Abstract
We describe a reporter mouse strain designed to fate-map cells that have activated IL-17A. Here we show that TH17 cells show distinct plasticity in different inflammatory settings. Chronic inflammatory conditions in EAE caused a switch to alternative cytokines in TH17 cells, whereas acute cutaneous infection with Candida albicans, did not result in deviation of TH17 to alternative cytokine production, although IL-17A production was shut off in the course of the infection. During development of EAE, IFN-γ and other pro-inflammatory cytokines in the spinal cord were produced almost exclusively by ‘ex-TH17’ cells whose conversion was driven by IL-23. Thus, this model allows relating the actual functional fate of effector T cells to TH17 developmental origin irrespective of IL-17 expression.
INTRODUCTION
IL-17-producing TH17 cells, were classified as a new effector CD4+ T cells subset on the basis of being independent of the transcription factors GATA-3 and T-bet that together with the marker cytokines interferon γ (IFN-γ) and IL-4 define TH1 and TH2 cells respectively1,2. The identification of IL-6 and TGF-β as differentiation factors3 as well as RORγt and RORα as lineage-defining transcription factors4,5 finalized acceptance of TH17 as a separate subset. Nevertheless, it was clear early on that TH17 cells displayed considerable plasticity and readily acquired the capacity to produce IFN-γ in addition to IL-17 production or completely shut off IL-17 production in vitro6-10, explaining the initial erroneous assumption that these cells may have diverged from a common TH1 precursor11. While extensive plasticity was attributed to in vitro-generated TH17 cells, it was suggested that TH17 cells developing in vivo retain their phenotype12. As many additional stimuli influence TH17 differentiation, including cytokines as well as environmental factors acting through the aryl hydrocarbon receptor (reviewed in13), it is conceivable that the requirements for full effector differentiation of TH17 cells are not met in vitro. Thus, it would be advantageous to analyse more closely TH17 cells that have developed in vivo to determine whether plasticity is also detectable under these conditions. We therefore decided to generate a TH17 reporter system that would allow not only identification, but also fate mapping of these cells in vivo. To trace noninvasively cells expressing IL-17, we inserted a Cre recombinase into the Il17a locus (Il17aCre) and crossed these mice to Rosa26-enhanced yellow fluorescence protein (R26ReYFP) reporter mice. In this mouse strain the fluorescent reporter would permanently label Il17Cre cells, thus allowing identification of cells that switched on the IL-17 program as well as alternative effector programs that might have been activated.
Here we show that eYFP expression was induced exclusively under TH17 conditions and gradually increased during effector differentiation in vivo and terminally differentiated effector cells all co-expressed IL-17 and eYFP. However, TH17 cells rapidly lost IL-17A expression in the course of inflammatory immune responses in vivo. Fate determination of eYFP+ ‘ex-TH17’ cells under different inflammatory conditions revealed distinct capacities for alternative fates depending on whether the inflammatory conditions were chronic or acute and rapidly resolved. For instance, in the course of experimental autoimmune encephalomyelitis (EAE) induction eYFP+ cells resident in the inflamed spinal cord did not express IL-17A protein anymore and a considerable proportion of instead produced IFN-γ. In contrast, in acute cutaneous infection with Candida albicans, IL-17A production also was shut down, but there was no deviation of eYFP+ cells to IFN-γ production. In this model eYFP+ IL-17− cells appeared quiescent with respect to cytokine production and rapidly decayed.
This suggests that the fate decisions of TH17 cells are shaped by different inflammatory conditions in vivo allowing distinct patterns of plasticity. Whereas pathogenicity in chronic inflammatory conditions is linked to the expression of additional pro-inflammatory cytokines, clearance of an infection that results in resolution creates an anti-inflammatory environment that precludes TH17 plasticity and the adoption of alternative cytokines.
RESULTS
Generation of IL-17A fate reporter mouse
To obtain an IL-17A-specific reporter that would allow tracing of Il17a expressing cells we generated a ‘knockin’ mouse strain bearing Cre recombinase in the Il17a gene locus (Il17aCre, Supplementary Fig.1). Cre activity was monitored by breeding with R26ReYFP reporter mice, thus allowing the visualization of cells that had activated the IL-17 program irrespective of current production of this cytokine. Analysis of tissues from non-immune reporter mice revealed eYFP+ cells in haematopoietic cells of spleen and small intestine lamina propria in line with previously described distribution of IL-17A-producing T cells both of CD4+ and γδ T cell lineage4. Also a CD4−CD8− T cell receptor-positive NKT population in the spleen was marked by eYFP, but no reporter expression in NKp46+ cells was detected (Supplementary Fig.2a). A CD45+ population of lineage-negative cells containing lymphoid tissue-inducer-like (LTi) cells (reviewed in 14) some of which expressed CD4+ was also found marked by eYFP (Supplementary Fig.2b). In the skin a sizeable fraction of γδ T cells, but very few CD4+ T cells, were eYFP+, (Supplementary Fig.2c).
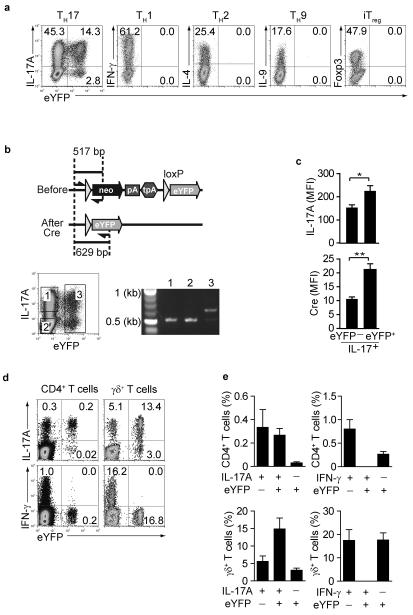
In vitro stimulation of FACS purified naïve CD4+ T cells under TH17 conditions generated a population of TH17 cells that were detectable by intracellular staining for IL-17A as well as eYFP expression. There was no induction of eYFP under conditions that led to TH1, TH2, TH9 or iTreg polarization (Fig.1a). Intracellular IL-17 expression without eYFP expression was exaggerated following restimulation with PdBU-ionomycin, which may induce early commitment to IL-17 production before full effector status is achieved. In contrast anti-CD3 stimulation showed a higher concordance between IL-17 and YFP expression (Supplementary Fig.3).
Figure 1. Induction of fate reporter eYFP+ cells in IL-17-producing cells.
(a) Naïve CD4+CD44loCD25− T cells were cultured under TH1, TH2, TH9, TH17 or iTreg conditions for 4 days and stained for indicated intracellular cytokines. Dot plots show intracellular cytokine expression vs eYFP (b) Schematic representation of PCR primer location used for assessment of Cre-mediated recombination at the ROSA26 eYFP locus. Cells cultured under TH17 conditions in vitro were sorted according to the gates indicated and DNA from sorted populations were tested for recombination at the ROSA26 eYFP locus. (c) mean fluorescence intensity for IL-17A(top) and Cre (bottom) in IL-17A+eYFP− and IL-17A+eYFP+ populations. Mean values and SD are shown. *denotes P value 0.03, ** denotes P value 0.01 (d) LN cells from non-immune mice were stained for CD4 and γδ TCR, followed by intracellular IL-17A and IFN-γ staining. Dot plots show intracellular staining vs eYFP expression. The FACS plots are representative for three independent experiments and bar graphs showing experimental variability are shown in (e).
To investigate whether this discrepancy was caused by aberrant expression of eYFP from the recombined R26ReYFP locus, we performed PCR from genomic DNA isolated from purified eYFP− CD4+ IL-17+ T cells (Fig.1b gate 1), eYFP IL-17A− CD4+ T cells (Fig.1b gate2) as well as eYFP+CD4+ T cells (Fig.1b gate 3). Analysis of the R26R26eYFP locus showed that eYFP− IL-17A+ cells had not undergone Cre-mediated recombination (gate 1) similar to cells that had not switched on IL-17A production at all (gate 2) and in marked contrast to eYFP+ cells in gate 3. Since the amount of intracellular IL-17A protein was lower in TH17 cells that failed to express eYFP, it suggests that levels of IL-17A transcription and therefore protein concentrations of Cre-recombinase were below a critical threshold to induce recombination (Fig.1c top panel). In line with this assumption, intracellular staining for Cre protein was much lower in eYFP− IL-17-producing cells compared with IL-17+ eYFP+ cells (Fig.1c bottom panel). About 50% of PdBU-ionomycin-stimulated TH17 cells isolated from naive mice co-expressed eYFP and IL-17A ex vivo, whereas more than 70% of γδ T cells showed co-expression of IL-17A and eYFP. Neither CD4+ nor γδ eYFP+ cells isolated from naive mice expressed IFN-γ (Fig. 1d,e). Thus, IL-17A is faithfully marked by eYFP once TH17 cells have acquired full effector function.
In vivo kinetics of eYFP and IL-17 expression
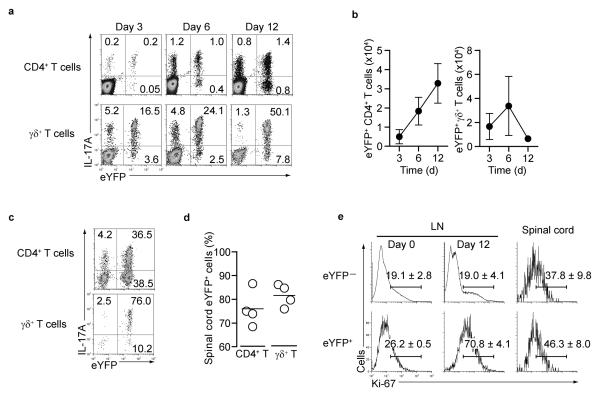
To evaluate the kinetics of eYFP reporter expression and the stability of IL-17 cytokine expression in vivo, mice were immunized with myelin oligodendrocyte glycoprotein (MOG) peptide in complete Freund's adjuvant (CFA) to induce experimental autoimmune encephalomyelitis (EAE), a TH17-dependent chronic autoimmune disease. IL-17A protein and eYFP expression in CD4+ T cells and γδ T cells isolated from draining lymph nodes of MOG-CFA immunized mice are shown (Fig.2a and Supplementary.Fig.4a). In both T cell subsets the percentage of double IL-17A eYFP expressing cells increased over time, with co-expression more pronounced and complete in γδ T cells than in TH17 cells. Absolute numbers of eYFP+ CD4+ TH17 cells increased over time, whereas eYFP+ γδ T cells, although becoming the dominant population within the total γδ T cell pool, did not increase in absolute numbers (Fig.2b). In the spinal cord of mice that succumbed to disease the vast majority of IL-17A-expressing T cells also expressed eYFP (Fig.2c,d and Supplementary Fig.4b), but γδ T cells represented only a minority of infiltrating T cells. Staining for Ki-67, a cellular marker for proliferation showed eYFP+ TH17 cells from the draining lymph node exhibited an increased proliferation rate compared to eYFP− cells at day 12 (Fig.2e). In contrast basal proliferation in non-immune mice (day 0) and proliferation in the spinal cord were similar in eYFP− and eYFP+ cells.
Figure 2. Kinetics of eYFP and IL-17A expression during EAE induction.
(a) Draining LN cells from MOG/CFA immunized mice were stained for CD4 and γδ TCR and assessed for eYFP and intracellular IL-17A at the indicated days after immunization. (b) Absolute numbers of eYFP+ CD4 and γδ T cells in the draining LN. Data show mean + SD from three mice. (c) Expression of eYFP and IL-17A of infiltrating CD4+ and γδ T cells in the spinal cord. (d) The percentage of eYFP positive CD4+ T cells or γδ T cells in the spinal cord (day 16 after EAE induction) is shown. The bars indicate mean derived from four immunized mice. Data in (a) and (c) represent three independent experiments. (e) FACS analysis for expression of the proliferation marker Ki-67 performed on gated CD4 eYFP− or eYFP+ cells from lymph node (day 0 and day 12) and spinal cord (day15) with experimental variation in three independent experiments indicated by % values shown in the plots.
A sizeable fraction of the eYFP+ TH17 cells in the draining lymph nodes were already negative for IL-17A early after immunization whereas most γδ T cells remained double positive for eYFP and IL-17A (Fig.2a). Similarly, in the spinal cord of mice with disease (around day 15) only a fraction of the CD4+ eYFP+ cells still expressed IL-17A protein, whereas most γδ T cells remained IL-17+ (Fig.2c).
IFN-γ expression by eYFP+ ‘ex-TH17’cells in EAE
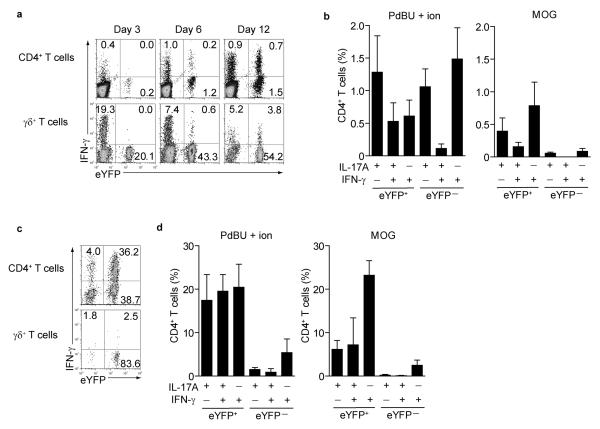
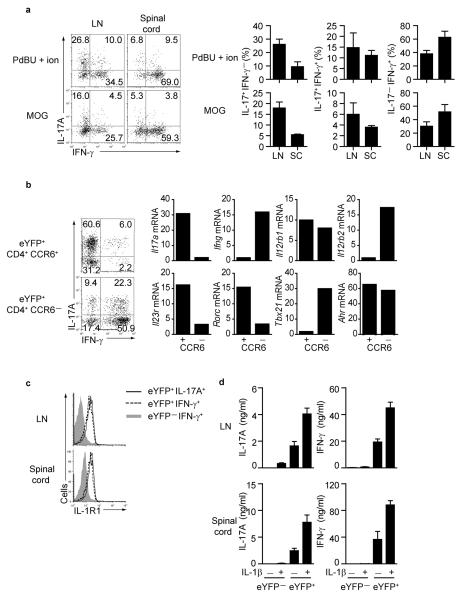
Other reports have shown the presence of IL-17A and IFN-γ double producers in inflamed tissues4,15, but their developmental origin has remained unclear. We therefore tested the source of IFN-γ producers in lymph nodes and spinal cord of MOG-CFA immunized mice. At day 3, CD4+ T cells in the draining lymph nodes expressed little IFN-γ and originated exclusively from eYFP− TH1 cells. A higher proportion of γδ T cells expressed IFN-γ, again mostly from the eYFP− fraction (Fig.3a left panels). IFN-γ expression increased over time primarily in eYFP+ CD4+ T cells, whereas IFN-γ expression decreased in γδ T cells (Fig.3a middle and right panels and Supplementary Fig.4a). We compared the effect of restimulation by PdBU-ionomycin with antigen-specific restimulation using MOG peptide or anti-CD3 restimulation and observed that antigen-specific re-stimulation of cells from draining lymph nodes resulted in much higher concordance between IL-17 expression and eYFP expression (representative FACS plots in Supplementary Fig.5a). In the spinal cord, half of the eYFP+ CD4+ T cells switched off IL-17A expression and virtually all IFN-γ in CD4+ T cells was derived from eYFP+ ‘ex-TH17’ cells. In contrast, eYFP+ γδ T cells remained IL-17A+ and did not switch to IFN-γ expression (Fig.3c and Supplementary Fig.5b). Restimulation with MOG peptide compared with PdBU-ionomycin (Fig.3d) confirmed that MOG -specific IFN-γ was mostly derived from ‘ex-TH17’ cells with only a minute fraction of eYFP− TH1 cells contributing to expression of this cytokine. There was no switch of eYFP+ cells to Foxp3 expression showing that eYFP+ cells that cease production of IL-17 do not deviate towards a regulatory phenotype (Supplementary Fig.5c). Thus, our data show that under chronic inflammatory conditions in EAE, TH17 cells rapidly switch to production of IFN-γ and represent the major source of this cytokine.
Figure 3. IFN-γ expression and antigen specificity in eYFP+ and eYFP− CD4 T cells.
(a) Draining LN cells from MOG/CFA immunized mice were stained for CD4 and γδ TCR and assessed for eYFP and intracellular IFNγ at the indicated days after immunization. (b) bar graphs showing % of eYFP+ or eYFP− CD4 T cells from draining lymph nodes at day 12 expressing cytokines following restimulation with PdBU-ionomycin (left panel) or MOG peptide (right panel). Mean values +/− SD of three individual mice are shown. (c) Representative FACS plots showing expression of eYFP and IFN-γ of infiltrating CD4+ and γδ T cells in the spinal cord. (d) bar graphs showing % of eYFP+ or eYFP− CD4 T cells from spinal cord at day 15 expressing cytokines following restimulation with PdBU-ionomycin (left panel) or MOG peptide (right panel).
CD4+ T cell cytokine production during EAE
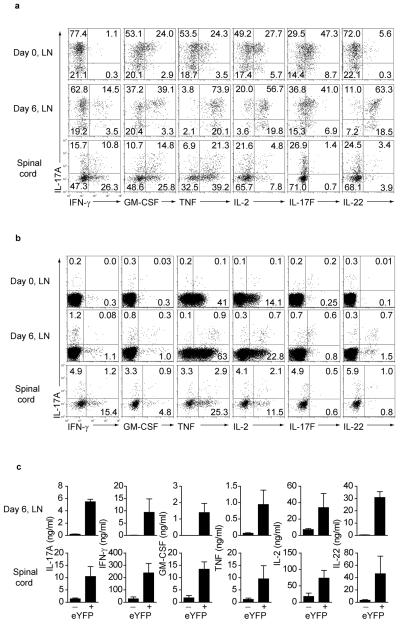
We next analysed both eYFP+ and eYFP− CD4+ T cells isolated from lymph nodes 6 days after MOG-CFA immunization and from spinal cord (day 15) to evaluate expression of additional cytokines. FACS plots and bar graphs are shown (Fig 4 and Supplementary Fig 6).
Figure 4. Cytokine expression in eYFP+ and eYFP− CD4+ T cells in the draining LN and spinal cord.
(a) Lymph nodes from non-immune mice (day 0) as well as draining LN and spinal cord cells 6 and 15 days after EAE induction, respectively, were stained for CD4 and intracellular cytokines as indicated. The dot plots show cytokine expression profiles in gated eYFP+ CD4+ T cells (a) and gated eYFP− CD4+ T cells in (b). The data are representative for at least three independent experiments. (c) Cytokine concentrations measured in supernatant of 2×104 sorted eYFP+ or eYFP− CD4 T cells isolated from day 6 lymph nodes or day 15 spinal cord and restimulated in vitro with anti-CD3/anti-CD28 for 24h. Data show mean values +/− SD of cytokines measured from three individual mice.
The majority of eYFP+ cells from lymph nodes of non-immunized mice expressed IL-17 without any IFN-γ and also showed some co-expression of GM-CSF, TNF, IL-2, IL-17F but very little IL-22 (Fig.4a top panels). A proportion of eYFP+ cells acquired IFN-γ expression at day 6 after immunization. These cells mostly co-expressed IL-17A and exhibited substantially increased expression of GM-CSF, IL-2 and IL-22. All IL-17+ cells co-expressed TNF at that stage, whereas expression of IL-17F remained at baseline levels (Fig.4a middle panels). In the spinal cord IL-17A expression was reduced and although co-expression of IL-17A with IFN-γ, GM-CSF and TNF was still evident, a sizeable proportions of cell produced the latter cytokines in the absence of IL-17A expression. Interestingly there was no IL-17F expression in the spinal cord population and greatly reduced proportions of IL-22-producing cells (Fig.4a bottom panel). With the exception of TNF and IL-2, the eYFP− fraction showed low expression of all cytokines (Fig.4b).
Measurement of cytokine protein concentrations from sorted, restimulated eYFP+ and eYFP− cells confirmed predominant cytokine secretion from the eYFP+ population. IL-17A, IFN-γ, GM-CSF and TNF were about 10-fold higher in the spinal cord compared to draining lymph nodes despite the lower expression profiles seen by intracellular staining (Fig.4c). The progressive development of eYFP+ cells from predominantly IFN-γ− IL-17A-secreting cells towards IFN-γ+ IL-17A+ cells and finally to IFN-γ expression alone suggests alternative cell fate decisions are adopted during effector cell development. Thus, pro-inflammatory cytokines produced by effector cells in the spinal cord were almost exclusively derived from ‘ex-TH17’ cells with no apparent contribution by TH1 cells.
Transcriptional changes in ‘ex-TH17’ cells
The eYFP+ population was heterogeneous consisting of both IL-17A+ IFN-γ− and IL-17A− IFN-γ+ cells. To distinguish these populations and to unequivocally prove that the source of IFN-γ was a cell with characteristics of a TH17 rather than a TH1 program, we used an adoptive transfer model. Because loss of IL-17A protein expression is accompanied by loss of CCR6 expression, we FACS purified CD4+ CCR6+_eYFP+ cells from draining lymph nodes of mice immunized four days previously with MOG-CFA. To optimize the cell yield and adoptive transfer we used reporter mice that expressed the 2D2 transgenic T cell receptor specific for MOG16. We then transferred 1 × 104 CCR6+ eYFP+ CD4+ T cells into RAG-1-deficient mice that were immunized with MOG-CFA to mimic the chronic inflammation of EAE. At the same time we performed single cell PCR from the starting inoculum of CCR6+ eYFP+ CD4+ T cells to determine ongoing transcription of Il17a and Ifng (Supplementary Table 1). About 30% of the adoptively transferred eYFP+ TH17 cells produced IFN-γ, in the lymph nodes compared to 60% in the spinal cord (Fig.5a). Single cells RT-PCR confirmed the majority of cells expressed Il17a and little Ifng at the time of transfer (Supplementary Table 1).
Figure 5. Transcriptional changes in eYFP+ CD4+ T cells.
(a) CCR6+ eYFP+ CD4+ T cells from 2D2 IL-17-reporter mice were purified 4 days after MOG-CFA immunization and adoptively transferred into immunized Rag-deficient mice. FACS plots and bar graphs of IL-17A and IFN-γ expression in eYFP+ CD4+ T cells from draining lymph nodes and spinal cord (day 16) restimulated with PdBU-ionomycin (upper panels) or MOG peptide (lower panels). Histograms show mean values for individual mice +/− SD.
(b) CCR6− and CCR6+ eYFP+ CD4+ T cells from spinal cord were sorted for qPCR analysis. Representative FACS plots show expression of IL-17A and IFN-γ. Relative gene expression in sorted (not restimulated) cells normalized to the expression of Hprt is shown. (c) CD4+eYFP−IFN-γ+, representing TH1, (shaded gray), CD4+eYFP+IFN-γ+ (ex-TH17 -dotted line), and CD4+eYFP+IL-17A+ (TH17- solid line) from draining LN and spinal cord cells 15 days after MOG-CFA immunisation were gated and assessed for IL-1R1 expression. The data represent at least three independent experiments. (d) Cytokine levels measured in supernatant of purified eYFP+ or eYFP− CD4 T cells from draining lymph nodes or spinal cord (day 15) restimulated with anti-CD3 +/− 20 ng/ml IL-1β for 24 hr. Data show mean values ± SD of cytokines from three individual mice. The data represent at least three independent experiments.
Next we induced EAE in reporter mice and isolated CD4+ CCR6+ eYFP+ and CD4+ CCR6− eYFP+ cells from the spinal cord to analyse their transcriptional profiles. As shown in the FACS plots of the sorted populations (Fig.5b), the eYFP+ CCR6+ population contained the most single IL-17A producers with few double producers of IFN-γ and IL-17A. In contrast, the eYFP+ CCR6− fraction contained the majority of double IFN-γ and IL-17A producers as well as IFNγ single producers but few IL-17A single producers. CCR6− eYFP+ cells downregulated mRNA for Il17a and upregulated Ifng consistent with the protein expression data. Il12rb1 mRNA was expressed at equal amounts in CCR6+ and CCR6− eYFP+ cells, whereas only CCR6− cells upregulated IL-12-specific Il12rb2 receptor gene expression and T-bet, while downregulating Rorgt and Il-23r. Similar transcriptional changes were reported previously in TH17 cells generated in vitro with the notable exception of IL-12Rβ2 which is not switched off in vitro7 but is absent on IL-17A expressing cells developing in vivo (Fig.5b). Importantly, IFN-γ producing ‘ex-TH17’ cells could be distinguished from TH1 producers of IFN-γ by AhR expression, which remained high in CCR6− eYFP+ cells and by IL-1R1 expression. The latter is absent on TH1 cells in lymphoid tissue and expressed in low amounts on TH1 cells from the spinal cord. In contrast eYFP+ IL-17A as well as IFNγ expressing cells express high amounts of IL-1R1 (Fig.5c). To test the functional_significance of IL-1R expression, we FACS purified eYFP+ and eYFP− CD4 T cells from draining lymph node and spinal cord of MOG-CFA immunized mice and stimulated the cells in the presence of IL-1β. This resulted in a marked enhancement of IL-17A as well as IFN-γ production exclusively from the eYFP+ fraction in agreement with the expression pattern of IL-1R (Fig5d). Thus, IL-1R expression could potentially be used as a marker for non-TH1 derived IFN-γ producers.
Effector deviation depends on IL-23 signalling
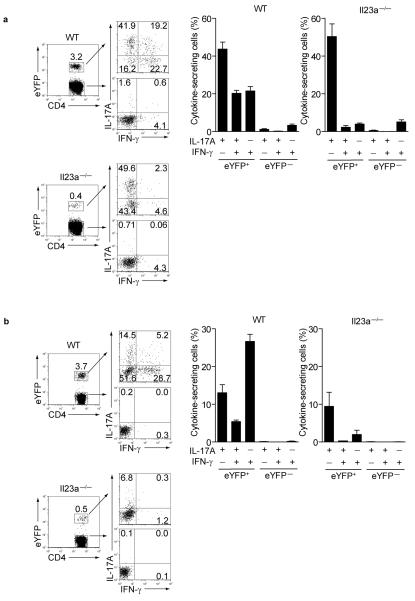
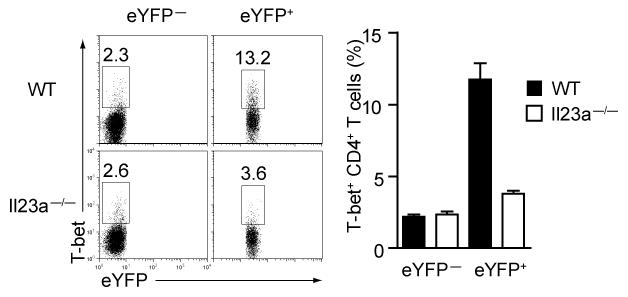
The importance of IL-23 signalling for TH17 effector differentiation is well-established 17. Furthermore, on the basis of in vitro studies IL-23 is thought to contribute to functional deviation of TH17 cells8. To determine the role of IL-23 signalling in our fate reporter model we immunized IL-23p19-deficient IL-17A reporter mice with MOG-CFA. Although IL-23p19-deficient reporter mice developed a much smaller proportion of eYFP+ CD4+ T cells than wild-type reporter mice, the proportion of IL-17A-single producers after PdBU-ionomycin stimulation was similar (Fig.6a representative FACS plots and bar graphs). However, the eYFP+ cells from IL-23p19-deficient reporter mice lacked IL-17A+ IFN-γ+ double producers as well as IFN-γ+ single producers compared to wild-type mice. This phenotype was more pronounced following restimulation with MOG peptide, where, even in wild-type mice, the proportion of double producers in eYFP+ cells was much lower than that observed after PdBU-ionomycin stimulation and completely absent in IL-23p19-deficient reporter mice (Fig.6b). To pinpoint the mechanistic link between IL-23 and deviation towards a TH1-like profile, we tested for T-bet expression in eYFP+ and eYFP− CD4+ T cells from wild-type or IL-23p19-deficient reporter mice. Absence of IL-23 signalling in TH17 cells prevented T-bet upregulation, suggesting this transcription factor is controlled by IL-23.
Figure 6. IL-23 signalling is required for acquisition of eYFP+ IFN-γ+ profile.
(a) Representative FACS plots (left) and bar graphs showing expression of IL-17A and IFNγ in eYFP+ and eYFP− CD4 T cells from day 12 draining lymph nodes of wild-type or IL-23 p19−/− reporter mice following restimulation with either PdBU/ionomycin or (b) MOG peptide. Data show mean values +/− SD of three individual mice.
Taken together these data support our initial assumption that developmental progression of effector cell differentiation proceeds via an eYFP+ stage before IL-17A is switched off and alternative fates are adopted. Furthermore, they suggest that IL-23 stimulation is not only crucial for attaining full effector function as shown before, but is also necessary for double expression of IL-17A and IFNγ, induction of T-bet and the consecutive deviation towards IFN-γ production.
TH17 cell plasticity in different inflammatory conditions
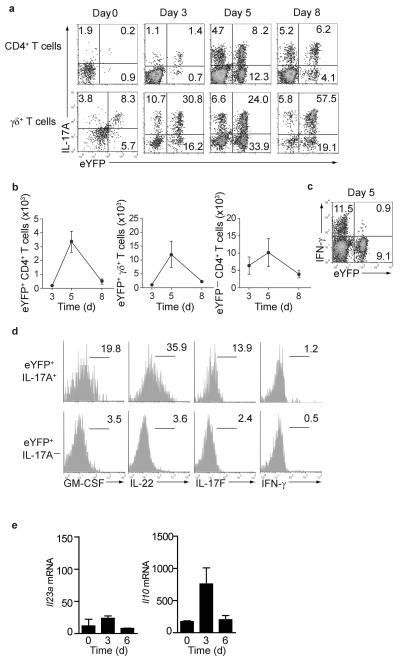
The developmental plasticity of TH17 cells towards a TH1 like phenotype has been well documented (reviewed in18), but it is unclear whether these cells can adopt other effector fates in a different inflammatory context. Reporter mice were cutaneously infected with Candida albicans hyphae. IL-17-producing γδ T cells and TH17 cells are important for the defence against this fungal infection, as revealed by the severe mucocutaneous infections with Candida in patients with defective IL-17 responses19-21. In contrast to chronic inflammation in EAE, infection with Candida albicans leads to an acute infection that is rapidly cleared. Infection was accompanied by infiltration of neutrophils (Supplementary Fig.7) and an early innate IL-17A response mediated by γδ T cells as well as a progressive involvement of TH17 cells (Fig.8a). By day 3 after infection the majority of both γδ T cells and CD4+ T cells expressing IL-17A also expressed eYFP. However, by day 5 when Candida was cleared (data not shown), a large fraction of eYFP+ cells had switched off IL-17A expression and by day 9 the absolute numbers of infiltrating CD4+ T cells (both eYFP+ or eYFP−) and γδ T cells had drastically declined (Fig.8b). Candida infection also elicited a substantial IFN-γ response by CD4+ T cells, but in marked contrast to the situation we encountered in the inflamed spinal cord during EAE, virtually none of these were eYFP+, indicating their TH1 origin (Fig.8c). The eYFP+ population that had switched off IL-17A appeared quiescent with respect to cytokine expression as neither IL-22, nor GM-CSF or IL-17F were expressed once IL-17A expression was extinguished (Fig.8d). qPCR analysis of CD11b+ and CD11c+ cells isolated from infected skin showed a strong Il10 and relatively weak Il23a expression suggesting that the milieu in the surrounding skin tissue is biased towards an anti-inflammatory profile that may curtail TH17 deviation towards alternative fates (Fig.8e). This data suggest that in contrast to chronic inflammation, acute inflammation promotes an anti-inflammatory environment in which TH17 cells downregulate Il17a transcriptional activity without activating alternative cytokine loci. Thus, resolution of acute inflammation goes hand in hand with disappearance of TH17 and γδ T cells rather than a switch to alternative effector fates that we characterized during chronic inflammation.
Figure 7. T-bet expression is curtailed in the absence of IL-23.
Representative FACS plots and bar graphs showing expression of eYFP and T-bet in eYFP+ and eYFP− CD4 T cells from day 6 draining lymph nodes of wildtype or IL-23p19 deficient reporter mice. The bar graphs show means ± SD of three individual mice.
Figure 8. Cutaneous infection with Candida albicans.
Skin (1cm2) of IL-17A fate reporter mice infected with Candida albicans hyphae was analyzed at the timepoints depicted. (a) intracellular IL-17A and eYFP expression in CD4+ and γδ T cells is shown. (b) absolute numbers of eYFP positive CD4+ and γδ T cells and eYFP− cells in the skin at the indicated time points. Mean + SD values from 3-5 mice per time point are shown. (c) intracellular IFN-γ and eYFP expression in CD4+ T cells. Data are representative of two independent experiments (d) histograms showing cytokine expression in gated eYFP+ IL-17+ and eYFP+ IL-17− CD4 T cells on day 5 after infection. The data are representative of two independent experiments. (e) CD11b+ and CD11c+ cells were MACS sorted from the infected skin for qPCR analysis. Relative gene expression in sorted cells normalized to the expression of Hprt is shown. The bar graphs show means ± SD of three individual mice. Data are representative of two independent experiments.
DISCUSSION
Lineage commitment and fate determination are fundamental biological processes that have been extensively studied in a variety of biological systems. In T cell biology these issues combined with the question of terminal differentiation, stability or plasticity have been intensively discussed and investigated in recent years. To some extent the unusual biology of the TH17 subset triggered these questions, although they are by no means restricted to TH17 cell biology and indeed are now explored in other effector T cell subsets as well (reviewed in 22,23). Given the documented rapid fate changes of cultured TH17 in vitro or following adoptive transfer, driven by epigenetic instability at transcription factor as well as cytokine gene loci24,25, the logical choice was to generate a reporter mouse which would allow permanent tracing of cells that had activated the Il17a locus as one indicator implying mandatory expression of this locus as a pre-requisite for TH17 fate determination. The reporter mouse we generated is to our knowledge the first fate reporter mouse for IL-17A, as existing mice generated by BAC transgenesis so far only monitor transcriptional activity at the closely linked Il17f locus26. IL-17F is largely co-expressed with IL-17A in lymphoid organs and may play a special role in lung inflammation, but we found little IL-17F expression in neuronal inflammation in line with the reported absence of a phenotype for IL-17F-deficient mice in EAE (reviewed in 27)
We provided experimental evidence that induction of the eYFP reporter correlated well with effector function. Our data also indicate that in vitro generated TH17 cells do not attain the same level of effector differentiation as seen in vivo during the course of inflammatory immune responses that lead to IL-17A induction. Even the few IL-17A-expressing CD4+ and γδ T cells found in lymphoid organs of mice without deliberate immunization contained a higher proportion of eYFP+ cells than we detected during in vitro differentiation of TH17 cells. It is likely that many factors that shape TH17 differentiation in vivo are not adequately mimicked by in vitro conditions.
A recent study suggested that in contrast to TH17 cells differentiated in vitro, Th17 cells generated in vivo appeared developmentally stable12. From our study it was evident that TH17 cells activated in vivo also rapidly shut off IL-17 production. This process closely followed the acquisition of eYFP in Il17aCre reporter mice since there was virtually no evidence for a direct progression of IL-17 to IFN-γ producers, bypassing the eYFP+ effector stage. Interestingly, analysis of TH17 development in IL-23p19-deficient reporter mice not only showed reduced acquisition of eYFP, but also lack of the double positive IL17+ IFN-γ+ population that usually develops following immunization in vivo. This suggests that TH17 cells require prior effector cell differentiation to produce IFN-γ. Similar observations were made previously by investigating intestinal TH17 responses in IL-23 receptor-deficient mice28. Since T-bet expression was strongly curtailed in TH17 cells from IL-23p19-deficient reporter mice, it is likely that IL-23 is upstream of T-bet and is required for its expression. IL-23 is abundant during chronic inflammation29, but is downregulated during resolution of the inflammatory response making IL-23 perhaps the major decision point for TH17 cell plasticity.
Furthermore it was evident that the eYFP+ population constituted the main source of other cytokines. Notably GM-CSF which is strongly implicated in EAE30,31 was produced exclusively by eYFP+ TH17 and ‘ex-TH17’ cells. The propensity for a switch to IFN-γ production was pronounced in the setting of EAE and accompanied by upregulation of IL-12Rβ2 and T-bet and downregulation of RORγt, IL-23R and CCR6. Interestingly, expression of AhR was retained, thus making IFN-γ producing ‘ex-TH17’ cells susceptible to environmental stimuli. In contrast to in vitro generated TH17 cells from mice or humans, which retain expression of both IL-12R chains7,32, TH17 cells developing in vivo did not express the IL-12Rβ2 chain (Fig.5b), making such cells refractory to the action of IL-12. Furthermore, stimulation of AhR, which is highly expressed on TH17 cells, leads to upregulation of both IL-12Rβ1 and IL-12Rβ233. Recent data showed that TH2 cells, which switch off IL-12Rβ2 during differentiation in vitro34 re-express the receptor when exposed to type I interferon or IFN-γ35. Similar mechanisms may contribute to re-induction of IL-12Rβ2 in TH17 cells during EAE with a potential involvement of plasmacytoid dendritic cells as source of type I interferon36,37. In this respect it is also worth noting that interferon alpha receptor-dependent inhibition of intracellular osteopontin may regulate IL-27 expression and subsequent inhibition of IL-1738. The relative roles of IL-17A versus IFN-γ in the pathology of EAE as well as multiple sclerosis in humans remain controversial with evidence both supporting a protective as well as pathogenic involvement of IFN-γ in CNS inflammation39-42. Effector cytokines produced by ‘ex-TH17’ cells during the chronic stage may thus play different roles in the pathogenesis of autoimmune diseases.
Our data reconcile seemingly conflicting results obtained by adoptive transfer of in vitro generated TH17 or TH1 cells43-45,42 that seemed to refute the important role of TH17 cells in various autoimmune models compared with earlier data that highlighted their crucial importance in these models (as reviewed in46). They also explain how T-bet-deficient mice are protected from EAE, which seemed at odds with an important role for TH17 cells in CNS pathology. It appears that IL-23 mediated T-bet induction resulting in IFN-γ expression by ‘ex-TH-17’ cells is an important step in the pathophysiology of EAE, since IFN-γ represents by far the most abundant cytokine in the spinal cord of mice with EAE. These data also explain our earlier findings that mice with transgenic expression of a dominant negative TGFβRII are protected from EAE and contain very few TH1 cells in the spinal cord despite an overabundance of TH1 cells throughout the lymphoid organs29. Thus, IL-17-producing cells, including innate γδ T cells or NKT cells47 might play an early role in recruitment of cells involved in setting up inflammatory responses, whereas the conversion to a TH1-like cytokine profile may be the major pathological driver. Interestingly, pathogenic effector cells TH17 cells must lose responsiveness to TGF-β to allow effective T-bet expression. It is possible that this could explain the recent report of pathogenic TH17 cells that arise in the apparent absence of TGF-β signaling48.
In contrast to the situation in EAE, a switch of IL-17A to IFN-γ production was not evident following Candida albicans infection of the skin, suggesting that TH17 plasticity depends on the inflammatory milieu. Whereas immunization with MOG in CFA induces chronic inflammatory response because of the depot effect of the immunogen emulsion, Candida infection of the skin was rapidly cleared in wild-type mice. The infection caused an early influx of neutrophils sustained by the IL-17A response, which was shut off in parallel with clearance of the neutrophil infiltration. At that stage the skin milieu was characterized by high expression of IL-10 and relatively modest IL-23 expression, suggesting a balance in favour of an anti-inflammatory microenvironment. The surviving eYFP+, but IL-17A− CD4 population showed minimal detectable cytokine secretion. Such a population of ‘ex-TH17’ cells would be invisible by any other means of detection, as IL-17A secretion was not even induced by PdBU-ionomycin stimulation. This would explain a previous report49, suggesting that TH17 cells cannot be detected in the pool of long-lived CD4+ memory T cells. It remains to be seen whether surviving eYFP+ cells carry memory of their initial induction and are able to re-initiate a TH17 program upon re-encounter with the original pathogen in similar inflammatory settings.
Expression of IL-1R1 on ‘ex-TH17’ cells could be a useful marker for identifying cells which develop though a TH17 program as neither TH1 nor TH2 cells normally express significant levels of this receptor, which has been shown to be crucial for pathogenisis of autoimmune diseases such as EAE50. Thus, our data reconcile a host of literature that identifies various pro-inflammatory cytokines, including GM-CSF, IFNγ, IL-1 in the pathogenisis of autoimmune diseases, as they are all linked to T cells that have started their effector stage as TH17 cells.
Taken together our data suggest that fate decisions of TH17 cells are shaped by different inflammatory conditions in vivo allowing distinct patterns of plasticity. Thus, IL-17A fate reporter mice constitute a valuable tool for dissection of effector cytokine programs that may have originated initially from TH17 cells and will facilitate mechanistic analysis of the inflammatory drivers that shape acquisition of the functional fate of effector T cells.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Medical Research Council UK (Ref. U117512792 for B.S. and U117563359 for A.J.P.) and the European Research Council (Ref.232782). We would like to thank our Large Scale Facility for growing Candida albicans, Graham Preece for expert cell sorting and Biological Services for breeding and maintenance of our mouse strains.
ONLINE METHODS
Mice
Codon-improved Cre recombinase (iCre)51 was inserted into the first exon of the Il17a locus in R1 embryonic stem cells by homologous recombination to express iCre under the control of the endogenous Il17a promoter. Details of the gene targeting and the Il17a targeting strategy are given in Supplementary Fig. 1a, b. To visualize Cre-mediated recombination, R26ReYFP mice52 were intercrossed with Il17aCre mice generating Il17aCre R26ReYFP mice. This strain was further bred with Il23a−/− mice to obtain Il23a−/− Il17aCre R26ReYFP or with 2D2 transgenic mice. All reporter mice used in this study had been backcrossed to C57BL/6 for at least six generations. RAG-1-deficient mice on B6 background were bred in our animal facility and all mice were kept under specified pathogen free conditions. All animal experiments were done according to institutional guidelines and Home Office regulations.
In vitro T cell differentiation and cytokine determination
FACS purified naïve T cells (CD4+CD25−CD44lo) were cultured in Iscove's modified Dulbecco medium (IMDM, Sigma) supplemented with 5% FCS, 2×10−3M L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 5×10−5 M mercaptoethanol (all Sigma) in the presence of anti-CD3 (1 μg/ml) and 10 μg/ml anti-CD28 (both plate bound). Cytokines for effector cell differentiation were: 3 ng/ml IL-12 for TH1, 10 ng/ml IL-4 for TH2, 5 ng/ml TGF-β for iTreg, 1 ng/ml TGF-β, 20 ng/ml IL-6 and 10 ng/ml IL-1 for TH17 and 10 ng/ml IL-4 and 1 ng/ml TGF-β for TH9. Cells were cultured for 4 days and then restimulated for 4 h with PdBU/ionomycin (both at 500 ng/ml) in the presence of brefeldin A (1 μg/ml) before intracellular staining for cytokines. Alternatively, cells were cultured with MOG peptide (50 μg/ml) or 1 μg/ml anti-CD3 overnight with brefeldin A present for the last 5 hours. All antibodies for surface and intracellular staining were purchased from Biolegend. IL-22 was detected by staining with either monoclonal antibody AM22.3 (kindly provided by Dr. J.-C. Renauld, Brussels) or alternatively by goat anti-mouse IL-22 (Biolegend). Determination of cytokines in supernatants of FACS purified cells from lymph node or spinal cord at the adjusted concentration of 5×104/ml was done after overnight culture with plate-bound anti-CD3 and anti-CD28 in the absence or presence of IL-1β (20ng/ml) using FlowCytomix (Bender Medsystems).
Preparation of tissue-resident lymphocytes
Briefly, lymphocytes in the lamina propria were prepared by cutting the small intestine into 1 cm pieces, shaking for at 37°C in 10 ml ‘IEL buffer’ (PBS supplemented with 10% FCS, 1 mM pyruvate, 20 μM HEPES, 10 mM EDTA, and penicillin/streptomycin, 10 μg/ml Polymyxin B) to remove epithelial and intraepithelial cells and then digesting the remaining tissue using 1 mg/ml collagenase D (Roche) and 10 U/ml DNase1 (Sigma) at 37°C for 1 hr followed by separation on a 36.5% Percoll gradient. Lymphocytes in the spinal cord from mice with clinical score 3-4 at day 12-16 after MOG/CFA immunization were prepared by mashing the spinal cord through 70 μm mesh filter and separation on a 36.5% Percoll gradient.
EAE induction
Mice were injected subcutaneously at two sites with a total of 100 μl emulsion of IFA containing 250 μg MOG peptide 35-55 and 250 μg heat-killed Mycobacterium tuberculosis strain H37Ra. Mice received 200 ng Bordetella pertussis (Calbiochem) i.p. on the day of immunization and two days later. Clinical assessment of EAE was performed daily and clinical scores were assessed according to the following criteria: 0 = unaffected, 1 = flaccid tail, 2 = impaired righting reflex and/or gait, 3 = partial hind limb paralysis, 4 = total hind limb paralysis, 5 = total hind limb paralysis with partial fore limb paralysis. According to Home Office regulations all mice with a clinical score of 5 were culled.
Cutaneous infection with Candida albicans
Candida albicans strain SC5413 was obtained from the Institute of Medical Sciences, University of Aberdeen, UK. Hyphae were prepared by allowing cells to germinate in culture at 37°C, in 5% CO2, for 2 h in RPMI medium (resulting in >98% of hyphae formation). An area of 2 cm2 in the ventral skin of mice was shaved and superficial scratches were made using a needle. 2 × 105 Candida albicans hyphae were applied on the skin surface. At the relevant time point mice were culled and 1 cm2 of infected skin excised, minced and digested in IMDM containing 400 μg/ml liberase (Roche) and 1 mg/ml collagenase D (Roche) for 1 h at 37°C under continuous stirring. Cells were then collected and activated with PdBU, ionomycin and brefeldin A for 2.5 h before intracellular cytokine staining.
Real time PCR
RNA was extracted from FACS purified CCR6+ and CCR6− CD4 T cells isolated from spinal cord using Trizol and reverse transcribed with Omuniscript (Qiagen) according to the manufacturer's protocol. The cDNA served as template for the amplification of genes of interest and a housekeeping gene (Hprt1) by real-time PCR, using TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) and universal PCR Master Mix (Applied Biosystems, Warrington, UK) on a ABI-PRISM 7900 Sequence Analyzer (Applied Biosystems, Foster City, CA). Target gene expression was calculated using the comparative method for relative quantification upon normalization to Hprt1 gene expression.
Statistical Analysis
All statistical analysis was performed using a two-tailed Student T test.
Footnotes
Author contributions:
H.K., J.H.D.,H.A.,C.W. designed and performed experiments, M.V.,E.H.,Y.L.,M.T., U.M. and A.G. were involved in generating the construct, targeting ES cells and injections. A.J.P and B.S. are joint senior authors who designed the targeting construct and experiments and wrote the paper.
REFERENCES
- 1.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6(11):1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6(11):1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 5.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204(8):1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565–572. doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin-Orozco N, et al. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39(1):216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi G, et al. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181(10):7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005;201(2):169–171. doi: 10.1084/jem.20042279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lexberg MH, et al. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38(10):2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 13.Hirota K, Martin B, Veldhoen M. Development, regulation and functional capacities of Th17 cells. Semin Immunopathol. 2010;32(1):3–16. doi: 10.1007/s00281-009-0187-y. [DOI] [PubMed] [Google Scholar]
- 14.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 15.Luger D, et al. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J Exp Med. 2008;205(4):799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettelli E, et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197(9):1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 19.de Beaucoudrey L, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205(7):1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milner JD, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kisand K, et al. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207(2):299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy KM, Stockinger B. Effector T cell plasticity: flexibility in the fact of changing circumstances. Nat Immunol. 2010 doi: 10.1038/ni.1899. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mukasa R, et al. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32(5):616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30(1):155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Croxford AL, Kurschus FC, Waisman A. Cutting edge: an IL-17F-CreEYFP reporter mouse allows fate mapping of Th17 cells. J Immunol. 2009;182(3):1237–1241. doi: 10.4049/jimmunol.182.3.1237. [DOI] [PubMed] [Google Scholar]
- 27.Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46(1):7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahern PP, et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33(2):279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veldhoen M, Hocking RJ, Flavell RA, Stockinger B. Signals mediated by transforming growth factor-beta initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nat Immunol. 2006;7(11):1151–1156. doi: 10.1038/ni1391. [DOI] [PubMed] [Google Scholar]
- 30.Ponomarev ED, et al. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178(1):39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- 31.McQualter JL, et al. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194(7):873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boniface K, et al. Human Th17 Cells Comprise Heterogeneous Subsets including IFN-{gamma}-Producing Cells with Distinct Properties from the Th1 Lineage. J Immunol. 2010;185:679–687. doi: 10.4049/jimmunol.1000366. [DOI] [PubMed] [Google Scholar]
- 33.Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szabo SJ, Dighe AS, Gubler U, Murphy KM. Regulation of the interleukin (IL)-12R beta 2 subunit expression in developing T helper 1 (Th1) and Th2 cells. J Exp Med. 1997;185(5):817–824. doi: 10.1084/jem.185.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegazy AN, et al. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 2010;32(1):116–128. doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 36.Bailey-Bucktrout SL, et al. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180(10):6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Isaksson M, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39(10):2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]
- 38.Shinohara ML, Kim JH, Garcia VA, Cantor H. Engagement of the type I interferon receptor on dendritic cells inhibits T helper 17 cell development: role of intracellular osteopontin. Immunity. 2008;29(1):68–78. doi: 10.1016/j.immuni.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Axtell RC, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 16(4):406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stromnes IM, et al. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14(3):337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lees JR, et al. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205(11):2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinman L. A rush to judgment on Th17. J Exp Med. 2008;205(7):1517–1522. doi: 10.1084/jem.20072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connor RA, et al. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181(6):3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McGeachy MJ, et al. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8(12):1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 45.Suryani S, Sutton I. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J Neuroimmunol. 2007;183(1-2):96–103. doi: 10.1016/j.jneuroim.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 46.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28(4):445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 47.Michel ML, et al. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204(5):995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467(7318):967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pepper M, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sutton C, et al. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203(7):1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimshek DR, et al. Codon-improved Cre recombinase (iCre) expression in the mouse. Genesis. 2002;32(1):19–26. doi: 10.1002/gene.10023. [DOI] [PubMed] [Google Scholar]
- 52.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.