Multiple Eph receptor tyrosine kinases and ephrin ligands are expressed in stem cells and their niches, where their complex roles are under intense investigation. Nomura et al. (2010) reveal an unexpected function of Eph signaling in suppressing cell fate plasticity of niche cells in the adult brain.
Harnessing the power of stem cells to repair or replace damaged tissues is one of the great hopes of regenerative medicine. Use of stem cells as a therapy necessitates a thorough understanding of intrinsic programs and extrinsic factors that govern their maintenance, expansion and differentiation. Resident stem cells in the adult brain also play an integral role in tissue homeostasis, regeneration and brain plasticity. However, the ability of these cells to divide and maintain their multipotency is highly dependent on intimately associated niche cells in the microenvironment (Fig. 1A) (Zhao et al., 2008). Thus, preservation of stem and niche cells alike, is essential for the regulation and function of adult neurogenic zones. In this issue of Cell Stem Cell, Nomura et al. show that two niche cell lineages – ependymal cells and non-proliferating subventricular zone astrocytes – retain an important level of cell fate plasticity in the adult mouse brain that contributes to the natural resilience of the system. Unexpectedly, the work reveals that a portion of ependymal cells that line the lateral ventricular wall and astrocytes in the subventricular zone are capable of mutually replacing one another following an injury. In the normal brain, the ability of these cell types to interconvert is suppressed by signaling events downstream of Notch and EphB receptor tyrosine kinases. However, these pathways are overridden following injury (Fig. 1B).
Figure 1. Unleashing niche cell plasticity in the adult brain.
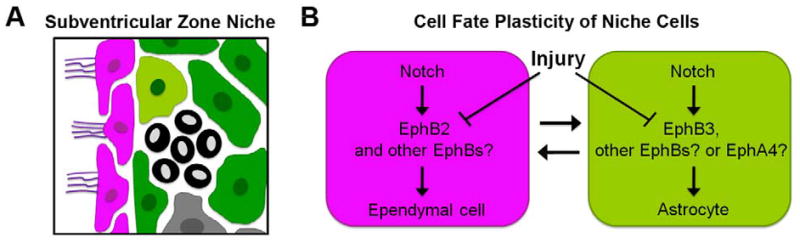
(A) Schematic showing the basic organization of the subventricular zone niche in the adult mouse brain. Ependymal cells (magenta) line the wall of the lateral ventricles as a single cell layer. Non-proliferating (light green) and proliferating (green and grey) astrocyte-like cells, as well as neuroblasts (black), are positioned within the niche. (B) Mild injury interferes with normal signaling downstream of Notch and EphB receptors and promotes intercellular conversion of ependymal cells (left) and non-proliferating astrocyte-like cells (right). Multiple EphB receptors (and possibly EphA4), each expressed in different subsets of cell types, are likely involved in these processes.
Using genetic fate mapping techniques, Nomura et al. find that normally quiescent ependymal cells and non-proliferating astrocytes acquire the ability to interconvert their cellular properties and location when the niche is perturbed, for example by a mild injury due to intraventricular injection of neuraminidase (Del Carmen Gomez-Roldan et al., 2008) or by disrupting Notch and Eph signaling. A small fraction of converted cells in each lineage (approximately 1% of astrocytes and 5% of ependymal cells monitored) appear to change their phenotype following neuraminidase-mediated damage. Most of the astrocytes that transform lose expression of the protein glial fibrillary acidic protein (GFAP) and gain the characteristics of ependymal cells, including becoming multiciliated and interposed in the ependymal cell layer. Conversely, lineage-labeled ependymal cells begin to express the astrocytic markers GFAP and glutamine synthetase but remain in the ependymal layer and retain features of ependymal cells, such as S100β expression. However, some ependymal cells that acquire astrocytic properties relocate to the subventricular zone, downregulate S100β, and take on morphological features of astrocytes in this region. Thus, cues derived from the new environment following a change in niche location may help drive full commitment to the new cell fate. Importantly, the mutual interconversion of ependymal cell and astrocyte populations occurs in the absence of cell proliferation, indicating that these are purely cell fate changes. This form of niche plasticity likely serves to balance these two niche cell populations when cells from each lineage are damaged or lost.
At the core of these cellular interconversion events is signaling through Notch and EphB receptors. Both of these receptor systems rely on membrane-tethered ligands on adjacent cells to trigger their activation. Canonical Notch signaling is critical for maintaining cell fate and quiescence of ependymal cells near the subventricular zone (Carlen et al., 2009), while EphB signaling regulates stem cell proliferation and survival as well as neuroblast migration (Conover et al., 2000; Furne et al., 2009; Genander and Frisen, 2010; Theus et al., 2010). Interestingly, Nomura et al. reveal that EphB2 expression in ependymal cells is positively regulated by Notch signaling in the normal brain. Following injury to the lateral ventricles, however, EphB2 levels are significantly reduced. Surprisingly, Notch signaling remains intact, indicating that another mechanism governs EphB2 expression in ependymal cells following injury. Inflammatory cytokines that are released as a result of brain injury may be among the factors that downregulate the levels of EphB receptors (including EphB2 and EphB3) in the niche to promote cellular conversion and stem/progenitor cell proliferation (Del Carmen Gomez-Roldan et al., 2008; Theus et al., 2010). It still remains to be determined if EphB receptor downregulation also plays a role in the astrocyte transformation to ependymal cells observed by Nomura and co-workers. Importantly, restoring EphB2 levels after injury or following inhibition of Notch signaling prevents conversion of ependymal cells to astrocytes, indicating that the loss of EphB signaling is directly involved in the transformative process of ependymal cells.
Multiple Eph receptors and ephrin ligands are expressed in combinatorial patterns in both ependymal cells and astrocyte-type cells of the subventricular zone (Conover et al., 2000; Furne et al., 2009; Genander and Frisen, 2010; Nomura et al., 2010; Theus et al., 2010). Nomura et al. found that global loss of EphB signaling causes a similar restructuring of the niche as mild injury, and promotes the conversion of ependymal cells to astrocytes and vice versa. Directly interfering with the ability of EphB receptors to transduce signals within ependymal cells (known as forward signaling) also triggers the cellular conversion event. In contrast, blocking the ability of the transmembrane ephrin-B ligands to convey their own signals within the cells through their cytoplasmic domain (known as reverse signaling) fails to convert ependymal cells to astrocytes. Thus, only signaling events downstream of EphB receptors are needed to preserve ependymal cell and astrocyte identity in the niche. The exact nature of these EphB signaling events still remains to be identified. Perhaps, EphB2 promotes the function or expression of genes involved in maintaining ependymal wall integrity, such as Numb and Numb-like (Kuo et al., 2006), or of Sox2, a stem cell transcription factor also expressed in ependymal cells and that has been recently linked to EphB2 signaling (Parrinello et al., 2010). Interestingly, EphB receptors as well as both Numb and Sox2 are known to promote cadherin-dependent intercellular adhesion, which may become disrupted following neuraminidase treatment of the ependymal layer (Del Carmen Gomez-Roldan et al., 2008). Thus, EphB signaling may be part of an active feedback loop that constitutively stabilizes the stem cell niche.
The full repertoire of Eph receptors and ephrins that participate in these processes still remains unclear. Is there molecular redundancy in the system or do different members of the large Eph receptor and ephrin families have distinct roles? It also is uncertain how the cell fate changes mediated by the ephrin-B/EphB system in ependymal cells and astrocytes interface with the previously reported roles of these proteins in decreasing Akt activation, promoting p53 expression, inhibiting proliferation of subventricular zone progenitors and regulating neuroblast migration (Conover et al., 2000; Furne et al., 2009; Theus et al., 2010). Furthermore, the impact of perturbation of EphB signaling on other niche cell types, such as microglia and vascular cells, requires further investigation.
The work by Nomura et al. confronts several unresolved aspects of niche homeostasis, and importantly uncovers some of the major signaling pathways involved. Whether or not these mechanisms play a role in niche plasticity following other brain injuries or during processes such as aging remains to be determined. However, the present findings along with other recent studies highlight the tremendous self-preservation capacity of adult neurogenic centers in the brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carlen M, Meletis K, Goritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Barnabe-Heider F, Yeung MS, Naldini L, et al. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat Neurosci. 2009;12:259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- Conover JC, Doetsch F, Garcia-Verdugo JM, Gale NW, Yancopoulos GD, Alvarez-Buylla A. Disruption of Eph/ephrin signaling affects migration and proliferation in the adult subventricular zone. Nature Neuroscience. 2000;3:1091–1097. doi: 10.1038/80606. [DOI] [PubMed] [Google Scholar]
- Del Carmen Gomez-Roldan M, Perez-Martin M, Capilla-Gonzalez V, Cifuentes M, Perez J, Garcia-Verdugo JM, Fernandez-Llebrez P. Neuroblast proliferation on the surface of the adult rat striatal wall after focal ependymal loss by intracerebroventricular injection of neuraminidase. J Comp Neurol. 2008;507:1571–1587. doi: 10.1002/cne.21618. [DOI] [PubMed] [Google Scholar]
- Furne C, Ricard J, Cabrera JR, Pays L, Bethea JR, Mehlen P, Liebl DJ. EphrinB3 is an anti-apoptotic ligand that inhibits the dependence receptor functions of EphA4 receptors during adult neurogenesis. Biochim Biophys Acta. 2009;1793:231–238. doi: 10.1016/j.bbamcr.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genander M, Frisen J. Ephrins and Eph receptors in stem cells and cancer. Curr Opin Cell Biol. 2010;22:611–616. doi: 10.1016/j.ceb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Goritz C, Catchpole T, Henkemeyer M, Frisen J. EphB signaling controls lineage plasticity of adult neural stem cell niche cells. Cell Stem Cell. 2010;7(this issue) doi: 10.1016/j.stem.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrinello S, Napoli I, Ribeiro S, Digby PW, Fedorova M, Parkinson DB, Doddrell RD, Nakayama M, Adams RH, Lloyd AC. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell. 2010;143:145–155. doi: 10.1016/j.cell.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theus MH, Ricard J, Bethea JR, Liebl DJ. EphB3 limits the expansion of neural progenitor cells in the subventricular zone by regulating p53 during homeostasis and following traumatic brain injury. Stem cells (Dayton, Ohio) 2010;28:1231–1242. doi: 10.1002/stem.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]


