Abstract
Many species of Myrtaceae are cultivated in home gardens throughout the tropics for their edible fruit, and have been used in traditional medicine to treat several inflammatory conditions. Fruit phenolics are important dietary antioxidant and anti-inflammatory constituents. We have investigated the antiradical activity, total phenolic content (TPC), and total anthocyanin content (TAC) of 14 underutilized Myrtaceae fruits, namely Eugenia aggregata, E. brasiliensis, E. luschnathiana, E. reinwardtiana, Myrciaria cauliflora, M. dubia, M. vexator, Syzygium cumini, S. curranii, S. jambos, S. javanicum, S. malaccense, S. samarangense, and S. samarangense var. Taiwan pink. An HPLC-PDA method was developed to quantify the amounts of cyanidin 3-glucoside (1), delphinidin 3-glucoside (2), ellagic acid (3), kaempferol (4), myricetin (5), quercetin (6), quercitrin (7), and rutin (8) present in MeOH extracts of the fruit. TPC ranged from 3.57 to 101 mg/g, TAC ranged from undetectable to 12.1 mg/g, and antiradical activity, measured as DPPH˙ IC50, ranged from very active (19.4 μg/ml) to inactive (389 μg/ml).
Keywords: HPLC-PDA, quantitative analysis, Myrtaceae, Myrciaria, Eugenia, Syzygium, antioxidant, flavonoid, anthocyanin, Folin-Ciocalteu, DPPH, polyphenolic
1. Introduction
According to the Centers of Disease Control and Prevention, over half of all deaths in the United States are caused by diseases of the heart and malignant neoplasms (Minino, Heron & Smith, 2006). Epidemiological evidence suggests that diets high in fruits and vegetables are linked to a reduced incidence of heart disease, cancer, and some neurodegenerative disorders (Youdim & Joseph, 2001; Hu & Willett, 2002; Arts & Hollman, 2005; Collins, 2005). Reactive oxygen species (ROS) are produced naturally in mammalian systems as a result of oxidative metabolism. However, ROS damage cell membranes and DNA, causing cancerous mutations, and the oxidation of low-density lipoprotein is a major factor in the promotion of heart disease. ROS are also implicated in activating redox-responsive pro-inflammatory transcription factors, nuclear factor (NF)-κB and activator protein (AP)-1 (Rahman & Adcock, 2006). Inflammation is a major factor in the promotion of chronic inflammatory diseases, as well as the etiology of cancers and heart disease (Middleton, Kandaswami & Theoharides, 2000; Hu et al., 2002). Oxidative damage is balanced by endogenous antioxidants, but additional protection, provided by nutritive and non-nutritive elements from food, is critical for disease chemoprevention.
Colourful fruits are a potentially rich source of many dietary phenolic antioxidants and are believed to play an important role in the prevention of many oxidative and inflammatory diseases (Arts et al., 2005). The anthocyanin pigments are responsible for many of the bright fruit and flower colours, and act as strong antioxidants and anti-inflammatories, with antimutagenic and cancer chemopreventative activities (Kong, Chia, Goh, Chia & Brouillard, 2003; Reynertson et al., 2006). Anthocyanins often account for much of the phenolic content of these fruits, but flavanols, procyanidins, phenolic acids, and ellagitannins may be the most predominant phenolics in some taxa. Polyphenolic compounds inhibit several oxidative and inflammatory enzymes (Middleton et al., 2000), and have shown antiallergenic, antiviral, antibacterial, antifungal, antitumor, and antihemorrhagic activities (Pietta, 2000). Flavonoids also inhibit the inflammatory transcription factors NF-κB and AP-1 (Rahman et al., 2006).
The plant family Myrtaceae is pan-tropical in occurrence, with concentrations in South America, Southeast Asia, and Australia. The fleshy-fruited subfamily, Myrtoideae, includes many economically important food plants, agricultural crops, and ornamentals, including the Mediterranean genus Myrtus (myrtle), spices such as clove (Syzygium aromaticum), allspice (Pimenta dioica), and bay rum (Pimenta racemosa), and the fruits of Psidium (guavas), Myrciaria, Eugenia, Syzygium, Plinia and Luma.
Blueberries, cranberries, strawberries, grapes, cherries, and other colourful fruits have been the focus of much current research on dietary chemoprevention of cancers and heart disease (Wang et al., 1999; Kähkönen, Hopia & Heinonen, 2001; Zheng & Wang, 2003; Seeram, Lee, Scheuller & Heber, 2006). In this report, we have analyzed the phenolic content and antiradical activity of 14 edible fruits from 13 species of Myrciaria, Eugenia and Syzygium, namely Eugenia aggregata Kiaersk., E. brasiliensis Lam., E. luschnathiana Klotzsch ex O.Berg, E. reinwardtiana (Bloom) DC, Myrciaria cauliflora (Mart.) O.Berg, M. dubia (Kunth) McVaugh, M. vexator McVaugh, Syzygium cumini (L) Skeels, S. curranii (C.B.Rob.) Merr., S. jambos (L) Alston, S. javanicum Miq., S. malaccense (L)Merr. & L.M.Perry, S. samarangense (Bloom) Merr. & L.M.Perry and S. samarangense var. Taiwan pink.
These fruits are mostly red to purple drupes, 2-4 cm in diameter, although some species produce larger or less pigmented fruit. In the tropics, these species are often cultivated in home gardens, small-scale agricultural plots, or wild-harvested. They are primarily eaten fresh or used to make jams, desserts, wines, liquors, and vinegars, and can be found in local markets. In addition to their use as food, many of these fruits have been used in divergent traditional medical practices for a variety of illnesses and conditions. Most notably, the seeds of the jamun (S. cumini) are an important Ayurvedic medicine for diabetes. The rose apple (S. jambos) has been used in India as a tonic for the brain and for liver problems, as an astringent, and digestive (Kirtikar & Basu, 1988), and distilled to make rosewater (Morton, 1987). In Brazil, E. brasiliensis leaves have been used for gastrointestinal disorders and rheumatism, and the jaboticaba fruit (M. cauliflora) has been used as a treatment for hemoptysis, asthma, diarrhea, and chronic inflammation of the tonsils (Morton, 1987). Leaves of S. malaccense have been used for a wide variety of inflammatory conditions in Western Samoa (Andersson Dunstan, Noreen, Serrano, Cox, Perera & Bohlin, 1997).
The majority of the phytochemical literature for these species has focussed on the aromatic terpenoid compounds found in the leaves (Wong & Lai, 1996), but phenolics have also been identified in some species. We previously reported flavonols, phenolic acids, anthocyanins, and depsides from M. cauliflora (Reynertson et al., 2006), as well as the occurrence of catechin and epicatechin in E. aggregata (Reynertson, Basile & Kennelly, 2005). Myrciaria dubia was reported to contain cyanidin 3-glucoside and delphinidin 3-glucoside (Zanatta, Cuevas, Bobbio, Winterhalter & Mercadante, 2005). Several flavonoids, ellagitannins, and phenolic acids have been identified from the fruits, seeds, and aerial parts of S. cumini (Bhatia, Bajaj & Ghangas, 1971; Bhatia & Bajaj, 1975; Mahmoud, Marzouk, Moharram, El-Gindi & Hassan, 2001), and flavonoids and ellagitannins have been found in the fruits of S. samarangense (Okuda, Yoshida, Hatano, Yazaki & Ashida, 1982; Nonaka, Aiko, Aritake & Nishioka, 1992; Srivastava, Shaw & Kulshreshtha, 1995; Nair, Krishnan, Ravikrishna & Madhusudanan, 1999). Ellagic acids and an anthocyanin were reported from S. malaccense (Andersson Dunstan et al., 1997). To the best of our knowledge, however, the phenolic constituents of Eugenia brasiliensis, E. luschnathiana, E. reinwardtiana, Myrciaria vexator, Syzygium curranii, S. javanicum, and S. samarangense var. Taiwan pink have not been reported in the literature, despite their widespread consumption in the tropics.
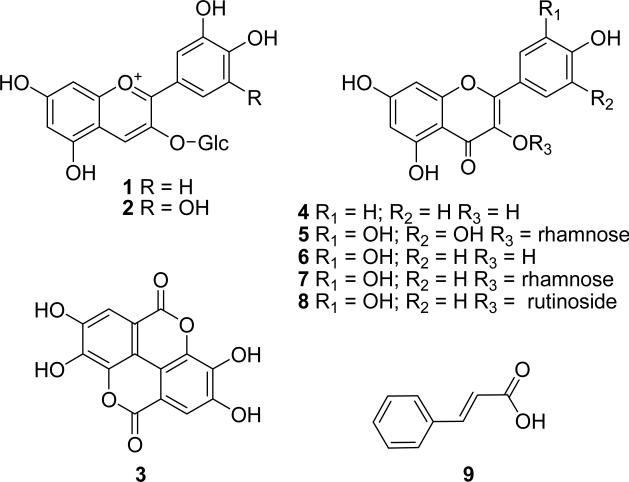
Here we report on the HPLC-PDA quantification of eight phenolic compounds in 14 fruits. Cyanidin 3-glucoside (1), delphinidin 3-glucoside (2), ellagic acid (3), kaempferol (4), myricetin (5), quercetin (6), quercitrin (7), and rutin (8) (Figure 1) have been quantified to better understand the phenolic profile of these fruits. In addition, the total phenolic content (TPC) and total anthocyanin content (TAC) were determined, and each extract was evaluated for antiradical activity using the DPPH˙ assay.
Figure 1.
Chemical structures of the phenolic compounds quantified or identified in the 14 Myrtaceae fruits. Compounds are numbered as follows: cyanidin 3-glucoside (1), delphinidin 3-glucoside (2), ellagic acid (3), kaempferol (4), myricetin (5), quercetin (6), quercitrin (7), rutin (8), and cinnamic acid (9)
2. Materials and methods
2.1. General experimental
HPLC analyses were performed on a Waters (Milford, MA, USA) Alliance 2695 using a 250 × 4.6 mm Phenomenex (Torrence, CA, USA) C18 Aqua column and monitored with a Waters 996 PDA scanning device from 210 to 600 nm. A Perkin-Elmer (Wellesley, MA, USA) Lambda 2 UV/VIS spectrophotometer was used for total anthocyanin content (TAC) determinations. A Molecular Devices (Sunnyvale, CA, USA) Versamax tunable absorbance detector was used for the 1,1-diphenyl-2-picrylhydrazyl (DPPH˙) antiradical assay and total phenolic content (TPC) measurements. Extractions were performed at room temperature, using an ultrasonicator from VWR (West Chester, PA, USA; model 50T, 45 W, 38.5 to 40.5kHz). Reversed-phase C18 solid phase extraction (SPE) cartridges (500 mg, 4 ml) from Alltech (Deerfield, IL, USA) were used for sample preparation. Compounds 3 – 8, DPPH, and 2N Folin-Ciocalteu reagent (FCR) were purchased from Sigma (St. Louis, MO, USA). Compounds 1 and 2 were previously isolated from Myrciaria cauliflora (Reynertson et al., 2006).
2.2. Plant material
Ripe fruits of Eugenia aggregata, E. brasiliensis, E. luschnathiana, E. reinwardtiana, Myrciaria cauliflora, M. vexator, Syzygium cumini, S. curranii, S. jambos, S. javanicum, S. malaccense, S. samarangense, and S. samarangense var. Taiwan pink were collected at the Fruit and Spice Park (Homestead, FL, USA), Rare Fruit and Vegetable Council of Broward County Research Garden (Southwest Ranches, FL, USA), The Kampong (Coconut Grove, FL, USA), and the University of Florida Tropical Resource and Education Center (Homestead, FL, USA). Fruits were frozen and shipped by overnight courier on dry ice to the laboratory, where they were kept in cold (-20 °C) dark storage until processed. Voucher specimens were prepared, identified, and deposited at the Steere Herbarium of The New York Botanical Garden (Bronx, NY, USA). Dried, powdered camu-camu fruit (M. dubia) was supplied by Essential Living Foods (Los Angeles, CA, USA).
2.3. Extraction and sample preparation
Approximately 10 g of freeze-dried fruit were homogenized in a blender with 200 ml of MeOH-formic acid (9:1) and sonicated for 1 h. Extracts were centrifuged, filtered, and the marc extracted two more times. Extracts were combined and concentrated under reduced pressure at temperatures not exceeding 40 °C and brought up to 100 ml total. Aliquots (2 to 4 ml) were prepared for each experiment and stored at – 20 °C until analyzed. Aliquots analyzed by HPLC and DPPH antiradical assay were dried, resuspended in 1 to 2 mL water, and subjected to SPE, eluting sequentially with water and MeOH. The MeOH fractions were collected, dried, and dissolved in DMSO for the DPPH˙ assay or MeOH-formic acid (9:1) for HPLC analysis.
2.4. Quantitative HPLC-PDA analysis
Following SPE, two aliquots of each fruit extract were analyzed in triplicate (n = 6). HPLC-PDA analysis was performed using a gradient solvent system of 1% formic acid (A) and acetonitrile (B) as follows: 90% to 75% A over 30 min; 75% to 40% A from 30 to 45 min at a flow rate of 1 ml/min. Results were monitored from 210 to 600 nm. Sample concentrations ranged from 1.5 to 7 mg/ml. Peak area for each compound quantified in the test samples was integrated from the HPLC-PDA chromatogram using Waters Empower software and the concentration was calculated using the equation for each standard curve.
2.5. Standard curves, limits of detection and quantification, recovery
Stock solutions of compounds 1 and 3 – 7 were prepared in MeOH-formic acid (9:1), and combined to make a standard mixture. This stock mixture was diluted to make six standard solutions (ranging from 0.95 to 888 μg/ml) for HPLC-PDA quantification. Peak area for each standard was integrated from HPLC-PDA chromatograms at 520 nm for anthocyanins and 254 nm for all other compounds using Waters Empower software and plotted against concentration to create a linear curve. Delphinidin 3-glucoside (2) was quantified based on cyanidin 3-glucoside equivalents.
Minimum limits of detection (LOD), at a signal-to-noise (S/N) ratio of 3:1, and minimum levels of quantification (LOQ), at a S/N ratio of 10:1, were determined experimentally.
The percent recovery for the analytical method was calculated for three representative compounds, (1, 3, and 8). Each standard was accurately weighed (0.1 mg) and added to 1 g of freeze-dried S. malaccense fruit. The material was extracted, prepared, and analyzed, following the same method as used for HPLC-PDA quantification.
2.6. Total phenolic content
Analysis was performed by the Folin-Ciocalteu method (Singleton & Rossi, 1965; Kähkönen et al., 1999). Three aliquots were analyzed in triplicate (n = 9). Samples or gallic acid (100 μl) and 1 ml of FCR (diluted 10-fold) were mixed in a vortexer and incubated for 5 min at room temperature (22 °C) prior to the addition of 1 ml of 10% Na2CO3 solution. This mixture was then allowed to stand for 90 min at room temperature, and the absorbance was determined at 765 nm. TPC was calculated as gallic acid equivalents (GAE) per g of dry weight.
2.7. Total anthocyanin content
Anthocyanin content was determined by the pH-differential method (Lee, Durst & Wrolstad, 2005). Pigment concentration is calculated and expressed as cyanidin 3-glucoside equivalents using the formula:
| (1) |
where A = (A520 nm – A700 nm)pH 1.0 – (A520 nm – A700 nm)pH 4.5; MW (molecular weight) = 449 g/mol; DF = dilution factor; 1 = cuvette pathlength in cm; ε= 26,900 l/mol/cm, molar extinction coefficient for cyanidin 3-glucoside.
2.8. Antiradical activity
Samples for the DPPH˙ antiradical assay were assayed in triplicate, as previously described, using 400 μM DPPH (Yang et al., 2003). Gallic acid was used as a positive control (IC50 = 30.0 ± 2.9 μM).
3. Results and discussion
3.1. General
Dark-coloured temperate fruits have recently generated considerable interest as a rich source of phenolic antioxidants. We have analyzed and quantified several antioxidative and anti-inflammatory flavonols, phenolic acids, and anthocyanins from 14 underutilized tropical Myrtaceae fruits. The anthocyanins are the most abundant compounds among those quantified (Tables 1 and 2) and are largely responsible for the bright colours of these fruits.
Table 1.
Results of antiradical DPPH˙ assay (IC50 in μg/ml), TPC (mg GAE/g dry weight), and TAC (mg C3G/g dry weight)
| DPPH˙ | TAC | TPC | |
|---|---|---|---|
| E. aggregata | 84.6 ± 2.63 | 1.26 ± 0.45 | 25.3 ± 1.54 |
| E. brasiliensis | 42.7 ± 5.92 | 8.37 ± 0.23 | 24.8 ± 1.06 |
| E. luschnathiana | 38.0 ± 4.86 | NDa | 22.0 ± 0.21 |
| E. reinwardtiana | 110 ± 8.31 | 0.08 ± 0.03 | 9.25 ± 0.78 |
| M. cauliflora | 19.4 ± 0.28 | 2.78 ± 0.17 | 31.6 ± 0.39 |
| M. dubia | 57.2 ± 5.61 | Trb | 101 ± 0.25 |
| M. vexator | 38.6 ± 2.40 | 6.84 ± 0.36 | 44.1 ± 1.21 |
| S. cumini | 389 ± 36.0 | 6.33 ± 0.10 | 9.95 ± 1.26 |
| S. curranii | 33.4 ± 2.52 | 12.1 ± 0.53 | 39.6 ± 0.77 |
| S. jambos | 92.0 ± 8.24 | ND | 8.69 ± 0.57 |
| S. javanicum | 81.4 ± 6.24 | 0.09 ± 4 × 10-3 | 3.57 ± 0.24 |
| S. malaccense | 269 ± 7.66 | Tr | 8.58 ± 0.12 |
| S. samarangense | 77.5 ± 4.19 | 0.07 ± 0.03 | 18.0 ± 0.70 |
| S. samarangense var. Taiwan pink | 157 ± 13.0 | 1.35 ± 0.10 | 23.8 ± 0.30 |
ND, not detected
Tr, trace (below 0.01 mg/g)
Table 2.
Minimum limits of detection and quantification of each standard compound
| LOQ (ng) | LOD (ng) | |
|---|---|---|
| cyanidin 3-glucoside (1)a | 10.0 | 3.0 |
| ellagic acid (3) | 8.3 | 2.5 |
| kaempferol (4) | 11.0 | 4.0 |
| myricetin (5) | 17.8 | 4.5 |
| quercetin (6) | 16.4 | 4.5 |
| quercitrin (7) | b | b |
| rutin (8) | 25.0 | 8.0 |
Cyanidin 3-glucoside was quantified at 520 nm, all others were quantified at 254 nm.
The LOD and LOQ were not determined for quercitrin.
3.2. Total phenolic content, total anthocyanin content and antiradical activity
Results for TPC, TAC, and antiradical activity are displayed in Table 1. The TPC for most species falls within the range of 3.57 (S. javanicum) to 44.1 mg GAE/g (M. vexator). The TPC for M. dubia (camu-camu), however, is over double the TPC for M. vexator at 101 mg GAE/g. The camu-camu, however, is known to have high levels of ascorbic acid (Aragao, Ikegaki, Sato, Oliveira & Park, 1996), which have been shown to interfere with the TPC measurement by the same oxidation/reduction reaction that detects phenolics (Singleton et al., 1965; Prior, Wu & Schaich, 2005). TAC values range from undetected (E. luschnathiana and S. jambos) to 12.1 mg/g equivalents (S. curranii). Antiradical activity ranges from very active (M. cauliflora, 19.4 μg/ml) to inactive (389 μg/ml, S. cumini). Myrciaria cauliflora is the most active extract in the DPPH˙ assay, with only a slightly higher than average TPC (31.6 mg GAE/g) and TAC (2.78 mg/g). Syzygium curranii is the highest in anthocyanin content according to both the TAC method and HPLC method. In our analysis, TAC did not correlate strongly with antiradical activity (γ2 = 0.1147), possibly because the anthocyanins make up only a portion of the overall phenolic profile. TPC was more closely correlated with antiradical activity, but did not reach significance (γ2 = 0.4542). There was no correlation between the individual phenolics quantified and the antiradical activity, suggesting that the antiradical activity of these extracts is a complicated synergy that may also involve compounds not identified or quantified. The DPPH˙ antiradical activity of the standard compounds quantified from these fruits has been previously reported in the literature (Luo, Basile & Kennelly, 2002; Ma, Yang, Basile & Kennelly, 2004; Reynertson et al., 2006). The activities of these compounds are rather similar, and a comparison of their concentrations in these extracts does not point to any obvious trends.
3.3. Standard curves, limits of detection and quantification, recovery
All standard curves had a correlation coefficient (γ2) value > 0.9998. The LOD and LOQ for each compound are given in Table 2. Syzygium malaccense was chosen as a representative species for the recovery experiment because the experimental matrix being analyzed (lyophilized, deseeded fruit) was the same for each species. When using a relatively small amount of material (1 g), the constituents of S. malaccense were not sufficiently concentrated to interfere with the recovery analysis. Using the same analytical methods as for the HPLC-PDA quantification, it was determined that the average recovery of cyanidin 3-glucoside (1) was 113 ± 12.6%; ellagic acid (3) was 80.1 ± 9.5%; and rutin (8) was 96.1 ± 9.6%.
3.4. Quantitative HPLC-PDA analysis
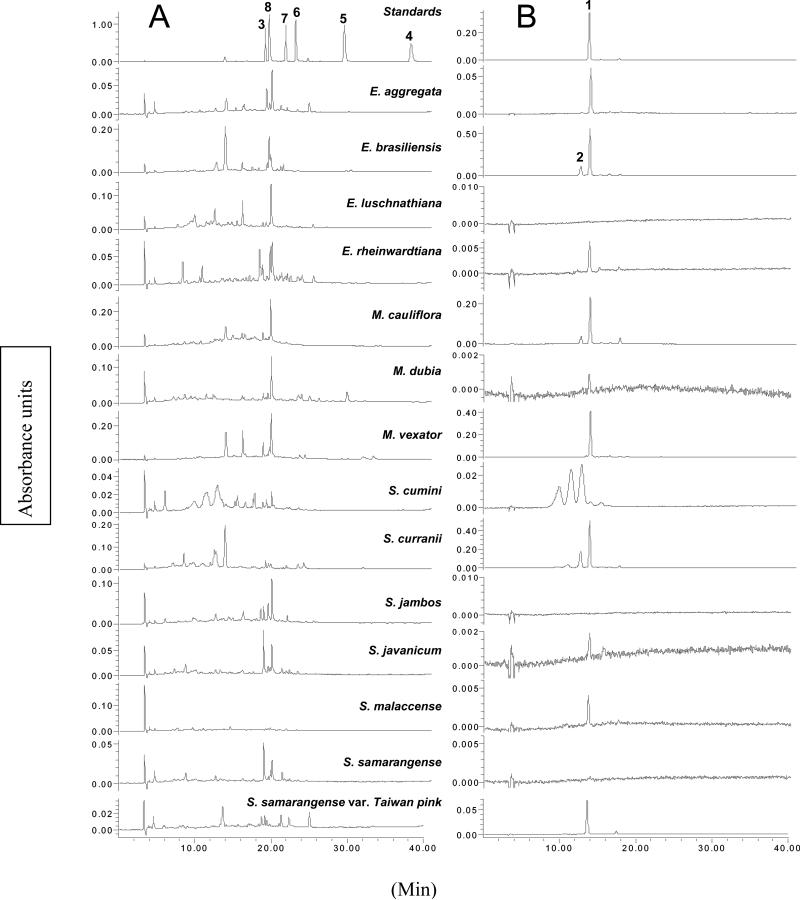
Chromatograms of the standard mixture and the 14 fruit extracts are presented in Figure 2. In our HPLC analysis, 1 is the most abundant compound in E. aggregata, E. brasiliensis, M. cauliflora, M. vexator, S. curranii, S. malaccense, and S. samarangense var. Taiwan pink (Table 3). It was detected or quantified in all but the two species that do not produce fruit coloured dark-purple or red; the orange fruit of E. luschnathiana and yellow fruit of S. jambos have no detectable anthocyanins by HPLC-PDA or TAC measurement. Eugenia brasiliensis, M. vexator and S. curranii have far greater levels of 1 (10.2, 13.1, and 9.56 mg/g, respectively) than the next-highest species (M. cauliflora, 4.33 mg/g). The purple-skinned fruits E. brasiliensis, E. aggregata, M. cauliflora, M. vexator, S. curranii and S. cumini contain both 1 and delphinidin 3-glucoside (2) and are unsurprisingly the highest in TAC (Table 1). The only reddish fruit that contains both 1 and 2 is M. dubia; the remaining red to pink fruits E. reinwardtiana, S. malaccense, S. samarangense and S. samarangense var. Taiwan pink, contain only 1. Syzygium curranii has far higher levels of delphinidin 3-glucoside than have any other fruit in the analysis (4.83 mg/g). Syzygium cumini, which is used by Ayurvedic practitioners in India as an antidiabetic medicine (Grover, Yadav & Vats, 2002), has more 2 than 1, based on the HPLC analysis, and the HPLC chromatogram indicates that the fruit may contain several delphinidin glycosides. Compound 2 is a potent antiradical compound (DPPH˙ IC50 = 26.3 μM), so it is remarkable that S. cumini fruit pulp is essentially inactive in the DPPH˙ assay. However, while the fruits may contain many delphinidin glycosides, the TPC (9.95 mg/g) was below average for the 14 fruits tested, and comparable with other fruits with low antiradical activity (IC50 > 100 μg/ml). Seed extracts of S. cumini, the part most often used in Ayurvedic medicine, were previously shown to have high levels of total phenolics and good activity in the trolox equivalent antioxidant capacity (TEAC) and ferric reducing antioxidant power (FRAP) antioxidant assays (Luximon-Ramma, Bahorun & Crozier, 2003).
Figure 2.
HPLC-PDA chromatograms at (A) 254 nm and (B) 520 nm of the standard mixture and methanolic extracts of 14 edible Myrtaceae fruits. Compounds are numbered as follows: cyanidin 3-glucoside (1), delphinidin 3-glucoside (2), ellagic acid (3), kaempferol (4), myricetin (5), quercetin (6), quercitrin (7), and rutin (8).
Table 3.
Results of HPLC-PDA quantification of common phenolics in 14 edible Myrtaceae fruits
| Cyanidin 3-glc Delphinidin 3-glc | |||||||
|---|---|---|---|---|---|---|---|
| (1) | (2)a | Ellagic acid (3) | Myricetin (5) | Quercetin (6) | Quercitrin (7) | Rutin (8) | |
| E. aggregata | 1.42 ± 0.17b | 0.04 ± 4.7 × 10-3 | NDc | 0.04 ± 3.6 × 10-3 | 0.04 ± 4.3 × 10-3 | 0.06 ± 7.8 × 10-3 | 0.48 ± 0.04 |
| E. brasiliensis | 10.2 ± 0.42 | 2.58 ± 0.13 | 0.26 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.06 ± 0.01 | 0.08 ± 0.01 |
| E. luschnathiana | ND | ND | 0.40 ± 0.01 | Trd | 0.04 ± 2.98 × 10-3 | 0.07 ± 2.31 × 10-3 | 0.18 ± 0.01 |
| E. reinwardtiana | 0.04 ± 2.2 × 10-3 | ND | ND | 0.01 ± 0.7 × 10-3 | 0.03 ± 0.6 × 10-3 | 0.05 ± 5.6 × 10-3 | 0.04 ± 5.6 × 10-3 |
| M. cauliflora | 4.33 ± 0.24 | 0.81 ± 0.06 | 0.52 ± 0.22 | 0.02 ± 2.6 × 10-3 | 0.04 ± 7.6 × 10-3 | 0.11 ± 0.03 | 0.21 ± 0.02 |
| M. dubia | 0.02 ± 3.2 × 10-3 | Tr | 0.45 ± 0.04 | ND | 0.24 ± 0.02 | 0.06 ± 7.2 × 10-3 | 0.13 ± 7.0 × 10-3 |
| M. vexator | 13.1 ± 3.17 | 0.29 ± 0.06 | 0.64 ± 0.26 | 0.03 ± 4.4 × 10-3 | 0.08 ± 0.02 | 0.05 ± 0.02 | 0.11 ± 0.02 |
| S. cumini | 0.14 ± 0.02 | 1.61 ± 0.50 | 0.03 ± 6.2 × 10-3 | ND | 0.01 ± 8.0 × 10-3 | Tr | 0.13 ± 0.02 |
| S. curranii | 9.56 ± 0.84 | 4.83 ± 0.41 | 0.09 ± 0.01 | Tr | 0.28 ± 9.0 × 10-3 | 0.17 ± 0.02 | 0.32 ± 0.01 |
| S. jambos | ND | ND | 0.05 ± 0.02 | ND | 0.01 ± 0.3 × 10-3 | 0.03 ± 4.0 ± × 10-3 | ND |
| S. javanicum | 0.01 ± 1.4 × 10-3 | ND | 0.05 ± 0.02 | ND | 0.01 ± 2.2 × 10-3 | 0.02 ± 0.9 × 10-3 | Tr |
| S. malaccense | 0.02 ± 1.5 × 10-3 | ND | 0.01 ± 1.7 × 10-3 | ND | Tr | 0.02 ± 0.01 | 0.02 ± 1.4 × 10-3 |
| S. samarangense | Tr | ND | 0.09 ± 0.01 | Tr | 0.02 ± 2.2 × 10-3 | 0.02 ± 6.0 × 10-3 | Tr |
| S. samarangense var. Taiwan pink | 1.56 ± 0.10 | ND | ND | ND | 0.07 ± 0.02 | 0.18 ± 6.6 × 10-3 | 0.16 ± 0.02 |
Amount of compound per dry weight of plant material expressed as mg/g.
Mean ± SD (n = 9).
ND, not detected.
Tr, amount was less than 0.01 mg/g.
Quercetin (6) and its glycosides are prevalent in the 14 studied fruits. These flavonols are important dietary constituents, with many chemopreventive activities (Formica & Regelson, 1995). The concentration of 6 is < 0.01 mg/g only in S. malaccense. Syzygium curranii and M. dubia had levels of 6 approximately six times higher (0.28 and 0.24 mg/g, respectively) than the other test fruits. Quercitrin (7) was also detected in every species, from < 0.01 mg/g (S. cumini) to 0.18 mg/g (S. samarangense var. Taiwan pink). Syzygium curranii had levels of 7 (0.17 mg/g) similar to S. samarangense var. Taiwan pink. Rutin (8), detected in every fruit but S. jambos, was found at the highest levels in E. aggregata (0.48 mg/g).
Ellagic acid (3) and its derivatives are also abundant in this taxon (Lowry, 1968; Okuda et al., 1982). It is likely that ellagic acid derivatives are present in many of these extracts, and contribute considerably to the overall TPC and antiradical activity. In our analysis, 3 was identified and quantified. The three Myrciaria species have the highest levels of 3 (M. cauliflora, 0.52; M. dubia, 0.45; and M. vexator, 0.64 mg/g). It was found in every species except E. aggregata, E. reinwardtiana, and S. samarangense var. Taiwan pink. Generally, it was the most abundant compound by HPLC-PDA analysis in the red, orange, and yellow-fruited species (E. luschnathiana, S. javanicum, S. jambos, S. samarangense and M. dubia).
Myricetin (5) was found in each of the Eugenia and Myrciaria species tested but M. dubia while, among the Syzygium species, it was detected only in S. curranii and S. samarangense at levels below 0.01 mg/g. Kaempferol (4) was detected in E. brasiliensis, E. luschnathiana and S. curranii, but at levels below 0.01 mg/g. M. cauliflora, S. malaccense and S. jambos were also found to contain t-cinnamic acid (9) by HPLC-PDA, but it was not quantified.
With cancers and heart disease being the leading causes of death in the United States, studies indicate that diets high in naturally occurring antioxidants and anti-inflammatories are important as a first-line strategy of chemoprevention (Youdim et al., 2001; Hu et al., 2002; Arts et al., 2005; Collins, 2005). We have shown that edible fruits in the Myrtaceae are a rich source of biologically active phenolic compounds, which is similar to other well-studied berries and fruits (Kähkönen et al., 2001).
Anthocyanins, flavonoids, phenolic acids and tannins, primarily, play ecological roles to attract pollinators and seed disbursers, and act as antifeedants and defence compounds against microbial infection (Middleton et al., 2000). Tropical Myrtaceae fruits are grown under conditions of high oxidative stress from intense sunlight and heat, and phenolic compounds inhibit lipid peroxidation and ultraviolet damage in plant tissues (Lee & Gould, 2002) in addition to protecting mammalian systems when eaten as fruit.
Acknowledgements
This work was supported in part by NIH-NCCAM grant number F31AT000801 (K.A.R.) and NIHNIGMS grant number S06GM08225 (E.J.K.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. We would like to thank Chris Rollins, Director of the Fruit and Spice Park, Larry Schokman, Director of The Kampong, Dr. Jonathan H. Crane of the Tropical Resource and Education Center, and the Broward County Rare Fruit and Vegetable Council for allowing us to collect fruit. Essential Living Foods is thanked for supplying the camu-camu (M. dubia) powder.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersson Dunstan C, Noreen Y, Serrano G, Cox PA, Perera P, Bohlin L. Evaluation of some Samoan and Peruvian medicinal plants by prostaglandin biosynthesis and rat ear oedema assays. Journal of Ethnopharmacology. 1997;57(1):35–56. doi: 10.1016/s0378-8741(97)00043-3. [DOI] [PubMed] [Google Scholar]
- Aragao C, Ikegaki M, Sato H, Oliveira IM, Park YK. Determination of ascorbic acid concentration in acerola and camu-camu fruit juices by the ascorbate oxidase method. Ciencia e Tecnologia de Alimentos. 1996;16(2):175–176. [Google Scholar]
- Arts ICW, Hollman PCH. Polyphenols and disease risk in epidemiologic studies. American Journal of Clinical Nutrition. 2005;81(1):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- Bhatia IS, Bajaj KL. Chemical constituents of the seeds and bark of Syzygium cumini. Planta Medica. 1975;28(4):346–352. doi: 10.1055/s-0028-1097868. [DOI] [PubMed] [Google Scholar]
- Bhatia IS, Bajaj KL, Ghangas GS. Tannins in black plum seeds. Phytochemistry. 1971;10(1):219–220. [Google Scholar]
- Collins AR. Antioxidant intervention as a route to cancer prevention. European Journal of Cancer. 2005;41(13):1923–1930. doi: 10.1016/j.ejca.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food and Chemical Toxicology. 1995;33(12):1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- Grover JK, Yadav S, Vats V. Medicinal plants of India with anti-diabetic potential. Journal of Ethnopharmacology. 2002;81(1):81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- Hu FB, Willett WC. Optimal diets for prevention of coronary heart disease. Journal of the American Medical Association. 2002;288(20):2569–2578. doi: 10.1001/jama.288.20.2569. [DOI] [PubMed] [Google Scholar]
- Kähkönen M, Hopia A, Vuorela H, Rauha J, Pihlaja K, Kujala T, Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. Journal of Agricultural and Food Chemistry. 1999;47:3954–3962. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- Kähkönen MP, Hopia AI, Heinonen M. Berry phenolics and their antioxidant activity. Journal of Agricultural and Food Chemistry. 2001;49(8):4076–4082. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- Kirtikar KR, Basu BD. Indian Medicinal Plants. International Book Distributors; Dehradun: 1988. [Google Scholar]
- Kong J-M, Chia L-S, Goh N-K, Chia T-F, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- Lee DW, Gould KS. Anthocyanins in leaves and other vegetative organs: an introduction. In: Gould KS, Lee DW, editors. Advances in Botanical Research: Anthocyanins in Leaves. Vol. 37. Academic Press; London: 2002. pp. 2–12. [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. Journal of AOAC International. 2005;88(5):1269–1278. [PubMed] [Google Scholar]
- Lowry JB. Distribution and potential taxonomic value of alkylated ellagic acids. Phytochemistry. 1968;7(10):1803–1813. [Google Scholar]
- Luo X-D, Basile MJ, Kennelly EJ. Polyphenolic antioxidants from the fruits of Chrysophyllum cainito L. (star apple). Journal of Agricultural and Food Chemistry. 2002;50(6):1379–1382. doi: 10.1021/jf011178n. [DOI] [PubMed] [Google Scholar]
- Luximon-Ramma A, Bahorun T, Crozier A. Antioxidant actions and phenolic and vitamin C contents of common Mauritian exotic fruits. Journal of the Science of Food and Agriculture. 2003;83(5):496–502. [Google Scholar]
- Ma J, Yang H, Basile MJ, Kennelly EJ. Analysis of Polyphenolic Antioxidants from the Fruits of Three Pouteria Species by Selected Ion Monitoring Liquid Chromatography-Mass Spectrometry. Journal of Agricultural and Food Chemistry. 2004;52(19):5873–5878. doi: 10.1021/jf049950k. [DOI] [PubMed] [Google Scholar]
- Mahmoud II, Marzouk MSA, Moharram FA, El-Gindi MR, Hassan AMK. Acylated flavonol glycosides from Eugenia jambolana leaves. Phytochemistry. 2001;58(8):1239–1244. doi: 10.1016/s0031-9422(01)00365-x. [DOI] [PubMed] [Google Scholar]
- Middleton E, Jr., Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacology Reviews. 2000;52(4):673–751. [PubMed] [Google Scholar]
- Minino AM, Heron MP, Smith BL. Deaths: Preliminary data for 2004. National Vital Statistics Reports. 2006;54(19):1–49. [PubMed] [Google Scholar]
- Morton J. Fruits of Warm Climates. Julia Morton; Winterville, NC: 1987. [Google Scholar]
- Nair AGR, Krishnan S, Ravikrishna C, Madhusudanan KP. New and rare flavonol gycosides from leaves of Syzygium samarangense. Fitoterapia. 1999;70(2):148–151. [Google Scholar]
- Nonaka G.-i., Aiko Y, Aritake K, Nishioka I. Tanins and related compounds. CXIX. Samarangenins A and B, novel proanthocyanidins with doubly bonded structures, from Syzygium samarangense and S. aqueum. Chemical and Pharmaceutical Bulletin. 1992;40(10):2671–2673. [Google Scholar]
- Okuda T, Yoshida T, Hatano T, Yazaki K, Ashida M. Ellagitannins of the Casuarinaceae, Stachyuraceae, and Myrtaceae. Phytochemistry. 1982;21(12):2871–2874. [Google Scholar]
- Pietta P-G. Flavonoids as antioxidants. Journal of Natural Products. 2000;63:1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. Journal of Agricultural and Food Chemistry. 2005;53(10):4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. European Respiratory Journal. 2006;28(1):219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- Reynertson KA, Basile MJ, Kennelly EJ. Antioxidant potential of seven Myrtaceous fruits. Ethnobotany Research & Applications. 2005;3:25–35. [Google Scholar]
- Reynertson KA, Wallace AM, Adachi S, Gil RR, Yang H, Basile MJ, D'Armiento J, Weinstein IB, Kennelly EJ. Bioactive depsides and anthocyanins from jaboticaba (Myrciaria cauliflora). Journal of Natural Products. 2006;69(8):1228–1230. doi: 10.1021/np0600999. [DOI] [PubMed] [Google Scholar]
- Seeram NP, Lee R, Scheuller HS, Heber D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chemistry. 2006;97:1–11. [Google Scholar]
- Singleton VL, Rossi JA., Jr. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965;16(3):144–158. [Google Scholar]
- Srivastava R, Shaw AK, Kulshreshtha DK. Triterpenoids and chalcone from Sysygium samarangense. Phytochemistry. 1995;38(3):687–689. [Google Scholar]
- Wang H, Nair MG, Strasburg GM, Chang YC, Booren AM, Gray JI, DeWitt DL. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. Journal of Natural Products. 1999;62(2):294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- Wong KC, Lai FY. Volatile constituents from the fruits of four Syzygium species grown in Malaysia. Flavour and Fragrance Journal. 1996;11:61–66. [Google Scholar]
- Yang H, Protiva P, Cui B, Ma C, Baggett S, Hequet V, Mori S, Weinstein IB, Kennelly EJ. New bioactive polyphenols from Theobroma grandiflorum (“cupuacu”). Journal of Natural Products. 2003;66:1501–1504. doi: 10.1021/np034002j. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: A multiplicity of effects. Free Radical Biology and Medicine. 2001;30(6):583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- Zanatta CF, Cuevas E, Bobbio FO, Winterhalter P, Mercadante A. Determination of anthocyanins from camu-camu Myrciaria dubia) by HPLC-PDA, HPLC-MS, and NMR. Journal of Agricultural and Food Chemistry. 2005;53:9531–9535. doi: 10.1021/jf051357v. [DOI] [PubMed] [Google Scholar]
- Zheng W, Wang SY. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. Journal of Agricultural and Food Chemistry. 2003;51(2):502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]