Abstract
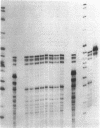
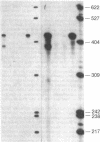
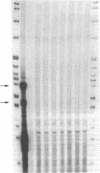
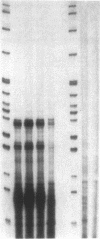
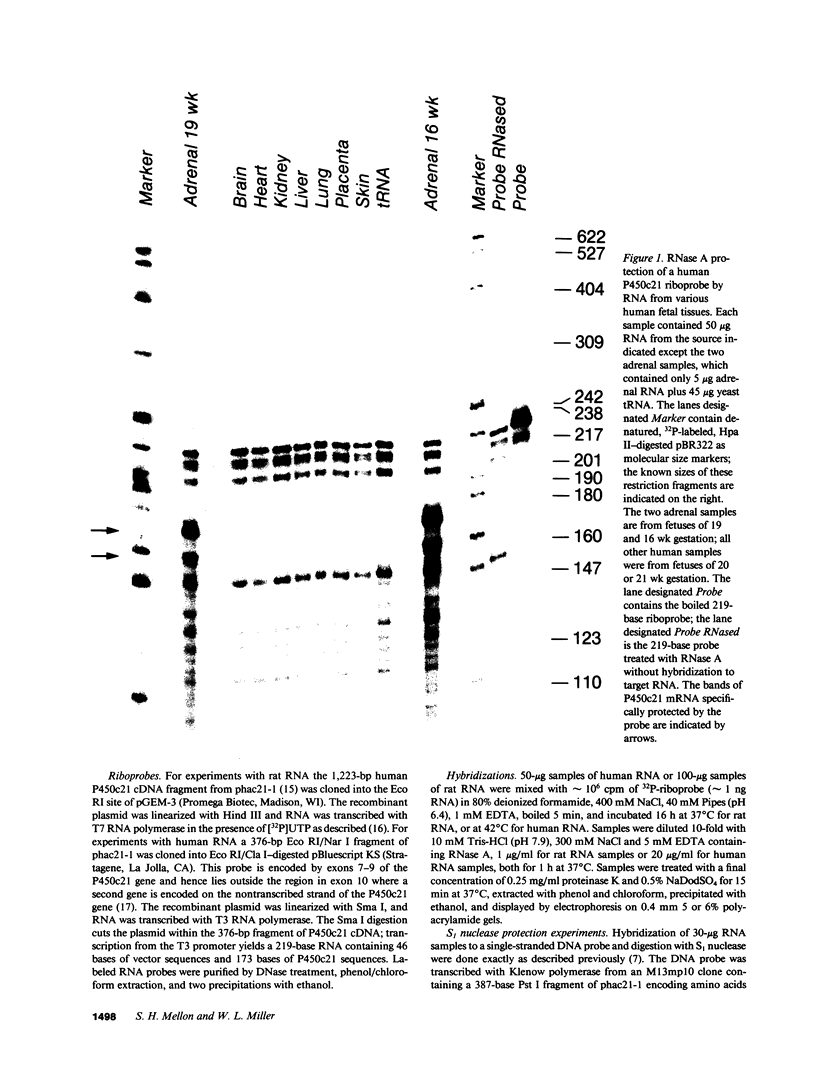
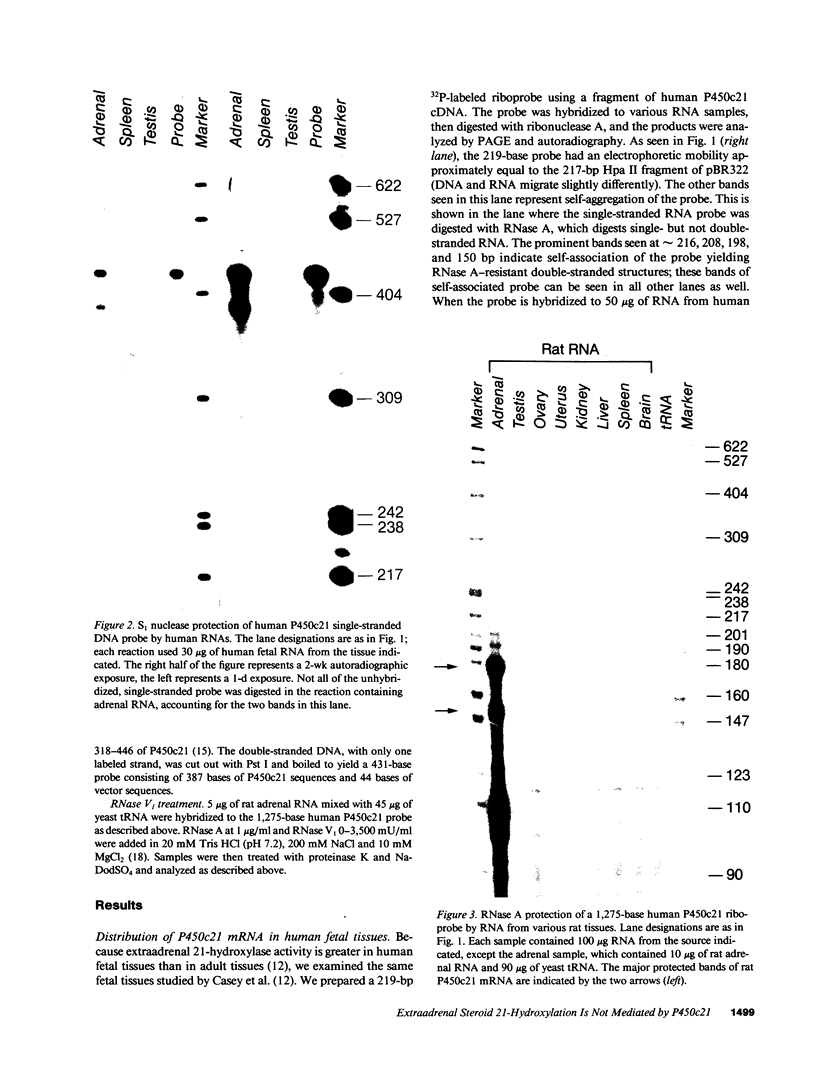
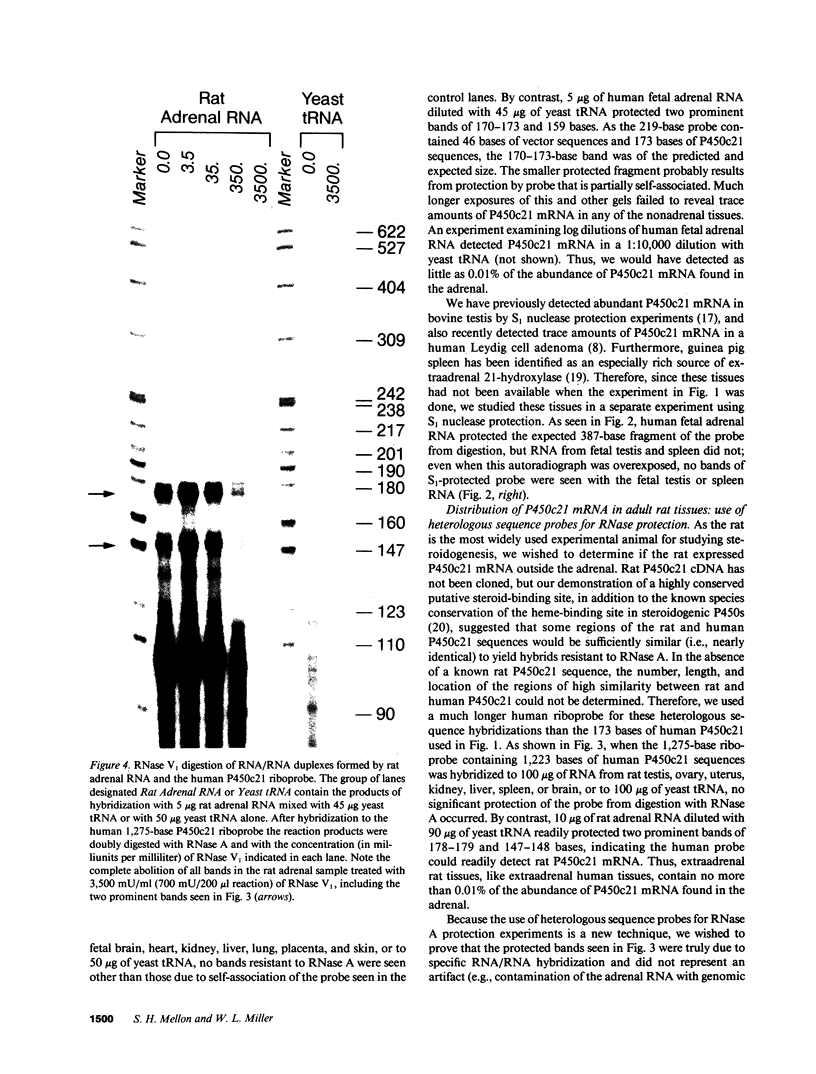
The 21-hydroxylation of progesterone to deoxycorticosterone (DOC) and of 17-hydroxyprogesterone to 11-deoxycortisol in the human adrenal cortex is mediated by a single enzyme termed P450c21. Extraadrenal tissues can clear circulating progesterone and progesterone sulfate by 21-hydroxylation to DOC and DOC-sulfate. It has previously been established that such extraadrenal 21-hydroxylase activity is widely distributed in adult and fetal tissues, but it has not been known if extra-adrenal 21-hydroxylation is mediated by the same P450c21 enzyme found in the adrenal. We examined human RNA from fetal adrenal, liver, kidney, lung, brain, heart, skin, spleen, testis, and placenta by solution hybridization to human P450c21 probes transcribed from cloned human P450c21 cDNA, followed by nuclease protection and acrylamide gel electrophoresis. No P450c21 mRNA was detectable in any extraadrenal tissue. The sensitivity of the assay would have detected P450c21 mRNA at 0.01% of its abundance in the human fetal adrenal. Similar experiments in rats showed no P450c21 mRNA in brain, heart, kidney, liver, lung, testis, ovary, or uterus. These results clearly demonstrate that one or more enzymes other than the classical adrenal 21-hydroxylase are responsible for human and rat extraadrenal 21-hydroxylation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amor M., Tosi M., Duponchel C., Steinmetz M., Meo T. Liver mRNA probes disclose two cytochrome P-450 genes duplicated in tandem with the complement C4 loci of the mouse H-2S region. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4453–4457. doi: 10.1073/pnas.82.13.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey M. L., MacDonald P. C. Extraadrenal formation of a mineralocorticosteroid: deoxycorticosterone and deoxycorticosterone sulfate biosynthesis and metabolism. Endocr Rev. 1982 Fall;3(4):396–403. doi: 10.1210/edrv-3-4-396. [DOI] [PubMed] [Google Scholar]
- Casey M. L., Winkel C. A., MacDonald P. C. Conversion of progesterone to deoxycorticosterone in the human fetus: steroid 21-hydroxylase activity in fetal tissues. J Steroid Biochem. 1983 Apr;18(4):449–452. doi: 10.1016/0022-4731(83)90064-x. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Chung B. C., Matteson K. J., Miller W. L. Structure of a bovine gene for P-450c21 (steroid 21-hydroxylase) defines a novel cytochrome P-450 gene family. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4243–4247. doi: 10.1073/pnas.83.12.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner J. M., Hintz R. L., Luetscher J. A. The role of renin and angiotensin in salt-losing, 21-hydroxylase-deficient congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1979 May;48(5):776–783. doi: 10.1210/jcem-48-5-776. [DOI] [PubMed] [Google Scholar]
- Kominami S., Ochi H., Kobayashi Y., Takemori S. Studies on the steroid hydroxylation system in adrenal cortex microsomes. Purification and characterization of cytochrome P-450 specific for steroid C-21 hydroxylation. J Biol Chem. 1980 Apr 25;255(8):3386–3394. [PubMed] [Google Scholar]
- Lieberman S., Greenfield N. J., Wolfson A. A heuristic proposal for understanding steroidogenic processes. Endocr Rev. 1984 Winter;5(1):128–148. doi: 10.1210/edrv-5-1-128. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Kumar A. Mapping tRNA structure in solution using double-strand-specific ribonuclease V1 from cobra venom. Nucleic Acids Res. 1981 Oct 10;9(19):5125–5140. doi: 10.1093/nar/9.19.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson K. J., Phillips J. A., 3rd, Miller W. L., Chung B. C., Orlando P. J., Frisch H., Ferrandez A., Burr I. M. P450XXI (steroid 21-hydroxylase) gene deletions are not found in family studies of congenital adrenal hyperplasia. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5858–5862. doi: 10.1073/pnas.84.16.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon S. H., Vaisse C. cAMP regulates P450scc gene expression by a cycloheximide-insensitive mechanism in cultured mouse Leydig MA-10 cells. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7775–7779. doi: 10.1073/pnas.86.20.7775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L., Leisti S., Johnson L. K. Synthesis of growth hormone, prolactin, and proopiomelanocortin by intact adult ovine pituitary tissue in vitro. Endocrinology. 1982 Oct;111(4):1358–1367. doi: 10.1210/endo-111-4-1358. [DOI] [PubMed] [Google Scholar]
- Miller W. L. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988 Aug;9(3):295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Morel Y. The molecular genetics of 21-hydroxylase deficiency. Annu Rev Genet. 1989;23:371–393. doi: 10.1146/annurev.ge.23.120189.002103. [DOI] [PubMed] [Google Scholar]
- Morel Y., Bristow J., Gitelman S. E., Miller W. L. Transcript encoded on the opposite strand of the human steroid 21-hydroxylase/complement component C4 gene locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6582–6586. doi: 10.1073/pnas.86.17.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J., Miller W. L. Homologous sequences in steroidogenic enzymes, steroid receptors and a steroid binding protein suggest a consensus steroid-binding sequence. Mol Endocrinol. 1988 Nov;2(11):1145–1150. doi: 10.1210/mend-2-11-1145. [DOI] [PubMed] [Google Scholar]
- Stoner E., Dimartino-Nardi J., Kuhnle U., Levine L. S., Oberfield S. E., New M. I. Is salt-wasting in congenital adrenal hyperplasia due to the same gene as the fasciculata defect? Clin Endocrinol (Oxf) 1986 Jan;24(1):9–20. doi: 10.1111/j.1365-2265.1986.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Tukey R. H., Okino S., Barnes H., Griffin K. J., Johnson E. F. Multiple gene-like sequences related to the rabbit hepatic progesterone 21-hydroxylase cytochrome P-450 1. J Biol Chem. 1985 Oct 25;260(24):13347–13354. [PubMed] [Google Scholar]
- Voutilainen R., Miller W. L. Developmental expression of genes for the stereoidogenic enzymes P450scc (20,22-desmolase), P450c17 (17 alpha-hydroxylase/17,20-lyase), and P450c21 (21-hydroxylase) in the human fetus. J Clin Endocrinol Metab. 1986 Nov;63(5):1145–1150. doi: 10.1210/jcem-63-5-1145. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Casey M. L., Worley R. J., Madden J. D., MacDonald P. C. Extraadrenal steroid 21-hydroxylase activity in a woman with congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1983 Jan;56(1):104–107. doi: 10.1210/jcem-56-1-104. [DOI] [PubMed] [Google Scholar]
- Winkel C. A., Wade C. E., Danley D. L., MacDonald P. C., Casey M. L. Conversion of progesterone to deoxycorticosterone in guinea pig spleen: an animal model for the study of steroid 21-hydroxylase activity in extra adrenal sites. J Steroid Biochem. 1983 Nov;19(5):1635–1638. doi: 10.1016/0022-4731(83)90382-5. [DOI] [PubMed] [Google Scholar]