Abstract
Serum components inhibit DNA polymerase, thereby obviating direct detection of serum viral DNA sequences by the polymerase chain reaction (PCR). This has necessitated extraction of nucleic acid from sera before performing PCR and has resulted in loss of sensitivity. By adsorbing virus to a solid surface (microcentrifuge tubes or antibody coated microparticles) followed by proteinase K digestion, as little as three viruses per 200 microliters serum may be directly detected by PCR without nucleic acid extraction. The sensitivity is dependent on the surface area of the adsorptive surface and is increased by having antibodies on the adsorptive surface. The nucleic acid sequence of the amplified DNA fragments may be directly determined by the dideoxy method. Of 24 plasma samples from HBsAg+ volunteer blood donors, HBV DNA was detected in 7 by dot blot assay, 7 by liquid hybridization, and 9 by PCR. PCR detected DNA in every sample that was positive by another assay. Analysis of serial samples of two patients with acute self-limited hepatitis B found detectable HBsAg and pre-S2 antigenemia before HBV DNA by the PCR method. These results suggest that surface antigenemia may precede viremia during acute hepatitis.
Full text
PDF
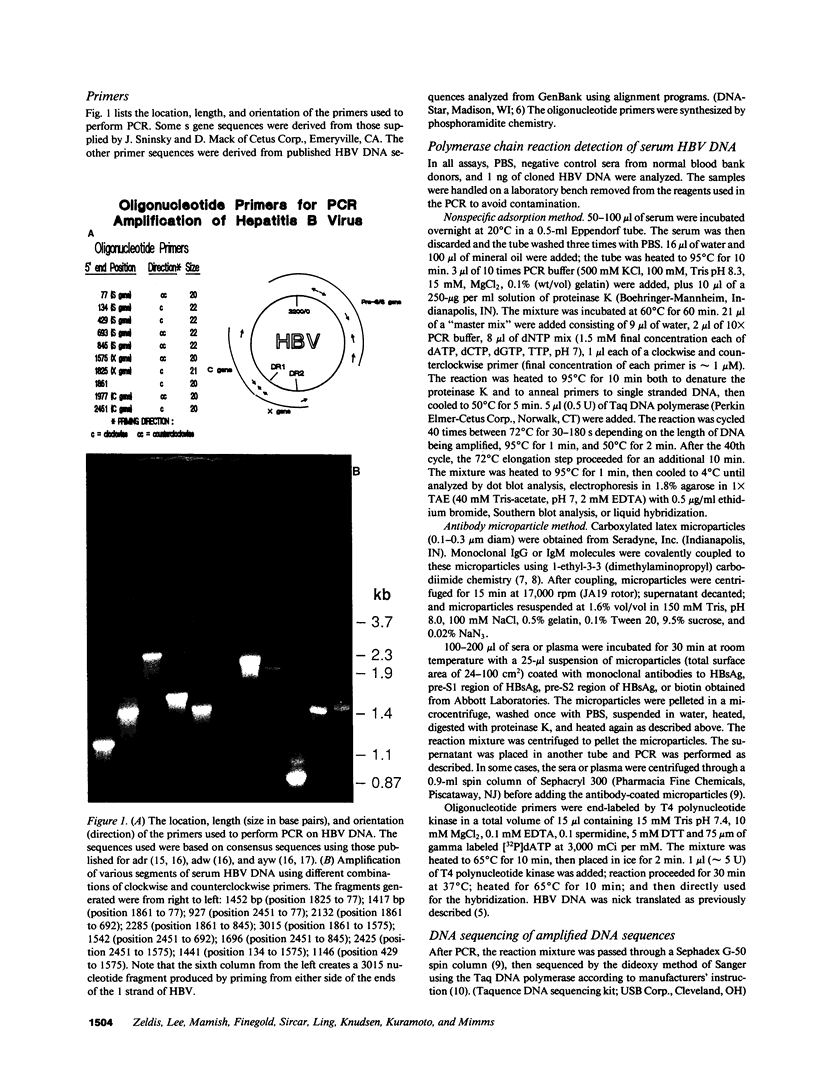
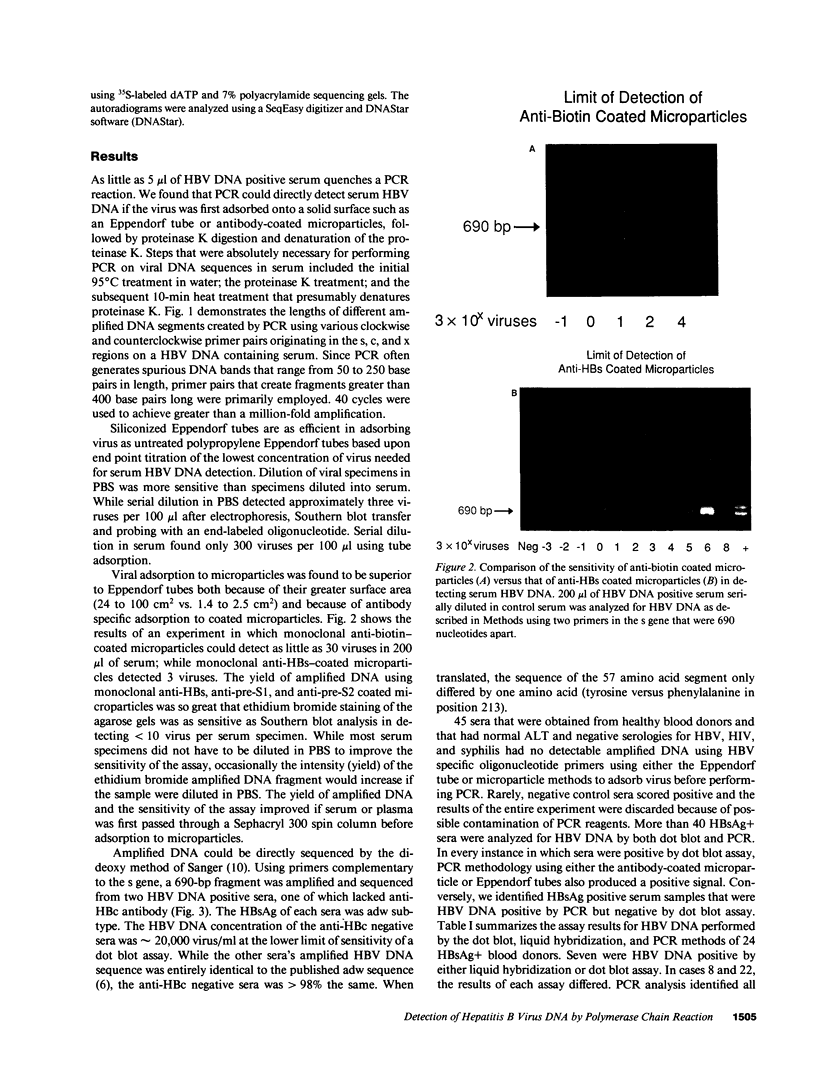

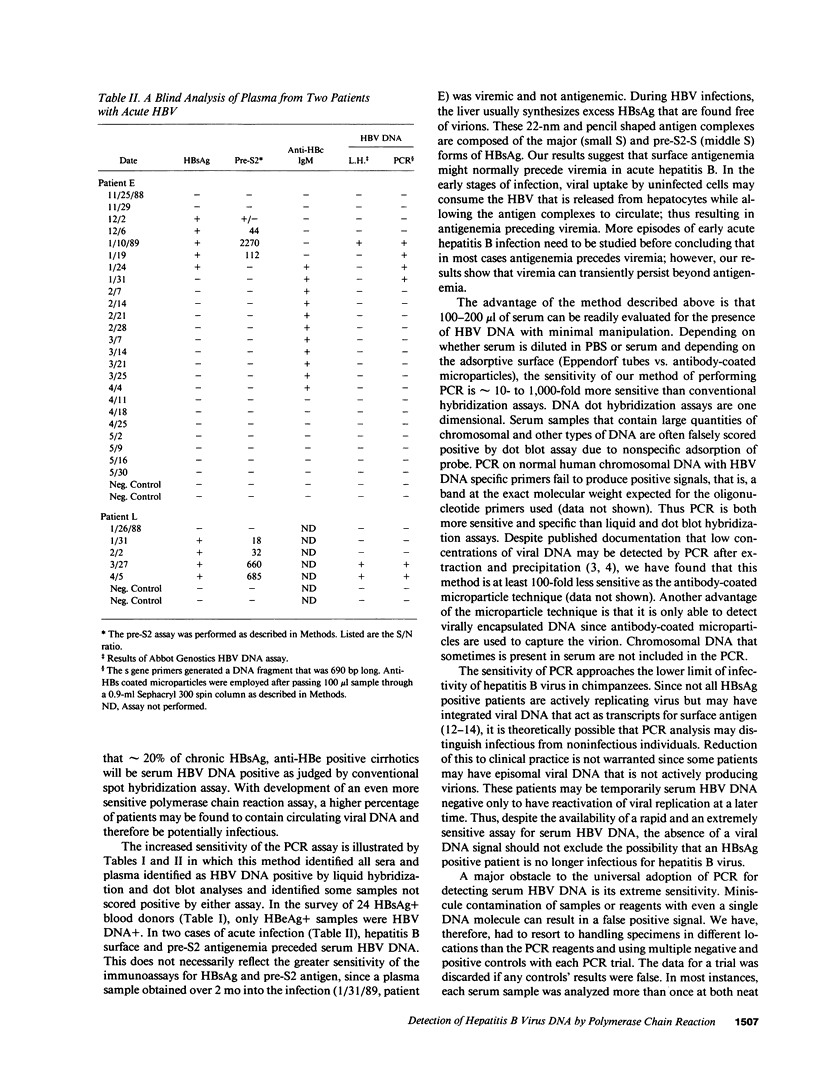

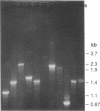
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bichko V., Pushko P., Dreilina D., Pumpen P., Gren E. Subtype ayw variant of hepatitis B virus. DNA primary structure analysis. FEBS Lett. 1985 Jun 3;185(1):208–212. doi: 10.1016/0014-5793(85)80771-7. [DOI] [PubMed] [Google Scholar]
- Brechot C., Hadchouel M., Scotto J., Degos F., Charnay P., Trepo C., Tiollais P. Detection of hepatitis B virus DNA in liver and serum: a direct appraisal of the chronic carrier state. Lancet. 1981 Oct 10;2(8250):765–768. doi: 10.1016/s0140-6736(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Miyanohara A., Nozaki C., Yoneyama T., Ohtomo N., Matsubara K. Cloning and structural analyses of hepatitis B virus DNAs, subtype adr. Nucleic Acids Res. 1983 Jul 11;11(13):4601–4610. doi: 10.1093/nar/11.13.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Hadziyannis S. J., Lieberman H. M., Karvountzis G. G., Shafritz D. A. Analysis of liver disease, nuclear HBcAg, viral replication, and hepatitis B virus DNA in liver and serum of HBeAg Vs. anti-HBe positive carriers of hepatitis B virus. Hepatology. 1983 Sep-Oct;3(5):656–662. doi: 10.1002/hep.1840030505. [DOI] [PubMed] [Google Scholar]
- Kam W., Rall L. B., Smuckler E. A., Schmid R., Rutter W. J. Hepatitis B viral DNA in liver and serum of asymptomatic carriers. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7522–7526. doi: 10.1073/pnas.79.23.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko S., Miller R. H., Feinstone S. M., Unoura M., Kobayashi K., Hattori N., Purcell R. H. Detection of serum hepatitis B virus DNA in patients with chronic hepatitis using the polymerase chain reaction assay. Proc Natl Acad Sci U S A. 1989 Jan;86(1):312–316. doi: 10.1073/pnas.86.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Cohn Z. A. Antitumor effects of hydrogen peroxide in vivo. J Exp Med. 1981 Nov 1;154(5):1539–1553. doi: 10.1084/jem.154.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y., Onda H., Sasada R., Igarashi K., Sugino Y., Nishioka K. The complete nucleotide sequences of the cloned hepatitis B virus DNA; subtype adr and adw. Nucleic Acids Res. 1983 Mar 25;11(6):1747–1757. doi: 10.1093/nar/11.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou C. Y., Kwok S., Mitchell S. W., Mack D. H., Sninsky J. J., Krebs J. W., Feorino P., Warfield D., Schochetman G. DNA amplification for direct detection of HIV-1 in DNA of peripheral blood mononuclear cells. Science. 1988 Jan 15;239(4837):295–297. doi: 10.1126/science.3336784. [DOI] [PubMed] [Google Scholar]
- Quash G., Roch A. M., Niveleau A., Grange J., Keolouangkhot T., Huppert J. The preparation of latex particles with covalently bound polyamines, IgG and measles agglutinins and their use in visual agglutination tests. J Immunol Methods. 1978;22(1-2):165–174. doi: 10.1016/0022-1759(78)90069-8. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiers V., Nakajima E., Kremsdorf D., Mack D., Schellekens H., Driss F., Goudeau A., Wands J., Sninsky J., Tiollais P. Transmission of hepatitis B from hepatitis-B-seronegative subjects. Lancet. 1988 Dec 3;2(8623):1273–1276. doi: 10.1016/s0140-6736(88)92891-7. [DOI] [PubMed] [Google Scholar]
- Zeldis J. B., Ben-Porath E., Enat R., Kirsch K., Wands J. Correlation of HBV DNA and monoclonal reactivity to HBsAg in serum of patients with HBV infection. J Virol Methods. 1986 Sep;14(2):153–166. doi: 10.1016/0166-0934(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Zeldis J. B., Mugishima H., Steinberg H. N., Nir E., Gale R. P. In vitro hepatitis B virus infection of human bone marrow cells. J Clin Invest. 1986 Aug;78(2):411–417. doi: 10.1172/JCI112591. [DOI] [PMC free article] [PubMed] [Google Scholar]