleen cells from such mice, confirming the nonneoplastic nature of the hyperplasia induced by chronic G-CSF stimulation. These experiments also indicated the stable integration of MPZen vectors in infected cells, as evident from the continuous expression of the inserted gene for at least 6 mo, and from the ability of infected stem cells from the primary recipients to express the gene in lethally irradiated secondary recipients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowtell D. D., Johnson G. R., Kelso A., Cory S. Expression of genes transferred to haemopoietic stem cells by recombinant retroviruses. Mol Biol Med. 1987 Aug;4(4):229–250. [PubMed] [Google Scholar]
- Bowtell D. D. Rapid isolation of eukaryotic DNA. Anal Biochem. 1987 May 1;162(2):463–465. doi: 10.1016/0003-2697(87)90421-0. [DOI] [PubMed] [Google Scholar]
- Bronchud M. H., Scarffe J. H., Thatcher N., Crowther D., Souza L. M., Alton N. K., Testa N. G., Dexter T. M. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br J Cancer. 1987 Dec;56(6):809–813. doi: 10.1038/bjc.1987.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Metcalf D., Lang R. A., Gonda T. J., Johnson G. R. Nonneoplastic hematopoietic myeloproliferative syndrome induced by dysregulated multi-CSF (IL-3) expression. Blood. 1989 May 1;73(6):1487–1497. [PubMed] [Google Scholar]
- Cheng G. Y., Kelleher C. A., Miyauchi J., Wang C., Wong G., Clark S. C., McCulloch E. A., Minden M. D. Structure and expression of genes of GM-CSF and G-CSF in blast cells from patients with acute myeloblastic leukemia. Blood. 1988 Jan;71(1):204–208. [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R., Marsh J. C., Cartwright G. E., Wintrobe M. M. Quantitative studies of blood and bone marrow neutrophils in normal mice. Am J Physiol. 1968 Aug;215(2):353–360. doi: 10.1152/ajplegacy.1968.215.2.353. [DOI] [PubMed] [Google Scholar]
- Cohen A. M., Zsebo K. M., Inoue H., Hines D., Boone T. C., Chazin V. R., Tsai L., Ritch T., Souza L. M. In vivo stimulation of granulopoiesis by recombinant human granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2484–2488. doi: 10.1073/pnas.84.8.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler R. L., Johnson G. R., Nicola N. A. Preparation of human erythropoietin for tissue culture. Exp Hematol. 1985 Sep;13(8):796–801. [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchateau J., Lamy M. IVth International Meeting of Clinical Biology CH Sainte-Ode, Baconfoy, Belgium May 23-24th, 1987 clinical biology in intensive care medicine. Leukocyte aggregation and complement activation in respiratory distress syndrome (ARDS). Ann Biol Clin (Paris) 1988;46(4):267–268. [PubMed] [Google Scholar]
- Dührsen U., Villeval J. L., Boyd J., Kannourakis G., Morstyn G., Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988 Dec;72(6):2074–2081. [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Keller G., Paige C., Gilboa E., Wagner E. F. Expression of a foreign gene in myeloid and lymphoid cells derived from multipotent haematopoietic precursors. Nature. 1985 Nov 14;318(6042):149–154. doi: 10.1038/318149a0. [DOI] [PubMed] [Google Scholar]
- Lala P. K., Johnson G. R. Monoclonal origin of B lymphocyte colony-forming cells in spleen colonies formed by multipotential hemopoietic stem cells. J Exp Med. 1978 Dec 1;148(6):1468–1477. doi: 10.1084/jem.148.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Cuthbertson R. A., Lyons I., Stanley E., Kelso A., Kannourakis G., Williamson D. J., Klintworth G. K., Gonda T. J. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987 Nov 20;51(4):675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lopez A. F., Nicola N. A., Burgess A. W., Metcalf D., Battye F. L., Sewell W. A., Vadas M. Activation of granulocyte cytotoxic function by purified mouse colony-stimulating factors. J Immunol. 1983 Dec;131(6):2983–2988. [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Marsh J. L., Erfle M., Wykes E. J. The pIC plasmid and phage vectors with versatile cloning sites for recombinant selection by insertional inactivation. Gene. 1984 Dec;32(3):481–485. doi: 10.1016/0378-1119(84)90022-2. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Johnson G. R. Production by spleen and lymph node cells of conditioned medium with erythroid and other hemopoietic colony-stimulating activity. J Cell Physiol. 1978 Jul;96(1):31–42. doi: 10.1002/jcp.1040960105. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. Proliferative effects of purified granulocyte colony-stimulating factor (G-CSF) on normal mouse hemopoietic cells. J Cell Physiol. 1983 Aug;116(2):198–206. doi: 10.1002/jcp.1041160211. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Warren D. J. Synergy of interleukin 1 and granulocyte colony-stimulating factor: in vivo stimulation of stem-cell recovery and hematopoietic regeneration following 5-fluorouracil treatment of mice. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7134–7138. doi: 10.1073/pnas.84.20.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. A., Welte K., Gabrilove J., Souza L. M. Biological activities of recombinant human granulocyte colony stimulating factor (rhG-CSF) and tumor necrosis factor: in vivo and in vitro analysis. Haematol Blood Transfus. 1987;31:210–220. doi: 10.1007/978-3-642-72624-8_45. [DOI] [PubMed] [Google Scholar]
- Morstyn G., Campbell L., Souza L. M., Alton N. K., Keech J., Green M., Sheridan W., Metcalf D., Fox R. Effect of granulocyte colony stimulating factor on neutropenia induced by cytotoxic chemotherapy. Lancet. 1988 Mar 26;1(8587):667–672. doi: 10.1016/s0140-6736(88)91475-4. [DOI] [PubMed] [Google Scholar]
- Muraguchi A., Hirano T., Tang B., Matsuda T., Horii Y., Nakajima K., Kishimoto T. The essential role of B cell stimulatory factor 2 (BSF-2/IL-6) for the terminal differentiation of B cells. J Exp Med. 1988 Feb 1;167(2):332–344. doi: 10.1084/jem.167.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H. Molecular cloning and expression of cDNA for human granulocyte colony-stimulating factor. 1986 Jan 30-Feb 5Nature. 319(6052):415–418. doi: 10.1038/319415a0. [DOI] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Rosen C. A., Haseltine W. A., Lenz J., Ruprecht R., Cloyd M. W. Tissue selectivity of murine leukemia virus infection is determined by long terminal repeat sequences. J Virol. 1985 Sep;55(3):862–866. doi: 10.1128/jvi.55.3.862-866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
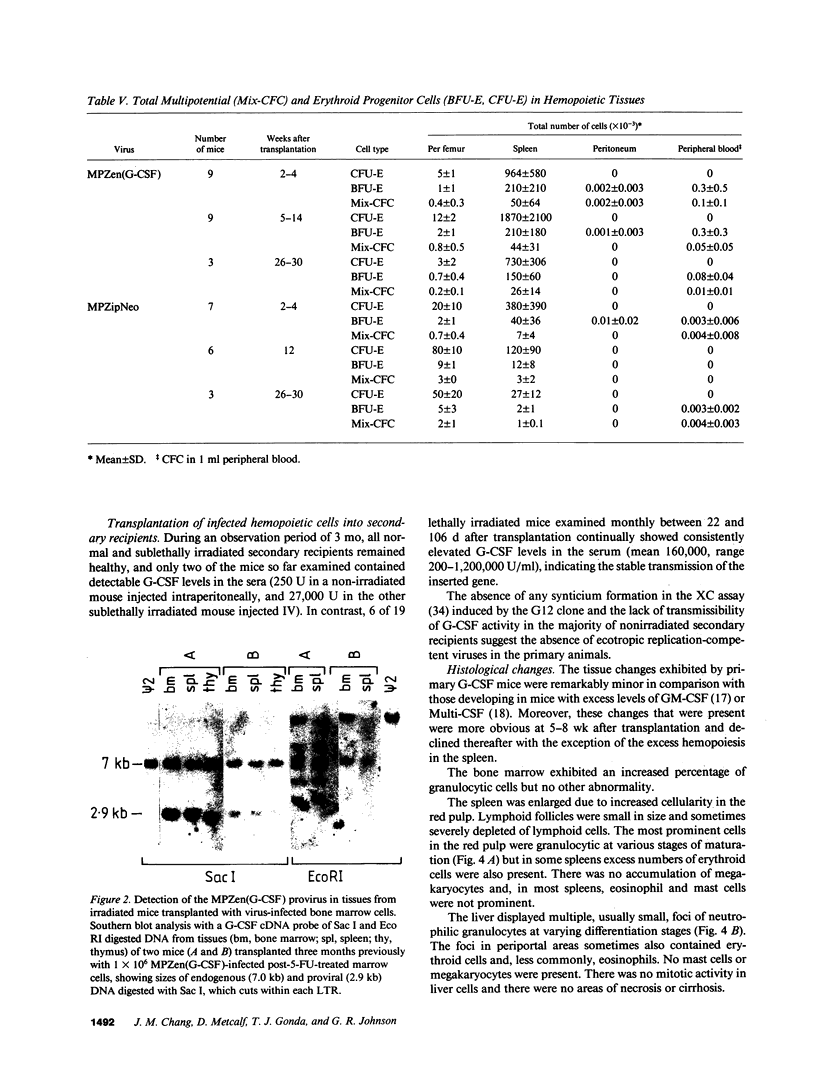
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Souza L. M., Boone T. C., Gabrilove J., Lai P. H., Zsebo K. M., Murdock D. C., Chazin V. R., Bruszewski J., Lu H., Chen K. K. Recombinant human granulocyte colony-stimulating factor: effects on normal and leukemic myeloid cells. Science. 1986 Apr 4;232(4746):61–65. doi: 10.1126/science.2420009. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Tamura M., Hattori K., Nomura H., Oheda M., Kubota N., Imazeki I., Ono M., Ueyama Y., Nagata S., Shirafuji N. Induction of neutrophilic granulocytosis in mice by administration of purified human native granulocyte colony-stimulating factor (G-CSF). Biochem Biophys Res Commun. 1987 Jan 30;142(2):454–460. doi: 10.1016/0006-291x(87)90296-8. [DOI] [PubMed] [Google Scholar]
- Tsuchiya M., Asano S., Kaziro Y., Nagata S. Isolation and characterization of the cDNA for murine granulocyte colony-stimulating factor. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7633–7637. doi: 10.1073/pnas.83.20.7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Bonilla M. A., Gillio A. P., Boone T. C., Potter G. K., Gabrilove J. L., Moore M. A., O'Reilly R. J., Souza L. M. Recombinant human granulocyte colony-stimulating factor. Effects on hematopoiesis in normal and cyclophosphamide-treated primates. J Exp Med. 1987 Apr 1;165(4):941–948. doi: 10.1084/jem.165.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte K., Platzer E., Lu L., Gabrilove J. L., Levi E., Mertelsmann R., Moore M. A. Purification and biochemical characterization of human pluripotent hematopoietic colony-stimulating factor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1526–1530. doi: 10.1073/pnas.82.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Dunbar C. E., Bodine D. M., Ruscetti S., Nienhuis A. W. Retrovirus-mediated transfer and expression of the interleukin-3 gene in mouse hematopoietic cells result in a myeloproliferative disorder. Mol Cell Biol. 1989 Feb;9(2):798–808. doi: 10.1128/mcb.9.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]