Abstract
Background
The growing number of novel candidate molecules for the treatment of allergic diseases imposed a dramatic increase in the demand for animal experiments to select immunogenic vaccines, a pre-requisite for efficacy. Because no in vitro methods to predict the immunogenicity of a protein are currently available, we developed an in vitro assay that exploits the link between a protein's immunogenicity and its susceptibility to endolysosomal proteolysis.
Methodology
We compared protein composition and proteolytic activity of endolysosomal fractions isolated from murine bone marrow- and human blood- derived dendritic cells, and from the dendritic cell line JAWS II. Three groups of structurally related antigen variants differing in their ability to elicit immune responses in vivo (Bet v 1.0101 and Bet v 1.0401, RNases A and S, holo- and apo-HRP) were subjected to in vitro simulated endolysosomal degradation. Kinetics and patterns of generated proteolytic peptides were evaluated by gel electrophoresis and mass spectrometry.
Results
Antigens displaying weak capacity of T cell priming in vivo were highly susceptible to endolysosomal proteases in vitro. As proteolytic composition, activity, and specificity of endolysosomal fractions derived from human and murine dendritic cells were comparable, the JAWS II cell line could be used as a substitute for freshly isolated human or murine cells in in vitro degradation assays.
Conclusions
Endolysosomal fractions prepared from the JAWS II cell line provide a reliable tool for in vitro estimation of protein immunogenicity. The rapid and simple assay described here is very useful to study the immunogenic properties of a protein, and can help to replace, reduce, and refine animal experiments in allergy research and vaccine development in general.
Introduction
The development of a novel vaccine is a highly complex and demanding process that, from the initial concept to a licensed product, can take up to decades. Once a candidate has evolved in the laboratory, it undergoes vast series of pre-clinical in vitro and in vivo examination and optimization procedures. Evidently, only a minority of candidates passes all these obstacles, is permitted to clinical trials, accepted by regulatory agencies, and converted into a commercial product. The development of allergy vaccines faces additional problems, because unlike prophylactic vaccination, allergen-specific immunotherapy (SIT) attempts to counteract an already established pathological immune response [1]. Severe anaphylactic side effects can result from interactions between the administered vaccine and allergen-specific IgE antibodies of the atopic patient. Moreover, the current use of extracts of undefined contents can lead to sensitizations against new allergens during conventional immunotherapy [2]. Thus, allergy research today focuses on strategies to improve both, safety and clinical efficacy of SIT.
Others and we have proposed the substitution of allergen extracts in immunotherapy by molecule-based vaccines in order to implement safer and patient-tailored treatment [2], [3]. However, in contrast to infectious disease antigens, many allergens have been reported to be weak immunogens [4]–[7], a property hampering therapeutic success. Notably, it has been shown that allergen isoforms can differ by both immunogenicity (T cell reactivity) and allergenicity (IgE reactivity). For example, the birch pollen major allergen Bet v 1 isoform 0401 activates T cells much more efficiently than Bet v 1.0101 [8] but displays reduced IgE reactivity (hypoallergen) [4], [9], [10]. As molecules with such properties would bypass IgE mediated side effects during SIT, they are considered ideal allergy vaccines. Besides naturally occurring hypoallergens, modern DNA technology facilitates genetic engineering of recombinant hypoallergens [1]–[3], [11], [12]. However, the structural manipulation required for hypoallergen generation [11] and the production procedures [13] can severely affect the immunogenicity of a recombinant protein [14]–[17]. For example, although differing only by a single amino acid, one out of two in silico designed recombinant Bet v 1 mutants with reduced IgE reactivity lost its immunogenicity in mice [18]. Moreover, from 400 chimera generated by DNA shuffling of 14 major tree pollen allergens, only 2 fulfilled the requirements for efficient vaccine candidates [19]. The screening of such large candidate libraries requires high-throughput methods. Although IgE reactivity can be easily evaluated by antibody-based in vitro experiments in microtiter format, such tests are lacking for immunogenicity assessment. The prediction of T cell reactivity (immunogenicity) is costly, time-consuming, can only be performed for a limited number of molecules, and depends on in vivo or cell-based ex vivo systems. Nevertheless, ever since the first purified recombinant allergen was available in the early 1990s [20], a multitude of proteins has been produced as candidates for SIT [3], [11], [12]. Hence, the fast development of new molecule-based allergy vaccines dramatically increases the demand for animal sacrifice, conflicting the 3Rs declaration of the European Partnership for Alternative Approaches to Animal Testing (EPAA).
Within the present study we established a degradation assay based on earlier studies showing that susceptibility to endolysosomal proteolysis by antigen presenting cells (APC) serves as in vitro marker for protein immunogenicity [15], [16]. This assay enables the pre-selection of molecules with highest immunogenicity out of a big repertoire of related candidate proteins, hence aiming to replace, reduce, and refine animal experiments in allergy vaccine development. Whereas previous work solely focused on the kinetics of endolysosomal protein decomposition, we also evaluated the in vitro generated antigen-derived peptides and performed comparative fingerprinting of microsomal proteases. Moreover, we compared the degradative potential of different types of human and murine DC's microsomes. In addition to previously investigated antigens (i.e. ribonucleases A and S, as well as holo- and apo-horseradish peroxidase) [15], [16], we included two well-described allergens, i.e. the high and low allergenic isoforms of the birch pollen major allergen Bet v 1.0101 and Bet v 1.0401 [8], to evaluate the general applicability of the assay. As source for the endolysosomal hydrolases we employed a commercially available murine dendritic cell (DC) line [21]. This approach enables high-throughput screening for immunogenic candidates in vaccine development saving time, costs, human blood donors, and animal sacrifice, and might be interesting for any study on protein immunogenicity.
Methods
Subjects
All blood-donors gave written consent before enrollment in this study, which was approved by the local Medical Ethical Committee of Vienna.
Mice
BALB/c mice were obtained from Charles River Laboratories (Wilmington, MA). All animal experiments were conducted according to National guidelines approved by the Austrian Ministry of Science (BMWF-66.012/0011-II/10b/2010).
Antigens
Recombinant Bet v 1.0101 (SwissProt accession: P15497) and Bet v 1.0401 (SwissProt accession: P43177) were produced in Escherichia coli as recently published [8]. Ribonuclease (RNase A (SwissProt accession: P61823), RNase S, and holo-HRP (SwissProt accession: P00433) were purchased from Sigma-Aldrich, St. Louis, MO. Apo-HRP was prepared according to previously described protocols [15].
Generation and culture of Dendritic Cells
Human mDCs (monocyte-derived DCs) obtained from heparinized blood samples and murine BMDCs (bone marrow-derived DCs) were generated and cultured as described elsewhere [8], [22]. The DC line JAWS II that has been established from bone marrow cells of a p53 knockout C57BL/6 mouse, was purchased from American Type Culture Collection (Manassas, VA) and cultured as previously described [21].
Subcellular Fractionation and Fingerprinting of Microsomes
Endosomes and lysosomes were isolated from DCs by differential centrifugation [16]. Briefly, cells (107 cells/protein) were homogenized in 10 mmol L−1 Tris/acetate pH 7 containing 250 mmol L−1 sucrose using a Dounce tissue grinder (Sigma-Aldrich, St. Louis, MO) and centrifuged for 10 minutes at 6,000× g. To obtain a total microsomal fraction, postnuclear supernatants were ultracentrifuged (60 minutes at 100,000× g). Microsomal content was released by 5 freeze-thaw cycles on liquid nitrogen and room temperature respectively, and stored at −20°C. For microsome fingerprinting 100 ng of microsomal proteins were reduced, alkylated, and trypsinized using the Calbiochem® ProteoExtract® All-in-One Trypsin digestion kit (EMD, Gibbstown, NJ) before injection into a one-dimensional capillary HPLC system (Model U3000, Dionex Benelux, Amsterdam, The Netherlands), equipped with a low-pressure gradient micro-pump, a micro-autoinjector and a capillary PS-DVB monolithic separation column (150×0.2 mm id). A 300 min gradient of 0–40% ACN in 0.05% aqueous TFA was applied using a flow rate of 1 µl min−1. The chromatographic setup was coupled to an ESI-LTQ Orbitrap mass analyzer (Thermo Fisher Scientific GmbH, Bremen, Germany). Each sample was analyzed in triplicate, while the peptides identified in one run were excluded from data dependent decisions in the following runs by the use of exclusion lists. Spectra were generated in positive mode in a mass range of m/z 500–2000. Fragmentation of a maximum of three precursors was realized in the ion trap using collision-induced dissociation. The Mascot search engine (Matrix Science, London, UK) and the software Proteome Discoverer (Thermo Fisher Scientific GmbH, Bremen, Germany) were used for peptide identification with the following parameters: taxonomy, all entries; Variable modification, methionine oxidation; fixed modification, carbamidomethyl (C), Enzyme, trypsin; peptide tolerance, ±10 ppm; MS/MS tolerance, ± 0.3 Da; maximum missed cleavages, 1; Human and murine samples were searched against species-specific SwissProt databases.
Degradation Assays
Endolysosomal degradation assays were performed with 0.25 µg µl−1 of substrates (Bet v 1.0101, Bet v 1.0401, RNase A, RNase S, holo-HRP, apo-HRP) and 0.4 µg µl−1 of isolated microsomal proteins in a final volume of 20 µl containing 100 mmol L−1 citrate buffer pH 4.8 and 2 mmol L−1 dithiothreitol. Reactions were conducted for 0, 0.5, 1, 3, 5, 12, 24, 36, and 48 h at 37°C and stopped by boiling for 5 min at 95°C followed by freezing at −20°C. Alternatively, in vitro degradations were performed using 5×10−4 U µl−1 of purified human Cathepsin S purchased from Sigma-Aldrich, St. Louis, MO. Assays were quantitatively evaluated by flatbed scanner densitometry of GelCode® Blue Reagent (Thermo Scientific, Waltham, MA) stained sodium dodecyl sulfate polyacrylamide gels [23]. Qualitative analysis was performed by mass spectrometry using an ESI-QTOF mass spectrometer fitted with a capillary reversed phase HPLC (Waters, Milford, MA) as described elsewhere [22].
Results
Human and Murine DC-Derived Microsomes Display Comparable Endolysosomal Degradomes
To conduct endolysosomal in vitro degradation assays we isolated total microsomal (endolysosomal) fractions from human mDCs, murine BMDCs, and the murine JAWS II DC line employing a differential centrifugation protocol. Endolysosomal total proteomes were analyzed by mass spectrometry-based fingerprinting. 600 different proteins were detected in the microsomal fractions of all three different DC samples. Proteins belonging to the endolysosomal degradome or that have been shown to be involved in antigen processing are listed in Table 1. Cathepsins A, B, C, D, L, S, and Z as well as lysosomal prolylcarboxypeptidase and tripeptidyl peptidase 1 could be identified in all microsomal fractions. By contrast, cathepsin K, lysosomal dipeptidyl peptidase 2, and asparagine endopeptidase (AEP) were not detectable in BMDCs, and cathepsin H was not measured in the DC line. In summary, JAWS II DCs contain all important lysosomal endo- and exopeptidases that have been shown to be involved in antigen processing [24], [25]. Besides proteases, endolysosomal fractions also contained a multitude of other molecules (non-proteolytic acidic hydrolases, membrane-associated proteins, and GTPases involved in vesicular trafficking) that are associated with endosomal and lysosomal compartments of APCs (Table 1).
Table 1. Mass spectrometry based fingerprinting of DC-derived endolysosomal fractions.
| Protein | mDC | BMDC | JAWS II | SwissProt accession | ||||
| pep. | % | pep | % | pep | % | human | murine | |
| Endoproteases | ||||||||
| Cathepsin D | 20 | 60.44 | 16 | 59.76 | 20 | 63.17 | P07339 | P18242 |
| Cathepsin K | - | - | - | - | 6 | 41.03 | - | P55097 |
| Cathepsin L | 4 | 29.73 | 7 | 42.81 | 2 | 15.87 | P07711 | P06797 |
| Cathepsin S | 13 | 48.94 | 9 | 54.12 | 10 | 47.94 | P25774 | O70370 |
| AEP (legumain) | - | - | 4 | 23.45 | 2 | 10.11 | - | O89017 |
| Endo- and Exoproteases | ||||||||
| Cathepsin B | 21 | 54.57 | 17 | 55.16 | 12 | 47.79 | P07858 | P10605 |
| Cathepsin H | 7 | 35.22 | 6 | 27.93 | - | - | P09668 | P49935 |
| Exoproteases | ||||||||
| Cathepsin A | 7 | 24.38 | 16 | 36.50 | 15 | 41.98 | P10619 | P16675 |
| Cathepsin C | 13 | 41.25 | 10 | 29.22 | 11 | 32.47 | P53634 | P97821 |
| Cathepsin Z | 9 | 41.25 | 12 | 50.33 | 13 | 49.67 | Q9UBR2 | Q9WUU7 |
| Dipeptidyl peptidase 2 | - | - | 2 | 6.32 | 3 | 9.88 | - | Q9ET22 |
| Prolylcarboxypeptidase | 6 | 16.73 | 4 | 21.59 | 3 | 10.59 | P42785 | Q7TMR0 |
| Tripeptidyl peptidase 1 | 7 | 25.93 | 6 | 23.31 | 5 | 23.21 | O14773 | O89023 |
| Non-proteolytic acidic hydrolases | ||||||||
| Acid lipase | 11 | 37.84 | 6 | 21.66 | 7 | 29.22 | P38571 | Q9Z0M5 |
| Acid phosphatase | 3 | 7.8 | 4 | 12.77 | 4 | 13.71 | P11117 | P24638 |
| Alpha glucosidase | 13 | 25.32 | 6 | 11.54 | 7 | 13.85 | P10253 | P70699 |
| Alpha mannosidase | 20 | 27.1 | 24 | 34.85 | 2 | 3.16 | O00754 | O09159 |
| Endolysosomal membrane-associated proteins | ||||||||
| Clathrin | 54 | 49.91 | 59 | 55.34 | 46 | 46.93 | Q00610 | Q68FD5 |
| LAMP 1 | 4 | 9.59 | 7 | 17.98 | 5 | 14.53 | P11279 | P11438 |
| LAMP 2 | 5 | 11.22 | 4 | 9.4 | 3 | 7.95 | P13473 | P17047 |
| LMP 2 | 3 | 10.46 | 3 | 12.97 | 4 | 16.74 | Q14108 | O35114 |
| VAMP 7 | 3 | 26.82 | 2 | 18.64 | - | - | P51809 | P70280 |
| GTPases involved in vesicular trafficking | ||||||||
| ARF 6 | 6 | 49.14 | 5 | 44.57 | - | - | P62330 | P62331 |
| Rab 5a | 3 | 20.93 | 3 | 16.28 | 4 | 27.44 | P20339 | Q9CQD1 |
| Rab 5c | 9 | 66.20 | 9 | 67.13 | 7 | 61.11 | P51148 | P35278 |
| Rab 7a | 14 | 57.97 | 13 | 72.46 | 11 | 64.73 | P51149 | P51150 |
%, percentage to which identified peptides cover the full length protein sequence; AEP, Asparagine endopeptidase; ARF, ADP-ribosylation factor; LAMP, Lysosome-associated membrane glycoprotein; LMP, lysosome membrane protein; pep., identified peptides; Rab, Ras-like protein; VAMP, Vesicle-associated membrane protein.
Kinetics of Endolysosomal Degradation Differ Between Structural Variants of the Same Antigen
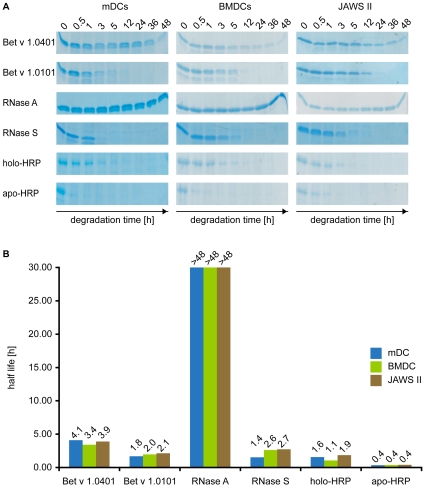
We compared the kinetics of endolysosomal decomposition for three pairs of antigens, i.e. structural variants of horseradish peroxidase (HRP), ribonuclease (RNase), and the major birch pollen allergen Bet v 1. The comparison of the average half lives (given in parenthesis) during endolysosomal in vitro degradation revealed that Bet v 1.0401 (3.8 h), RNase A (>48 h), and holo-HRP (1.5 h) were more resistant to proteolysis than Bet v 1.0101 (2 h), RNase S (2.2 h), and apo-HRP (0.4 h), respectively (Figures 1A and 1B). Thus, the decomposition of Bet v 1.0401 was around two-fold slower than observed for Bet v 1.0101. For the holo/apo-HRP and RNase A/S pairs endolysosomal half-life ratios were 3.8 and >20, respectively.
Figure 1. Kinetics of endolysosomal proteolysis.
2.5 µg of protein samples were analyzed by SDS-PAGE and GelCode® Blue staining after 0, 0.5, 1, 3, 5, 12, 24, 36 and 48 h of in vitro degradation using endolysosomal fractions isolated from human mDCs, murine BMDCs, and the DC line JAWS II (A). For each protein, the half-life during endolysosomal proteolysis was calculated from scanned and densitometrically quantified protein bands (B).
Structural Variants of the Same Antigen Show Similar Patterns of Proteolytic Fragments
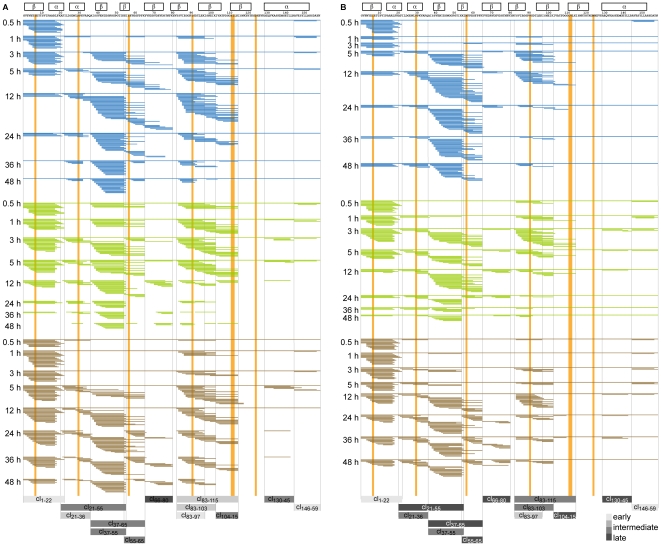
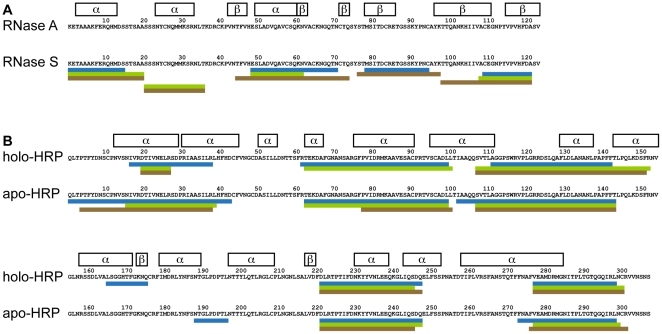
Mass spectrometry-based analysis showed that peptides generated by endolysosomal in vitro proteolysis formed nested clusters sharing a common central core but displaying variable flanking regions. These features are characteristic for MHC (major histocompatibility complex) class II-bound peptides [22], [26]. Notably, peptides derived from both Bet v 1.0101 and Bet v 1.0401 were of 5 to 30 amino acids in length and clustered into 13 different regions along the Bet v 1 sequence. However, central (region Bet v 121–65 and Bet v 183–115) and C-terminal (region Bet v 1130–159) peptide clusters of the more stable isoallergen Bet v 1.0401 appeared temporally delayed. Interestingly, amino acids differing between the two Bet v 1 isoforms were rather located apart from proteolytic cutting sites in central regions of the peptide clusters (Figures 2A and 2B). Proteolytic fragments from both holo- and apo-HRP clustered in 5 analogous regions of the full-length molecules. Due to its high resistance to endolysosomal hydrolases, we could not detect any peptides from RNase A, whereas proteolytic fragments from RNase S were found in 5 different regions (Figure 3). According to our observations, differences in proteolytic resistance between pairs of antigens with similar sequence and conserved fold do not translate into altered patterns of proteolytic fragments.
Figure 2. Chronology of Bet v 1 peptide cluster formation during endolysosomal proteolysis.
Peptides sequenced by mass spectrometry after 0.5, 1, 3, 5, 12, 24, 36, and 48 h of in vitro digestion with microsomal fractions from mDCs (blue), BMDCs (green) and JAWS II DCs (brown) are shown for Bet v 1.0101 (A) and Bet v 1.0401 (B), respectively. Regions of predominant peptide clusters (clx-x) are highlighted as bars colored in different shades of grey depending on their temporal occurrence (average appearance of early clusters: ≤1 h, intermediate clusters: >1 h and <5 h, and late clusters: ≥5 h. Bet v 1 secondary structures (α-helices and β-sheets) are indicated as framed boxes. Amino acids (n = 7) that differ between the 2 Bet v 1 isoforms are highlighted in orange.
Figure 3. Regions of peptide clusters generated by endolysosomal proteolysis.
Peptides sequenced by mass spectrometry after 0.5, 1, 3, 5, 12, 24, 36, and 48 h of in vitro digestion with microsomal fractions from mDCs (blue), BMDCs (green) and JAWS II DCs (brown) clustered in distinct regions along the protein sequence of RNase (A) and peroxidase (B) model antigens. Secondary structures (α-helices and β-sheets) are indicated as framed boxes.
Endolysosomal Degradation of Bet v 1 is Mediated by Cathepsin S
Strikingly, the majority of in vitro generated Bet v 1-derived peptide clusters (Figure 2) was flanked by lysine residues (K20, 54, 55, 65, 80, 97, 103, 115, and 129). This observation suggests that cathepsin S, a lysosomal cysteine endoprotease favoring substrates with glutamines or lysines at the P1 position [27], could be involved in Bet v 1 decomposition. To pursue this idea, we performed in vitro degradation assays of Bet v 1.0101 with purified cathepsin S, and identified 31 cathepsin S sites. As shown in Figure 4, cathepsin S sites seem to be progressively accessible by the protease in a time-dependent manner. Notably, 8 out of the 13 Bet v 1.0101 peptide clusters that appeared during endolysosomal proteolysis applying microsomal fractions could also be generated by cathepsin S alone (Figure 4). Initially occurring Bet v 1.0101 peptide clusters contained (cl1–22) or were flanked (cl146–59) by early-cleaved cathepsin S sites (Figure 4). Short-time degradation assays with human microsomes (data not shown) revealed that the N-terminal cluster cl1–22 appeared already after a few minutes of reaction. Thus, cathepsin S might be engaged in the initial steps of Bet v 1.0101 processing. Late-appearing peptide clusters, which corresponded to the middle region of the Bet v 1 sequence (cl83–115, cl83–103, and cl83–97), might be produced by cathepsin S and AEP, a cysteine endopeptidase that strictly cleaves on the C-terminal site of asparagine residues (e.g. Bet v 1.0101 N82) [24], [25].
Figure 4. Cathepsin S digest of Bet v 1.
Cathepsin S cleavage sites are depicted in the bar diagram showing their location in the Bet v 1.0101 sequence (x-axis) and the number of different peptides generated per cleavage site (y-axis). According to their temporal accessibility, cathepsin S sites are highlighted in red (early), orange (intermediate), and yellow (late) in the bar diagram. Generated peptides are shown as purple lines. Bet v 1.0101 secondary structures (α-helices and β-sheets) are indicated as framed boxes. Regions of Bet v 1.0101 peptide clusters (clx-x) generated by endolysosomal proteases isolated from DCs are depicted as grey boxes.
Discussion
The ability to efficiently induce an immune response is a most important quality of any protein vaccine. As no in vitro tools have been available so far, immunogenicity is usually assessed in vivo by animal experiments or by ex vivo cell-based systems. The immunogenicity of an antigen strongly depends on the character of its interaction with DCs. These specialized APCs constitute the interface between antigens and adaptive immunity. After internalization, DCs convert antigen into peptides that are bound to MHC class II molecules, transported to the cell surface, and presented to CD4+ T helper cells. Of note, there is evidence that various subsets of DCs differ by their capacities of antigen processing in vivo [28]. For instance, the CD8+ DEC205+ subset of murine splenic DC is more biased for cross-presentation on MHC class I molecules, whereas the CD8− DCIR2+ DC subset is specialized for MHC class II antigen presentation and displays increased expression of proteins involved in the exogenous antigen processing pathway, including cathepsins C, H, Z, and AEP. Further, it has been shown that compared to monocyte-derived DCs, conventional DCs are much more efficient in antigen processing and presentation independent from the route of antigen uptake [29].
Within the course of antigen processing, several steps (antigen uptake, DC activation, peptide generation, MHC-peptide complex stability and density) can be decisive regarding the immunogenicity of a given antigen. For instance, the internalization of ovalbumin conjugated to trimethyl chitosan by DCs was 5-fold increased leading to a 1,000-fold higher ovalbumin-specific IgG titers in immunized mice [30]. Apart from such adjuvant-dependent aspects, also properties intrinsic to the antigen (posttranslational modifications, structural features, and stability) can influence immunogenicity. For example, targeting receptor-mediated endocytosis, mannosylated ovalbumin induced a much stronger proliferation of ovalbumin-specific T cells than its unglycosylated counterpart [31]. Moreover, although structural discrepancies between isoforms of the birch pollen major allergen are minor (PDB accession numbers are given in parenthesis), uptake and endocytosis of Bet v 1.0101 (PDB: 1BV1) by murine BMDCs were significantly slower and less efficient than observed for Bet v 1.0401 (PDB: 3K78). The two isoforms also induced divergent patterns of DC activation and humoral responses, which have been attributed to cysteine-mediated dimerization due to a serine to cysteine exchange in Bet v 1.0401 [8]. In more detail, mice immunized with Bet v 1.0401 displayed a comparable IgE but a strongly elevated IgG and IgA response. On DCs Bet v 1.0401 elicited a significantly increased expression of CD80 and CD86 activation markers, whereas the secretion of cytokines antagonizing DC maturation and activation (IL-6) was enhanced in BMDCs stimulated with Bet v 1.0101. Notably, insufficient DC activation might be generally responsible for a shift towards the allergic immune response [32] and could in part explain hypoallergenicity of Bet v 1.0401.
Antigen uptake and DC activation might also affect the generation of T cell stimulatory peptides, a complex process that involves the sequential action of acidic hydrolases in endosomal and lysosomal compartments of the DC [24], [33]. Antigens that are rather stable to endolysosomal proteolysis can persistently provide peptides for binding to MHC molecules, thus ensuring efficient presentation to T cells and thus, high immunogenicity. By contrast, instable molecules fail to elicit an immune response due to rapid T cell epitope destruction [15], [16]. Hence, efficient antigen uptake of rather stable antigens might facilitate continuous delivery of peptides that can properly bind the MHC class II grove, thereby favoring a high density of MHC-peptide complexes on the surface of the DC for T cell presentation. Notably, the density of T cell epitopes is crucial for T cell activation [34] and could even be decisive in the development of a Th1 or allergic Th2 biased response [35]. Apart from such quantitative aspects, also the quality of peptides generated by endolysosomal proteases has a strong impact on the immunogenicity of proteins. For example, stabilization of MHC-peptide complexes by substituting single amino acids in a tumor antigen-derived T cell epitope increased specific T cell responses up to 50-fold [36]. Hence, the 7 amino acid exchanges in the Bet v 1.0401 isoform might also contribute to its stronger immunogenicity.
In the present study, we exploited the link between resistance to endolysosomal proteolysis and immunogenicity to establish a high-throughput screening procedure for rational protein vaccine development. Although the capacity of an antigen to induce an immune response depends on many parameters, according to our data susceptibility to endolysosomal proteases seems to be a key factor determining immunogenicity. Nevertheless, as cell-free endolysosomal in vitro degradations do not encompass important parameters of immunogenicity like antigen uptake, DC activation, and stability of the MHC-peptide complex, it cannot reflect the complex situation of antigen processing in vivo. Therefore, this method cannot be used for T cell epitope determination, and assessments on protein immunogenicity might deviate in some cases from in vivo obtained data. Still, the assay described here represents an excellent tool requiring only standard laboratory equipment, a tissue homogenizer, an ultracentrifuge, and a cell culture facility for rapid and simple assessment of protein immunogenicity. Because costs, time, up-scalability, and standardization problems limit the use of human as well as animal-derived DCs, we introduced the murine commercially available DC cell line JAWS II as source for endolysosomal proteases. Comparative mass spectrometry-based analysis of the protein composition provided strong evidence of the similarity between the different degradomes. To our knowledge, this represents the first study on the endolysosomal proteome from mice and men. We found that the proteome data nicely correlated with degradative activity and proteolytic specificity of the degradome using RNase A, HRP, and Bet v 1.0101 model antigens as substrates. Because structural features of proteins seem to be intimately connected to their immunogenic activity, as demonstrated earlier for structural variants of RNase A, HRP [15], [16], and Bet v 1 [8], we analyzed the kinetics and degradation patterns of RNase S, apo-HRP and Bet v 1.0401. Indeed, we observed remarkable differences in the proteolytic resistance and kinetics of degradation among the investigated antigens. It has been demonstrated that local structural flexibility is a general requirement for proteolytic sensitivity and that deletion of such elements enhances protein stability [37]. However, we did not observe a strict correlation between proteolytic cutting sites and loop structure elements in our model antigens and variants thereof. Of note, the flexible C-terminal loop of Bet v 1 (Bet v 1123–129) seems to be heavily degraded by lysosomal proteases, as we could not detect any in vitro generated peptides corresponding to this region. Thus, deletion of Bet v 1123–129 might increase Bet v 1.0101 stability and immunogenicity. Another possibility would be the targeting of frequently used and surface-exposed cathepsin S cleavage sites of Bet v 1.0101 (F19-K20, K65-Y66, G92-D93, K115-I116, and K134-A135) identified here.
Other factors influencing proteolytic sensitivity and immunogenicity are aggregation [8], integrity of the polypeptide backbone, and the presence of ligands [15], [16]. As described above, due to a serine to cysteine exchange at position 112, Bet v 1.0401 shows a high tendency to form disulfide-linked aggregates [8]. Presumably due to steric hindrance of proteolytic cutting sites, this isoform appears twice as stable in endolysosomal in vitro degradation assays than Bet v 1.0101. Accordingly, antibody and T cell responses are elevated in mice immunized with Bet v 1.0401. Similarly, RNase A induces more than 10,000-fold stronger T cell and IgG responses than RNase S, which is destabilized by a single subtilisin-mediated peptide bond cleavage between A20 and S21. During endolysosomal in vitro proteolysis RNase S was degraded more than 20-fold faster than RNase A. Moreover, apo-HRP lacking Ca2+ ions and the prosthetic heme group is a weak immunogen and displayed about 4-fold stronger sensitivity to endolysosomal proteases during in vitro degradation than fully equipped holo-HRP [15], [16].
In summary, our data convincingly showed that not only the proteome fingerprint but also the proteolytic activity in terms of specificity and kinetics of the analyzed degradomes is equivalent. Our findings are further supported by degradation experiments of Bet v 1.0101 using purified cathepsin S, an important endolysosomal protease. Remarkably, the patterns obtained by degradation with cathepsin S alone resembled those observed in reactions applying endolysosomal fractions. Despite the advantage of straightforward determination of proteolytic epitopes, the single protease approach cannot reliably mimic the complexity of a whole degradome since concerted action of proteases might be crucial in the kinetics of antigen degradation. As kinetics is the key indicator for assaying immunogenicity, this strategy would lack general applicability.
To conclude, the adoption of the JAWS II DC line for endolysosomal in vitro degradation facilitated the development of a simple, rapid, reliable, and easily upscalable comparative assay to select highly immunogenic allergy vaccine candidates from a pool of related molecules for pre-clinical evaluation. Data can be obtained within one week, and RNases A and S might serve as internal references. The method described here is very useful to study the immunogenic properties of a protein, and can help to replace, reduce, and refine animal experiments in allergy research and vaccine development in general.
Footnotes
Competing Interests: The role of Biomay AG in funding the research was via the Christian Doppler Research Association, a government Institution partially financed by industrial partners. This does not alter the authors‚ adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by the Christian-Doppler Research Association (CDG; www.cdg.ac.at), the Austrian Science Funds (www.fwf.ac.at; FWF Project L688), Biomay AG (www.biomay.com), and Land Salzburg (www.salzburg.gv.at). The funders had no rule in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rolland JM, Gardner LM, O'Hehir RE. Allergen-related approaches to immunotherapy. Pharmacol Ther. 2009;121:273–284. doi: 10.1016/j.pharmthera.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Egger M, Hauser M, Himly M, Wopfner N, Wallner M, et al. Development of recombinant allergens for diagnosis and therapy. Front Biosci (Elite Ed) 2009;1:77–90. doi: 10.2741/E9. [DOI] [PubMed] [Google Scholar]
- 3.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 4.Ferreira F, Hirtenlehner K, Jilek A, Godnik-Cvar J, Breiteneder H, et al. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J Exp Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linhart B, Jahn-Schmid B, Verdino P, Keller W, Ebner C, et al. Combination vaccines for the treatment of grass pollen allergy consisting of genetically engineered hybrid molecules with increased immunogenicity. Faseb J. 2002;16:1301–1303. doi: 10.1096/fj.01-1012fje. [DOI] [PubMed] [Google Scholar]
- 6.Saarelainen S, Zeiler T, Rautiainen J, Narvanen A, Rytkonen-Nissinen M, et al. Lipocalin allergen Bos d 2 is a weak immunogen. Int Immunol. 2002;14:401–409. doi: 10.1093/intimm/14.4.401. [DOI] [PubMed] [Google Scholar]
- 7.Scheiblhofer S, Stoecklinger A, Gruber C, Hauser-Kronberger C, Alinger B, et al. Gene gun immunization with clinically relevant allergens aggravates allergen induced pathology and is contraindicated for allergen immunotherapy. Mol Immunol. 2007;44:1879–1887. doi: 10.1016/j.molimm.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Zaborsky N, Brunner M, Wallner M, Himly M, Karl T, et al. Antigen aggregation decides the fate of the allergic immune response. J Immunol. 2010;184:725–735. doi: 10.4049/jimmunol.0902080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smole U, Balazs N, Hoffmann-Sommergruber K, Radauer C, Hafner C, et al. Immunobiology; 2010. Differential T-cell responses and allergen uptake after exposure of dendritic cells to the birch pollen allergens Betv1.0101, Betv1.0401 and Betv1.1001. [DOI] [PubMed] [Google Scholar]
- 10.Wagner S, Radauer C, Bublin M, Hoffmann-Sommergruber K, Kopp T, et al. Naturally occurring hypoallergenic Bet v 1 isoforms fail to induce IgE responses in individuals with birch pollen allergy. J Allergy Clin Immunol. 2008;121:246–252. doi: 10.1016/j.jaci.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Mutschlechner S, Deifl S, Bohle B. Genetic allergen modification in the development of novel approaches to specific immunotherapy. Clin Exp Allergy. 2009;39:1635–1642. doi: 10.1111/j.1365-2222.2009.03317.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallner M, Briza P, Thalhamer J, Ferreira F. Specific immunotherapy in pollen allergy. Curr Opin Mol Ther. 2007;9:160–167. [PubMed] [Google Scholar]
- 13.Wallner M, Himly M, Neubauer A, Erler A, Hauser M, et al. The influence of recombinant production on the immunologic behavior of birch pollen isoallergens. PLoS One. 2009;4:e8457. doi: 10.1371/journal.pone.0008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carra JH, Welcher BC, Schokman RD, David CS, Bavari S. Mutational effects on protein folding stability and antigenicity: the case of streptococcal pyrogenic exotoxin A. Clin Immunol. 2003;108:60–68. doi: 10.1016/s1521-6616(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 15.Delamarre L, Couture R, Mellman I, Trombetta ES. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med. 2006;203:2049–2055. doi: 10.1084/jem.20052442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–1634. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 17.Vidard L, Rock KL, Benacerraf B. Diversity in MHC class II ovalbumin T cell epitopes generated by distinct proteases. J Immunol. 1992;149:498–504. [PubMed] [Google Scholar]
- 18.Thalhamer T, Dobias H, Stepanoska T, Proll M, Stutz H, et al. Designing hypoallergenic derivatives for allergy treatment by means of in silico mutation and screening. J Allergy Clin Immunol. 2010;125:926–934 e910. doi: 10.1016/j.jaci.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 19.Wallner M, Stocklinger A, Thalhamer T, Bohle B, Vogel L, et al. Allergy multivaccines created by DNA shuffling of tree pollen allergens. J Allergy Clin Immunol. 2007;120:374–380. doi: 10.1016/j.jaci.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Ferreira FD, Hoffmann-Sommergruber K, Breiteneder H, Pettenburger K, Ebner C, et al. Purification and characterization of recombinant Bet v I, the major birch pollen allergen. Immunological equivalence to natural Bet v I. J Biol Chem. 1993;268:19574–19580. [PubMed] [Google Scholar]
- 21.Jiang X, Shen C, Rey-Ladino J, Yu H, Brunham RC. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect Immun. 2008;76:2392–2401. doi: 10.1128/IAI.01584-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mutschlechner S, Egger M, Briza P, Wallner M, Lackner P, et al. Naturally processed T cell-activating peptides of the major birch pollen allergen. J Allergy Clin Immunol. 2010;125:711–718, 718 e711-718 e712. doi: 10.1016/j.jaci.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 23.Tan HY, Ng TW, Liew OW. Effects of light spectrum in flatbed scanner densitometry of stained polyacrylamide gels. Biotechniques. 2007;42:474–478. doi: 10.2144/000112402. [DOI] [PubMed] [Google Scholar]
- 24.Colbert JD, Matthews SP, Miller G, Watts C. Diverse regulatory roles for lysosomal proteases in the immune response. Eur J Immunol. 2009;39:2955–2965. doi: 10.1002/eji.200939650. [DOI] [PubMed] [Google Scholar]
- 25.Matthews SP, Werber I, Deussing J, Peters C, Reinheckel T, et al. Distinct protease requirements for antigen presentation in vitro and in vivo. J Immunol. 2010;184:2423–2431. doi: 10.4049/jimmunol.0901486. [DOI] [PubMed] [Google Scholar]
- 26.Lippolis JD, White FM, Marto JA, Luckey CJ, Bullock TN, et al. Analysis of MHC class II antigen processing by quantitation of peptides that constitute nested sets. J Immunol. 2002;169:5089–5097. doi: 10.4049/jimmunol.169.9.5089. [DOI] [PubMed] [Google Scholar]
- 27.Lutzner N, Kalbacher H. Quantifying cathepsin S activity in antigen presenting cells using a novel specific substrate. J Biol Chem. 2008;283:36185–36194. doi: 10.1074/jbc.M806500200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 29.Kamphorst AO, Guermonprez P, Dudziak D, Nussenzweig MC. Route of antigen uptake differentially impacts presentation by dendritic cells and activated monocytes. J Immunol. 2010;185:3426–3435. doi: 10.4049/jimmunol.1001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slutter B, Soema PC, Ding Z, Verheul R, Hennink W, et al. Conjugation of ovalbumin to trimethyl chitosan improves immunogenicity of the antigen. J Control Release. 143:207–214. doi: 10.1016/j.jconrel.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Lam JS, Mansour MK, Specht CA, Levitz SM. A model vaccine exploiting fungal mannosylation to increase antigen immunogenicity. J Immunol. 2005;175:7496–7503. doi: 10.4049/jimmunol.175.11.7496. [DOI] [PubMed] [Google Scholar]
- 32.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez PA, Carreno LJ, Coombs D, Mora JE, Palmieri E, et al. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. Proc Natl Acad Sci U S A. 2005;102:4824–4829. doi: 10.1073/pnas.0500922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buatois V, Baillet M, Becart S, Mooney N, Leserman L, et al. MHC class II-peptide complexes in dendritic cell lipid microdomains initiate the CD4 Th1 phenotype. J Immunol. 2003;171:5812–5819. doi: 10.4049/jimmunol.171.11.5812. [DOI] [PubMed] [Google Scholar]
- 36.Baratin M, Kayibanda M, Ziol M, Romieu R, Briand JP, et al. Amino acid modifications in the wild type sequence p53 232-240 overcome the poor immunogenicity of this self tumour epitope. J Pept Sci. 2002;8:327–334. doi: 10.1002/psc.391. [DOI] [PubMed] [Google Scholar]
- 37.Carmicle S, Steede NK, Landry SJ. Antigen three-dimensional structure guides the processing and presentation of helper T-cell epitopes. Mol Immunol. 2007;44:1159–1168. doi: 10.1016/j.molimm.2006.06.014. [DOI] [PubMed] [Google Scholar]