Abstract
The canonical Wnt/β-catenin signaling plays essential role in development and diseases. Previous studies have implicated the canonical Wnt/β-catenin signaling in the regulation of normal palate development, but functional Wnt/β-catenin signaling and its tissue-specific activities remain to be accurately elucidated. In this study, we show that functional Wnt/β-catenin signaling operates primarily in the palate epithelium, particularly in the medial edge epithelium (MEE) of the developing mouse palatal shelves, consistent with the expression patterns of β-catenin and several Wnt ligands and receptors. Epithelial specific inactivation of β-catenin by the K14-Cre transgenic allele abolishes the canonical Wnt signaling activity in the palatal epithelium and leads to an abnormal persistence of the medial edge seam (MES), ultimately causing a cleft palate formation, a phenotype resembling that in Tgfβ3 mutant mice. Consistent with this phenotype is the down-regulation of Tgfβ3 and suppression of apoptosis in the MEE of the β-catenin mutant palatal shelves. Application of exogenous Tgfβ3 to the mutant palatal shelves in organ culture rescues the midline seam phenotype. On the other hand, expression of stabilized β-catenin in the palatal epithelium also disrupts normal palatogenesis by activating ectopic Tgfβ3 expression in the palatal epithelium and causing an aberrant fusion between the palate shelf and mandible in addition to severely deformed palatal shelves. Collectively, our results demonstrate an essential role for Wnt/β-catenin signaling in the epithelial component at the step of palate fusion during palate development by controlling the expression of Tgfβ3 in the MEE.
Keywords: Wnt/β-catenin signaling, Tgf-β3, palate fusion, cleft palate
Research Highlights.
The canonical Wnt signaling activity is detected in the developing palate, particularly in the MEE.
Epithelial inactivation of Catnb causes downregulation of Tgfβ3 and persistent midline seam, leading to a cleft palate formation.
Inclusion of exogenous Tgfβ3 in organ culture rescues the palate fusion defect in the K14Cre;CatnbF/F palate.
Ectopic activation of the caonical Wnt signaling in the palatal epithelium results in ectopic activation of Tgfβ3 and aberrant palate-mandible fusion.
Wnt/β-catenin signaling functions in the palatal epithelium to control palate fusion by regulating Tgfβ3 expression in the MEE.
Introduction
Palate development is a unique process during mammalian embryogenesis: two secondary palatal shelves outgrow from bilateral maxillary processes ab initio and then, together with the primary palate, fuse to form an intact structure. Palate fusion is a characteristic and crucial step of palatogenesis. To prepare for this step, the two secondary palatal shelves have to elevate to the horizontal position above the tongue and adhere to each other with their medial edge epithelium (MEE), which then develops into a single layered medial edge seam (MES). Progressive elimination of the MES ultimately leads to fusion of the two palatal shelves that become the definite palate. Subsequently, the definite secondary palate further fuses with the primary palate and the nasal septum, forming a complete palatal structure that separates the oral and nasal cavities (Ferguson, 1988). Abnormal persistence of MES prevents palate fusion, leading to a cleft palate formation, as exemplified in Tgfβ3 mutants (Kaartinent et al., 1995; Proetzel et al., 1995;Taya et al., 1999).
In humans, the cleft palate is a prevalent birth defect whose etiologies are still poorly understood. The mouse shares great similarity with the human in embryogenesis and its genome can be manipulated by sophisticated tools, allowing dissection of gene function in temporal and spatial manners. Recent studies have implicated complicated genetic networks in palatogenesis and demonstrated essential roles for growth factor signaling pathways in each step of this process (Gritli-Linde, 2007; Jugessur et al., 2009). For example, Bmp, Shh and Fgf signaling pathways are crucial for palate outgrowth and patterning, Pdgf signaling has a role in palate elevation, and Tgfβ1–3 engaged signaling cascade is required for MES disintegration and palate fusion (Gritli-Linde, 2007).
The canonical Wnt/β-catenin signaling pathway plays an essential role in multiple developmental processes, including craniofacial development (Grigoryan et al., 2008; Liu and Millar, 2010). Active Wnt/β-catenin signaling has been detected in the cranial neural crest cells, nasal ectoderm, taste papilla, and developing tooth (Lan et al., 2006; Liu et al., 2007a; Liu et al., 2008; Lohi et al., 2010; Mani et al., 2010). Wnt1-Cre mediated deletion of Catnb, which encodes β-catenin protein, leads to an absence of the cranial neural crest-derived structures, and epithelial specific inactivation of Catnb causes defective development of the tooth, hair follicle, and taste papilla (Brault et al., 2001; Huelsken et al., 2001; Liu et al., 2007a; Liu et al., 2008). In addition, targeted inactivation of Lrp6, a key receptor for Wnt/β-catenin signaling, causes severe craniofacial defects, including cleft lip and cleft palate in mice (Song et al., 2009)
Mutations in several WNT genes have been linked to cleft lip/palate defect in humans (Chiquet et al., 2008). In mice, expression of a number of Wnt ligands has been reported in the developing palate, and cleft palate phenotype has been shown in several mouse models deficient for Wnt signaling components, including Wnt5a, Wnt9b, Gsk3β, and Rspo2 (Brown et al., 2003; Lan et al., 2006; Liu et al., 2007b; He et al., 2008; Warner et al., 2009; Yamada et al., 2009; He et al., 2010a). Wnt5a was shown to regulate cell proliferation and cell migration in the developing palate via Ror2-mediated noncanonical pathway (He et al., 2008). The evidence for a direct involvement of β-catenin in palate development came from the studies in which tissue specific deletion of Catnb in the palatal mesenchyme produces a cleft palate defect (Chen et al., 2009). However, since functional Wnt/β-catenin signaling has not yet been evidenced in the developing palatal shelves, the requirement of β-catenin for the palatal mesenchyme could be attributed to its cell-adhesion function. In addition, despite strong β-catenin expression in the developing palatal epithelium, particularly in the MEE (Maritinez-Alvarez et al., 2000; Tudela et al., 2002; Nawshad and Hay, 2003; He et al., 2008), its role in the epithelial component for palatogenesis appears elusive. This is because a cleft palate phenotype was not reported in mice carrying Cre-mediated ablation of Catnb in the palatal epithelium in the previous studies (Huelsken et al., 2001; Liu et al., 2008).
To reveal a role for the canonical Wnt/β-catenin signaling in palatogenesis, we surveyed the expression of a number of Wnt signaling molecules, receptors, and antagonists, and examined activity of Wnt/β-catenin signaling in the developing palatal shelves. We found that the canonical Wnt/β-catenin signaling is primarily activated in the palatal epithelium, particularly in the MEE, consistent with the restricted expression of several canonical Wnt ligands and receptors, and β-catenin. We used K14Cre-mediated gene ablation to inactivate Catnb function in the palatal epithelium. The conditional knockout mice (K14Cre;CatnbF/F) exhibit a cleft palate defect due to failed palate fusion, consistent with a down-regulation of Tgfβ3 expression and suppression of apoptosis in the MEE cells. The persistent midline seam phenotype in the mutant palate could be rescued by exogenous Tgfβ3 in organ culture. Ectopic activation of Wnt/β-catenin signaling in the palatal epithelium induces ectopic Tgfβ3 expression, resulting in an aberrant palate-mandible fusion and ultimately a cleft palate formation. Our results indicate that functional Wnt/β-catenin signaling operates primarily in the epithelium to control palate fusion by regulating Tgfβ3 expression during palate development.
Materials and methods
Animals
TOPGAL (DasGupta and Fuchs, 1999), BATGAL (Maretto et al., 2003), and CatnbF/F (Brault et al., 2001) mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Genotyping of K14Cre and CatnbF(ex3) mice have been described previously (Andl et al., 2004; Harada et al., 1999). To inactivate Catnb specifically in the embryonic epithelium, K14Cre;CatnbF/+ mice were crossed to CatnbF/F mice to generate K14Cre;CatnbF/F mice. The Wnt/β-catenin signaling gain-of-function model (K14Cre;CatnbF(ex3)) was generated by intercrossing K14Cre mice with CatnbF(ex3) mice. Animals and procedures used in this study were approved by the Tulane University Institutional Animal Care and Use Committee.
In vitro organ culture
For in vitro palate fusion assay, paired palatal shelves were carefully dissected from embryonic day 13.5 (E13.5) K14Cre;CatnbF/F mutant and control embryos. K14Cre;CatnbF/F embryos at this stage can be easily identified by hypoplastic limb buds (data not shown), and confirmed late by a PCR-based genotyping. Paired palatal shelves were placed on a filter paper in Trowell type organ culture and were oriented and juxtaposed with the MEE facing each other closely, as described previously (Taya et al., 1999; Zhang et al., 2002). Samples were cultured in a chemical defined medium with or without recombinant Tgfβ3 (50 ng/ml) at 37º for 72 hrs (Taya et al., 1999). Medium was changed once after 48 hrs in culture. Samples were then harvested for fixation and histological analysis.
Histology, in situ hybridization, and X-gal staining
Mouse embryos were collected from timed pregnant females in ice-cold PBS and fixed in 4% paraformaldehyde (PFA)/PBS solution at 4°C overnight. Following dehydration through gradient ethanol, samples were embedded in paraffin and coronally sectioned at 10μm. Slides were subjected to either Hematoxylin/Eosin staining for histological analysis or to non-radioactive in situ hybridization, as described previously (St. Amand et al., 2000). For whole mount in situ hybridization, samples were dehydrated through gradient methanol after overnight fixation in 4% PFA, and were subjected to non-radioactive in situ hybridization assay as described before (Zhang et al., 1999). Whole mount and section X-gal staining of Wnt reporter embryos were carried out as described previously (Chai et al., 2000; He et al., 2010b).
Cell proliferation and TUNEL assays
To assess the cell proliferation rate, timed pregnant female mice were injected with BrdU solution (Bromodeoxyuridine (BrdU) Labeling and Detection Kit) (Roche Diagnostics Corporation, Indianapolis) at the concentration of 1.5 ml/100 g body weight. Embryos were harvested 1 hr later, Carnoy-fixed, paraffin-embedded, sectioned, and processed for immunodetection of BrdU labeling, as described previously (Xiong et al., 2009). BrdU-positive cells were counted in an arbitrary area in the palatal mesenchyme and epithelium, respectively. Nine continuous sections from three individual samples were counted, and the outcome of BrdU labeling was presented as percentage of BrdU-positive cells among total nuclei in the fixed area. To determine the significance of difference, data were subjected to Student’s t-test. TUNEL assay were performed to detect apoptotic cells as described previously (Alappat et al., 2005). TUNEL-positive cells were counted on palatal shelf sections from three mutant and control embryos, respectively.
Results
Wnt/β-catenin signaling activity and expression of Wnt/β-catenin signaling components in the developing secondary palate
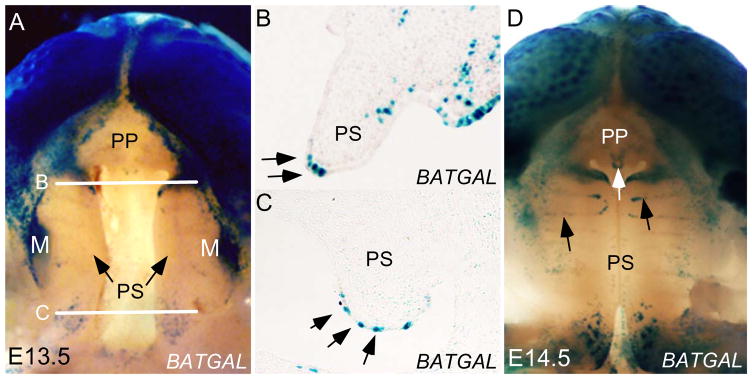
Previous studies have shown a restricted expression of Catnb in the epithelium of developing palatal shelves, implicating a role for Wnt/β-catenin signaling in palatogenesis (Maritinez-Alvarez et al., 2000; Tudela et al., 2002; Nawshad and Hay, 2003; He et al., 2008). However, by using the TOPGAL transgenic reporter mice, we failed to detect activity of the canonical Wnt/β-catenin signaling in the developing palatal shelves (He et al., 2008; He et al., 2010a). This result appears to argue against for an involvement of functional Wnt/β-catenin signaling in palate development. However, the concern that the TOPGAL transgenic allele is not sensitive enough to detect low signaling level prompted us to use a different transgenic Wnt reporter line, the BATGAL mice, and Axin2 expression as an indicator of Wnt/β-catenin signaling activity. Indeed, we observed Axin2 expression in the developing palatal shelves, primarily in the MEE region (Fig. 3C; Fig. 7G; He et al., 2010a). Consistent with this observation, we detected LacZ reporter expression in the MEE of the palatal shelves of BATGAL embryo at embryonic day 13.5 (E13.5), with a few sporadic positive cells in the anterior palatal mesenchyme and some above background positively stained cells in the posterior palatal mesenchyme (Fig. 1A–C). At E14.5, LacZ reporter expression expanded to the rugae and primary palate in BATGAL embryo (Fig. 1D). The lack of LacZ reporter expression in the middle portion of the developing palate is very likely due to relatively lower level of canonical Wnt signaling that is below the sensitivity of the BATGAL reporter. The variation in reporter activities in the developing palatal shelves of TOPGAL and BATGAL transgenic mice is not a surprise, since similarities and differences in the patterns of reporter activities during craniofacial development in these mice have been reported previously (Brugmann et al., 2007). Nevertheless, these results demonstrate the presence of functional Wnt/β-catenin signaling in the developing palate, especially in the MEE region.
Figure 3.
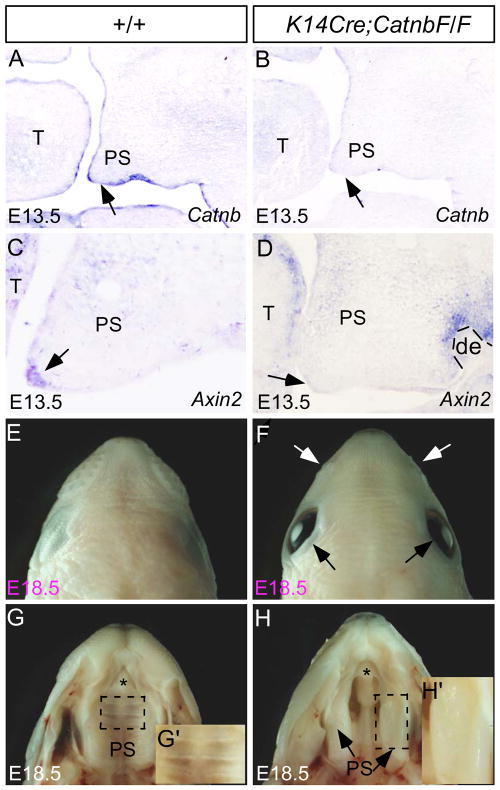
Epithelial ablation of Catnb generates a cleft palate defect. (A) At E13.5, Catnb is expressed in the palatal epithelium, including the MEE (arrow). (B) Catnb expression is undetectable in the palatal epithelium (arrow) as well as oral epithelium of an E13.5 K14Cre;CatnbF/F embryo. (C) Axin2 expressed is detected in the MEE (arrow) and in the palatal mesenchyme at a low level of an E13.5 control palate. (D) An E13.5 K14Cre;CatnbF/F palatal shelf show absent Axin2 expression in the MEE (arrow), but a weak expression in the palatal mesenchyme and a strong expression in the dental mesenchyme remain. (E, F) A dorsal view of an E18.5 K14Cre;CatnbF/F head (F) shows open eyelid (black arrows) and hypoplastic whisker pad (while arrows), as compared to a littermate control. (G, H) An E18.5 wild type embryo develops an intact palate (G) while the mutant exhibits a complete cleft secondary palate. (G’, H’) High magnification images of E18.5 wild type (G’) and mutant (H’) palate show rugae in the wild type control and the lack of rugal formation in the mutant. T, tongue; de, dental epithelium; PS, palatal shelf. Asterisk indicates the primary palate.
Figure 7.
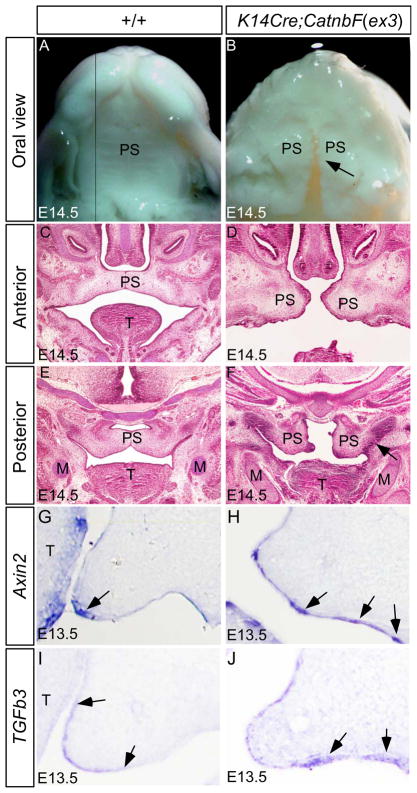
Ectopic Wnt/β-catenin signaling activity in the palatal epithelium activates ectopic Tgfβ3 expression and causes cleft palate defect. (A, B) At E14.5, wild type embryo develops an intact palate with visible rugal formation (A), but the palatal shelves remain separately in K14Cre;CatnbF(ex3) embryo, forming a clefting (arrow), and do not develop rugae (B). (C, E) Histological analysis reveals that the wild type palate has elevated and fused at E14.5, in both the anterior (C) and posterior region (E). (D, F) In E14.5 K14Cre;CatnbF(ex3) embryo, the anterior palatal shelves are positioned at the horizontal level, but are severely deformed and are too short to make contact at the midline (D); the posterior palate shelves fail to elevate and exhibit aberrant fusion (arrow) with the mandible (F). (G, H) At E13.5, Axin2 expression (arrow) is restricted to the MEE of wild type palate (G), but its expression is ectopically activated in the mutant palatal epithelium, particularly in the ventral palatal epithelium (arrows) (H). (I) Tgfβ3 expression is detected in the MEE of E13.5 control palate. Arrows define the expression domain in the palatal shelf. (J) Ectopic Tgfβ3 expression (arrows) is detected in the E13.5 K14Cre;Catnb(ex3) palatal epithelium. M, Meckel’s cartilage; T, tongue; PS, palatal shelf.
Figure 1.
Detection of Wnt/β-catenin signaling activity in the palatal epithelium. (A) Oral view of an E13.5 BATGAL reporter embryonic head (mandible removed) shows LacZ staining in the upper lip, developing molar, and the secondary palate, but not in the primary palate. Arrows point to the secondary palatal shelves. While lines indicate section levels shown in panel B and panel C, respectively. (B, C) Coronal sections of an E13.5 BATGAL embryo show that Wnt/β-catenin signaling activity is largely restricted to the MEE cells (arrows) in both the anterior (B) and posterior palatal shelf (C). Sporadic LacZ-positive cells are found in the anterior palatal mesenchyme. (D) Oral view of an E14.5 BATGAL reporter embryonic head with removal of mandible reveals that LacZ activity has expanded to the primary palate (white arrow) and rugae (black arrows) in addition to the patterns observed at E13.5. M, Molar; PP, primary palate; PS, secondary palatal shelf.
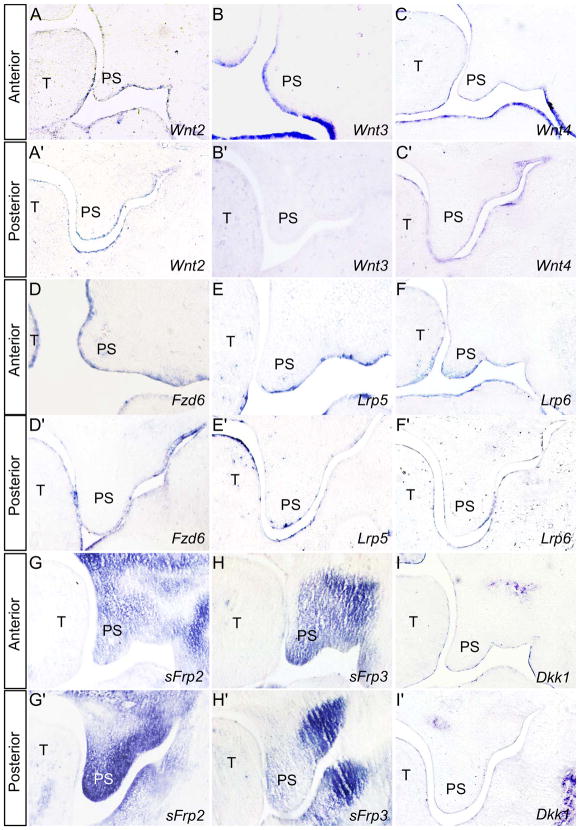
In a microarray survey of gene expression profile in E13.5 mouse palatal shelves, we identified 18 genes encoding components of Wnt signaling pathway (data not shown). We chose to examine in the developing palate the expression of some of these factors that act in the canonical Wnt/β-catenin signaling pathway, including ligands, receptors, and extracellular antagonists. At E13.5, all three canonical Wnt ligands examined show a restricted expression in the palatal epithelium, with Wnt2 and Wnt4 in both the anterior and posterior palate (Fig. 2A, 2A’, 2C, 2C’), and Wnt3 in the anterior palate (Fig. 2B, 2B’). Activation of Wnt/β-catenin signaling requires both Fzd receptors and LRP5/6 co-receptors. We have reported previously the expression of Fzd4 in the developing palate (He et al., 2008), and here we show that Fzd6, Lrp5 and Lrp6 mRNAs are expressed primarily in the palatal epithelium, with Lrp6 expression being also observed in anterior palatal mesenchyme (Fig. 2D–F’). We also confirmed by in situ hybridization the expression several Wnt signaling antagonists/regulators in the developing palate. Of these, expression of Sfrp2 and sFrp4 was detected dominantly in the palatal mesenchyme; both of them exhibit a gradient expression along the anterior-posterior axis, with higher level of sFrp2 in the posterior and sFrp3 in the anterior domain (Fig. 2G, 2G’, 2H and 2H’). Dkk1 expression was found to be restricted in the anterior palatal epithelium, consistent with its previously reported expression pattern (Lieven et al., 2010). Interestingly, almost all Wnt/β-catenin signaling components that were examined show a restricted expression in the palatal epithelium, while the extracellular antagonists sFrp2 and sFrp3but not Dkk are strongly expressed in the mesenchyme. The presence of these Wnt signaling antagonists appears to modulate and confine functional Wnt/β-catenin signaling to the MEE region, as demonstrated by BATGAL reporter activity and Axin2 expression.
Figure 2.
Expression of Wnt/β-catenin signaling components and antagonists in E13.5 developing palate. (A–C’) Wnt2 (A, A’) and Wnt4 (C, C’) are expressed in the epithelium of both anterior and posterior palate (A, A’, C, C’), respectively, while Wnt3 is expressed only in the anterior palate epithelium (B, B’). (D–F’) Wnt receptors Fzd6 (D, D’), Lrp5 (E, E’), and Lrp6 (F, F’) are expressed in palatal epithelium, and Lrp6 is also expressed in the anterior palatal mesenchyme (F). (G, G’) Sfrp2 expression is detected strongly in the palatal mesenchyme, forming an expression gradient from the posterior to the anterior domain. (H, H’) Sfrp4 expression in the palatal mesenchyme also forms a gradient with the highest level in the anterior palate. (I, I’) Dkk1 is expressed in the palatal epithelium. T, tongue; PS, palatal shelf.
Epithelial inactivation of β-catenin causes cleft palate by impairing palate fusion
Despite the fact that a cleft palate phenotype was not reported in mice with K14Cre-mediated epithelial ablation of Catnb in previous studies (Huelsken et al., 2001; Liu et al., 2008), the presence of Wnt/β-catenin signaling activity in the MEE let us to revisit potential role of Wnt/β-catenin signaling in the epithelium during palatogenesis. We generated K14Cre;CatnbF/F mice using a K14Cre line that exhibits Cre activity as early as E11.5 in the craniofacial epithelium including the palatal shelves (He et al., 2010b). Our in situ hybridization assay showed that at E13.5, Catnb expression is completely abolished, so is the expression of the Wnt/β-catenin signaling target Axin2, in the mutant palatal epithelium (Fig. 3A–D). These results confirm a successful ablation of Catnb and Wnt/β-catenin signaling activity. A low level of Axin2 expression remained in the palatal mesenchyme of K14Cre;CatnbF/F embryo further supports a tissue-specific inhibition of Wnt/β-catenin signaling activity. At E18.5, in general, we did not find significant alterations in craniofacial structures, but we did observe a slightly pointed head with open eyelid and hypoplastic whisker pad in K14Cre;CatnbF/F embryos (Fig. 3E–H). However, gross examination revealed a complete cleft of the secondary palate phenotype in K14Cre;CatnbF/F embryos with 82% penetrance (18/22), indicating an essential role for Wnt/β-catenin signaling in secondary palate development (Fig. 3H). The inconsistency of the K14 promoter activity could have contributed to this incomplete penetrance of the cleft palate defect in K14Cre;CatnbF/F mice. Since BATGAL expression is seen in the palatal rugae at E14.5 (Fig. 1D), suggesting a potential role for the canonical Wnt signaling in the regulation of rugal formation. In accordance with this assumption, a closer morphological examination revealed the absence of rugae in the mutant (Fig. H’). Thus possibility exists that the disrupted rugal formation may contribute to the cleft palate defect in K14Cre;CatnbF/F mice.
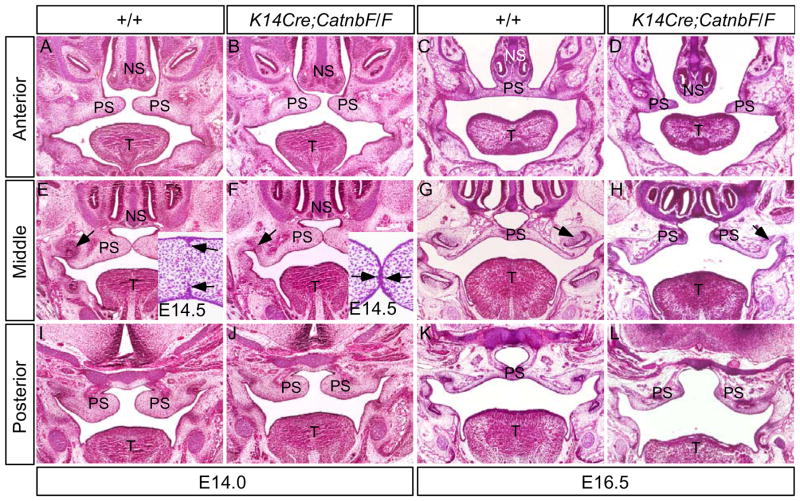
To characterize the cleft palate defects in K14Cre;CatnbF/F embryos, we performed histological analysis on both mutant and control embryos aging from E11.5 to E16.5. We found that the mutant palate showed structures comparable morphologically to littermate controls from E11.5 to E14.0 (Fig. 4; and data not shown). At E14.0, similar to littermate controls, the mutant palatal shelves had elevated to the horizontal position and made contact in the midline in the middle portion of the palate (Fig. 4A, 4B, 4E, 4F, 4I, and 4J). At E14.5, the MES in the control had begun to disappear (insert in Fig. E), but remained persistent in the mutant (insert in Fig. 4F). At E16.5, the control palate have completed fusion and formed an intact structure (Fig. 4C, 4E, 4K). In contrast, the mutant palatal shelves did not fuse, and stayed separately, forming a complete clefting along the anterior-posterior axis (Fig. 4D, 4H, 4L). This cleft palate phenotype appears to resemble that observed in Tgfβ3 mutant mice (Kaartinent et al., 1995; Proetzel et al., 1995)
Figure 4.
K14Cre;CatnbF/F palate shelves exhibit normal outgrow and elevation. (A, E, I) At E14.0, the wild type palate shelves have elevated horizontally and made contact at the middle position. (B, F, J) At the same stage, mutant palate shelves develop comparably to the control. Note molar tooth in mutant is arrested at the lamina stage (arrow in F). Insert in (E) shows disappearance of the midline seam at E14.5. Only residual epithelial seam (arrows) is present at this stage. Insert in (F) shows the persistent midline seam (arrows) in the E14.5 mutant palate (C, G, K) At E16.5, the wild type palate has fused to form an intact structure, and the molar has developed to the bell stage (arrow in G). (D, H, L) At E16.5 in mutant, the palatal shelves are separated, forming a complete cleft of the secondary palate, and the mutant molar development remains at the lamina stage (arrow in H). T, tongue; NS, nasal septum; PS, palatal shelf.
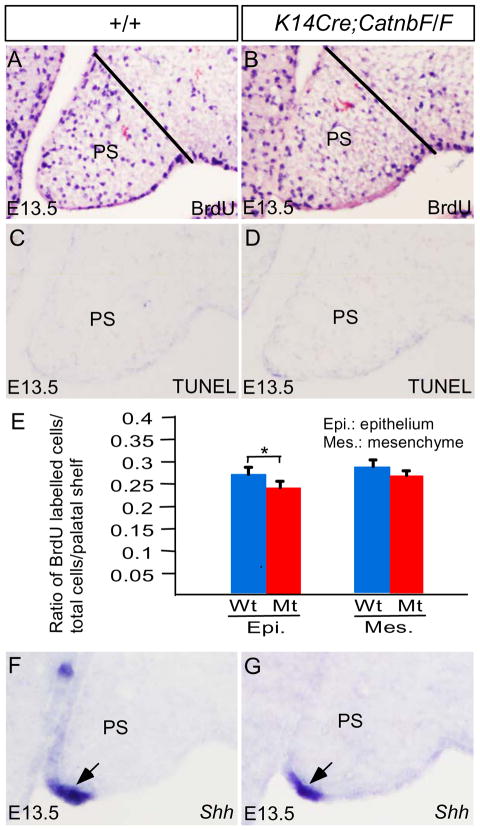
To investigate if deletion of Catnb in the palatal epithelium would alter cell proliferation rate and cause ectopic cell apoptosis in the palatal shelves, we conducted BrdU labeling and TUNEL assays, respectively, on control and mutant palate. We found that cell proliferation rate in the palatal mesenchyme remained comparable between the controls and mutants at E13.5, but the rate in the mutant palatal epithelium indeed decreased as compared to that in controls (P < 0.05) (Fig. 5A, 5B, 5E). Using TUNEL assay, we did not observe excessive apoptotic cells in the mutant palatal shelves at E12.5 and E13.5, as compared to controls (Fig. 5C, 5D; and data not shown). Shh, a known down-stream target of Wnt/β-catenin signaling in several developing organs, is expressed in the MEE of the anterior palate and rugae of the palatal shelves and acts in palatal mesenchyme to regulate cell proliferation (Zhang et al., 2002; Rice et al., 2004; Han et al., 2009; Lan and Jiang et al., 2009). Consistent with an unaltered cell proliferation rate in the palatal mesenchyme of K14Cre;CatnbF/F embryo, Shh expression remained unchanged in the MEE, as shown by in situ hybridization (Fig. 5F, 5G). Given the fact that the mutant palatal shelves did not exhibit an altered morphology and were able to make contact at the right time and right place (Fig. 4), it appears that the decreased cell proliferation rate in the palatal epithelium of mutants does not have significant impact on palatal growth, patterning, and elevation at the early developmental stage. However, we still cannot rule out the possibility that the decreased cell proliferation rate in the epithelium, even it is relatively mild, may contribute at certain extent to the formation of cleft palate in K14Cre;CatnbF/F mutant.
Figure 5.
Cell proliferation, apoptosis, and Shh expression in K14Cre;CatnbF/F palatal shelves. (A, B) E13.5 palatal shelves from control (A) and mutant (B) show BrdU labeling. Black lines demarcate the palatal region for counting of BdrU-positive cells in the epithelium and mesenchyme, respectively. (E) Comparison of BrdU-labeled cells in epithelium and mesenchyme of fixed area in control and mutant palatal shelves. *: P < 0.05. (F, G) Shh expression (arrow) is detected in the MEE of the palatal shelves from E13.5 control (F) and mutant (G). PS, palatal shelf.
Wnt/β-catenin signaling is required for Tgfβ3 expression in the MEE
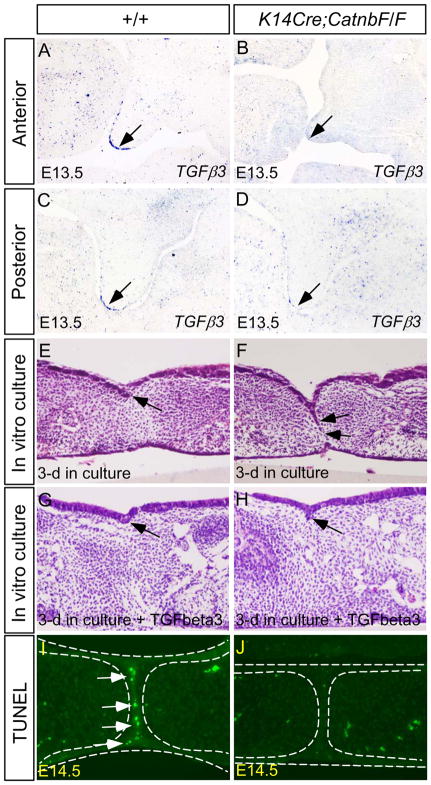
TGFβ signaling plays an essential role in palate fusion. Inactivation of Tgfβ3 or its receptors Alk2 and Alk5 in the palatal epithelium disrupts palate fusion (Kaartinent et al., 1995; Proetzel et al., 1995; Dudas et al., 2004; Martinez-Alvarez et al., 2004; Murillo et al., 2009; Xu et al., 2006; Yang and Kaartinen, 2007). On the other hand, ectopic Tgfβ3 activity leads to ectopic palatal epithelial cell death in both in vivo and in vitro conditions (Alappat et al., 2005; Murillo et al., 2009; Xu et al., 2006; He et al., 2010b). The similarity in cleft palate formation in Tgfβ3 mutants and K14Cre;CatnbF/F mice and the overlapped expression of Tgfβ3 with BATGAL activity and Axin2 expression in the MEE of the developing palatal shelves (Fig. 6A, 6C) prompted us to determine if the canonical Wnt signaling functions to regulate Tgfβ3 expression. As we expected, Tgfβ3 expression was almost, if not completely, abolished in the MEE of the K14Cre;CatnbF/F palatal shelves along the anterior-posterior axis, as compared to controls (Fig. 6B, 6D; Suppl. Fig. 1). To assess fusion ability of the K14Cre;CatnbF/F palatal shelves , paired palatal shelves isolated from E13.5 mutants and littermate controls were cultured in the Trowell-type organ culture set-up. After 72 hrs in culture, control palatal shelves underwent fusion, as indicated by the disappearance of the midline seam and establishment of the mesenchymal continuity (3/3) (Fig. 6E). In contrast, the mutant palatal shelves failed to fuse, evidenced by the persistent midline seam (MES) (4/4) (Fig. 6F). However, we noted that the midline seam in all cases was not fully persistent. This is very likely due to residual Tgfβ3 expression as the consequence of inconsistency of the K14 promoter and Cre activities in transgenic animals. Nevertheless, addition of exogenous Tgfβ3 protein in organ culture caused complete disappearance of the midline seam and rescued the fusion defect in the mutant (Fig. 6H), confirming that a down-regulation of Tgfβ3 is responsible for the persistent midline seam phenotype. Since programmed cell death is crucial for MES elimination and palate fusion (Cuervo and Covarrubias, 2004; Nawshad, 2008), we examined cell apoptosis in mutant and the control palate. At E14.5, intensive apoptotic cells were detected by TUNEL assay in the MES of control embryos (Fig. 6I). However, we detected rare apoptotic cells, if there was any, in the mutant MES at the same stage (Fig. 6J). These results indicate that suppression of cell apoptosis in the MES attributes to failed palate fusion, leading to a cleft palate formation in K14Cre;CatnbF/F mice. While the possibility exists that the cell adhesion role of β-catenin in the epithelium may contribute palate fusion, the down-regulation of Tgfβ3 expression and suppression of apoptosis in the MEE cells in the absence of functional Wnt/β-catenin signaling strongly support an essential role for the β-catenin-mediated signaling in palate development.
Figure 6.
Palate fusion in K14Cre;CatnbF/F embryo is disrupted by abnormal MES persistence due to downregulation of Tgfβ3 and failed cell apoptosis. (A, C) In E13.5 control palatal shelf, Tgfβ3 is expressed in the MEE cells (arrow) in the anterior (A) and posterior palatal shelf (B). (B, D) Tgfβ3 expression is not detectable in the MEE of the anterior (B) and posterior (D) palatal shelf of E13.5 mutant. (E) Palatal shelves from E13.5 wild type control have successfully fused after 3-day in culture. Arrow points to the remainder of the midline seam. (F) Palatal shelves from E13.5 mutant show limited fusion and the majority of MES is persistent (arrows). (G, H) Histological sections show disappearance of the midline seam in both control (G) and mutant (H) palatal shelves after 3-day in culture in the presence of exogenous Tgfβ3. Arrows point to the remainders of the midline seam. (I) TUNEL assay reveals extensive apoptotic cells in the palatal MES of E14.5 wild type embryo. (J) Apoptotic MES cells are hardly detected in the mutant MES at E14.5
Wnt/β-catenin signaling is sufficient to induce Tgfβ3 expression
To further confirm that it is β-catenin-mediated Wnt signaling rather than its cell adhesion function that regulates Tgfβ3 expression, we took a gain-of-function approach by ectopically activating a stabilized form of β-catenin in embryonic epithelium. We used an exon3 floxed Catnb allele (CatnbF(ex3)), which, upon Cre-mediated recombination, produces a stabilized β-catenin, leading to ectopic activity of the canonical Wnt signaling (Harada et al., 1999). To do this, we compounded the K14Cre allele to the CatnbF(ex3) alelle to generate K14Cre;CatnbF(ex3) embryos. At E14.5, when the control palate had completed elevation and fused, the mutant palatal shelves appeared too short to make contact at the midline, forming a complete cleft of the secondary palate (Fig. 7A, 7B). Histological analysis revealed that in mutant the anterior palatal shelves grew horizontally similar to the controls, but the posterior palatal shelves remained in a vertical position and fused to the mandible abnormally (Fig. 7C–F). This phenotype resembles that found in mice deficient for either Fgf10 Noggin; in both these mutants ectopic Tgfβ3 expression and excessive apoptotic cells were observed in the ventral palatal epithelium (Alappat et al., 2005; He et al., 2010b). This is indeed the case we found. In the palatal shelves of K14Cre;CatnbF(ex3) embryos at E13.5, we detected ectopic expression of Axin2 as well as Tgfβ3 in the palatal epithelium (Fig. 7G–J; Suppl. Fig. 1), indicating that ectopic Wnt/β-catenin signaling is sufficient activate Tgfβ3 expression. In addition to aberrant palate-mandible fusion, the palatal shelves of K14Cre;CatnbF(ex3) mice also displayed several morphological abnormalities (Fig. 7D, 7F), suggesting alteration in other signaling pathways. Indeed, elevated cell proliferation rate was found in the palatal mesenchyme of K14Cre;CatnbF(ex3) mice (data not shown). We are currently investigating the underlying mechanisms. It was also noticeable that the rugae failed to form in the K14Cre;CatnbF(ex3) palatal shelves at E14.5 (Fig. 7B). At E18.5, the mutant embryo developed severely hyper-keratinized epidermis, making it impossible to see if any rugal structure forms anyway (data not shown). Taken together, mis-regulated Wnt/β-catenin signaling in the palatal epithelium disrupts multiple developmental processes during palatogenesis, leading to cleft palate formation, but the aberrant palate-mandible fusion phenotype appears to be resulted from the ectopic activation of Tgfβ3 in the ventral palatal epithelium.
Discussion
Mutations in several WNT genes have been associated with cleft lip/palate in humans; however, the function of these genes and their engaged signaling pathways in palate development still remains to be elucidated (Chiquet et al., 2008). We previously reported that Wnt5a-activated noncanonical Wnt signaling is required for directional cell migration and normal cell proliferation during palate development (He et al., 2008). However, despite of increasing evidence, the lack of evidence for the presence of functional Wnt/β-catenin signaling in the developing palate makes it elusive as if the canonical Wnt/β-catenin signaling plays a role in palate development. In this paper, we show that the canonical Wnt/β-catenin signaling does operate in the palatal epithelium and has an essential role in palate fusion through regulating Tgfβ3 expression in the MEE.
β-catenin constitutes the core mediator of the canonical Wnt signaling, but it also has an important role in cell adhesion by linking actin-cytoskeleton to E-cadherin (Nelson and Nusse, 2004; Brembeck et al., 2006). Thus, the cleft palate defect in K14Cre;CatnbF/F could be attributed to either Wnt/β-catenin signaling deficiency or an defective adhesion, or both in the palatal epithelium. Taking account of our results and previously reported evidence, we tend to support a signaling role for β-catenin in palate development: 1) functional Wnt/β-catenin signaling is present in the palatal epithelium, especially in the MEE, overlapping with Tgfβ3 expression; 2) mice deficient for both the Wnt signaling effectors Tcf4 and Lef1 exhibit persistent palatal MES, resembling the phenotype observed in K14Cre;CatnbF/F mice (Brugmann et al., 2007); 3) mutation in Lrp6 leads to a cleft palate formation (Song et al., 2009); 4) while E-cadherin is expressed in the palatal epithelium, epithelial ablation of its function does not disrupt palate development (Luning et al., 1994; Tunggal et al., 2005). However, the cell adhesion function of β-catenin may still contribute to normal palate development. This is exemplified by the observation that inactivation of Catnb in the palatal mesenchyme where Wnt/β-catenin signaling activity is present at a relatively lower level causes a cleft palate formation (Chen et al., 2009).
Numerous previous studies have implicated a crucial role for β-catenin has been in regulation of cell proliferation and apoptosis. Deletion of β-catenin reduces proliferation rate and enhances cell apoptosis. In line with the role of β-catenin in regulating cell proliferation, a decreased level of cell proliferation was found in the palatal epithelium of K14Cre;CatnbF/F mice. However, cell proliferation rate remained unaltered in the palatal mesenchyme of K14Cre;CatnbF/F mice. Shh shares an overlapped expression with β-catenin in the palatal epithelium, and acts in the palatal mesenchyme to regulate cell proliferation (Zhang et al., 2002: Rice et al., 2002; Han et al., 2009; Lan and Jiang, 2009). Despite being a downstream target of the Wnt/β-catenin signaling in the hair follicle, tooth germ, and taste papilla (Huelsken et al., 2001; Silva-Vargas et al., 2005; Liu, et al., 2007a; Liu et al., 2008), inactivation of β-catenin did not affect Shh expression in the MEE, consistent with an unchanged level of cell proliferation in the palatal mesenchyme. Interestingly, we did not detect excessive cell death in the palatal epithelium of K14Cre;CatnbF/F mice, suggesting differential roles for β-catenin in different developing organs and at different developmental stages.
Tgfβ3 is expressed in the MEE and its essential role in palate fusion is widely accepted. Inactivation of Tgfβ3 or its receptors leads to cleft palate defect due to persistence of the MES, which is disintegrated during normal palate formation (Dudas et al., 2004; Martinez-Alvarez et al., 2004; Xu et al., 2006). While many studies have been conducted to look into molecular mechanism and downstream cascade of Tgfβ3 action in palate fusion (Martinez-Alvarez et al., 2004; Yang and Kaartinen, 2007; Nawshad, 2008), upstream regulators of Tgfβ3 are less understood. We have previously demonstrated that both Fgf10 and Noggin function to repress Tgfβ3 transcription in the palatal epithelium at ventral side (Alappat et al., 2005; He et al., 2010); however, upstream activators that regulate Tgfβ3 expression in the MEE remain unknown. It was reported previously that a TCF binding site exists in the Tgfβ3 upstream region, and ChIP assay further confirmed binding of β-catenin and TCF4 to this site in an in vitro system, suggesting that Wnt/β-catenin signaling regulates Tgfβ3 transcription directly (Medici et al., 2008). In this study, we show a co-localization of functional Wnt/β-catenin signaling and Tgfβ3 expression in the MEE. We further used both in vivo loss-of- and gain-of-function approaches to show that Wnt/β-catenin is essential and sufficient for activation of Tgfβ3 in the palatal epithelium. Based on these lines of evidence, we conclude that Wnt/β-catenin signaling regulates palate fusion by controlling Tgfβ3 expression in the MEE, most likely through a direct transcription activation mechanism.
Prior to our study reported here, mouse models with K14Cre-mediated ectodermal deletion of Catnb have been generated for studies of ectodermal organ development (Huelsken et al., 2001; Liu et al., 2007a; Liu et al., 2008). However, a palate phenotype was not reported these studies. Either the phenotype was ignored or not described, or did not exist at all. In one of these previous studies, K14Cre;CatnbF/F mice apparently survived to adulthood showing skin defect (Huelsken et al., 2001). The different outcome of ectodermal β-catenin deletion could attribute to the different efficiency and versatility of activation stage of different K14-Cre lines. In this study, we use a K14-Cre line whose Cre activity in the palatal epithelium could be detected as early as at E11.5, coinciding with onset of palate development (He et al., 2010b). Even though, this K14-Cre line could also exhibit inconsistent promoter activity to certain extent. This explains the incomplete penetrance of the cleft palate defect in K14Cre;CatnbF/F mice as well as the lack of fully persistent MES in cultured mutant palatal shelves.
In conclusion, in this study, we have examined the activity of Wnt/β-catenin signaling in the developing palate and demonstrated an essential role for Wnt/β-catenin signaling in palate fusion by regulating Tgfβ3 expression. Together with our previous finding that the Wnt5a/Ror2-mediated noncanonical signaling regulates cell proliferation and migration in palatal mesenchyme, we conclude that both the canonical and noncanonical Wnt signaling pathways are essential for palatogenesis, but regulate different steps of palate development, which could provides biological basis for the development of effective prevention and therapeutic treatments of cleft palate defects in humans with altered Wnt signaling.
Supplementary Material
Altered expression of Tgfβ3 and Axin2 in the developing palatal shelves of K14Cre;CatnbF/F and K14Cre;CatnbF(ex3) mice. (A) Whole mount in situ hybridization shows Tgfβ3 expression in the MEE (arrows) of E13.5 wild type palatal shelves. (B) Whole mount in situ hybridization shows a down-regulated Tgfβ3 expression in the MEE (arrows) of E13.5 K14Cre;CatnbF/F palatal shelves. Note that the expression of Tgfβ3 in the molar mesenchyme remains unaltered. (C) Whole mount in situ hybridization shows ectopic Tgfβ3 expression (arrows) in an E13.5 K14Cre;CatnbF(ex3) palatal shelves. (D–F) Whole mount in situ hybridization shows Aixn2 expression in the palatal shelves (arrows) and molars of an E13.5 wild type embryo (D), downregulated Axin2 expression (arrows) in the palatal shelves (arrows) but not in the molar of an E13.5 K14Cre;CatnbF/F embryo (E), and ectopic activation of Axin2 in the palatal shelves (arrows) and the snout of an E13.5 K14Cre;CatnbF(ex3) embryo (F). M, molar; PS, palatal shelf.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen YP. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev Biol. 2005;277:102–113. doi: 10.1016/j.ydbio.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Andl T, Ahn K, Kairo A, Chu EY, Wine-Lee L, Reddy ST, Croft NJ, Cebra-Thomas JA, Metzger D, Chambon P, Lyons KM, Mishina Y, Seykora JT, Crenshaw EB, Millar SE. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Bremback FH, Rosario M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Brown NL, Knott L, Halligan E, Yarram SJ, Mansell JP, Sandy JR. Microarray analysis of murine palatogenesis: Temporal expression of genes during normal palate development. Dev Growth Diff. 2003;45:153–165. doi: 10.1034/j.1600-0854.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Brugmann SA, Goodnough LH, Gregorieff A, Leucht P, ten Berge D, Fuerer C, Clevers H, Nusse R, Helms JA. Wnt signaling mediates regional specification in the vertebrate face. Development. 2007;134:3283–3295. doi: 10.1242/dev.005132. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Chen J, Lan Y, Baek JA, Gao Y, Jiang R. Wnt-beta-catenin signaling plays an esential role in activation of odontogenic mesenchyme during early tooth development. Dev Biol. 2009;334:174–185. doi: 10.1016/j.ydbio.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet BT, Blanton SH, Burt A, Ma D, Stal S, Mulliken JB, Hecht JT. Variation in WNT genes is associated with non-syndromic cleft lip with or without cleft palate. Hum Mol Genet. 2008;17:2212–2218. doi: 10.1093/hmg/ddn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Dudas M, Nagy A, Laping NJ, Moustakas A, Kaartinen V. Tgfβ3 induced palatal fusion is mediated by Alk-5/Smad pathway. Dev Biol. 2004;266:96–108. doi: 10.1016/j.ydbio.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ. Palate development. Development. 1988;103(suppl):41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of {beta}-catenin in mice. Genes Dev. 2008;22:2308–2341. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A. Molecular control of secondary palate development. Dev Biol. 2007;301:309–326. doi: 10.1016/j.ydbio.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo M. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Mayo J, Xu X, Li J, Bringas P, Jr, Maas RL, Rubenstein JL, Chai Y. Indirect modulation of Shh signaling by Dlx5 affects the oral-nasal patterning of palate and resuces cleft palate in Msx1-null mice. Development. 2009;136:4225–42. doi: 10.1242/dev.036723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Yu X, Espinoza-Lewis R, Liu C, Gu S, Nishita M, Suzuki K, Yamada G, Minami Y, Chen YP. Wnt5a regulates directional cell migration and cell proliferation via Ror2-mediated noncanonical pathway in mammalian palate development. Development. 2008;135:3871–3879. doi: 10.1242/dev.025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Popkie AP, Xiong W, Li L, Wang Y, Phiel CJ, Chen YP. Gsk3β is required in the epithelium fr palatal elevation but does not function by modulating Wnt/β-catenin signaling. Dev Dyn. 2010a;239:3235–3246. doi: 10.1002/dvdy.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, Klingensmith J, Chen YP. Modulation of BMP signaling by Noggin is requried for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol. 2010b;247:109–121. doi: 10.1016/j.ydbio.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for beta-Catenin in Anterior-Posterior Axis Formation in Mice. J Cell Bio. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. [beta]-Catenin Controls Hair Follicle Morphogenesis and Stem Cell Differentiation in the Skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ishitani T, Ninomiya-Tsuji J, Nagai S, Nishita M, Meneghini M, Barker N, Waterman M, Bowerman B, Clevers H, Shibuya H. The TAK1–NLK–MAPK-related pathway antagonizes signalling between β-catenin and transcription factor TCF. Nature. 1999;399:798–802. doi: 10.1038/21674. [DOI] [PubMed] [Google Scholar]
- Jugessur A, Farlie P, Kilpatrick N. The genetics of isolated orofacial clefts: from genotypes to subphenotypes. Oral Dis. 2009;15:437–453. doi: 10.1111/j.1601-0825.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Volcken JK, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interactions. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Lan Y, Jiang R. Sonic hedgehog signaling regulates reciprocal epithelial-mesenchymal interactions controlling palatal outgrowth. Development. 2009;136:1387–1396. doi: 10.1242/dev.028167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y, Ryan R, Zhang Z, Bullard S, Bush J, Maltby K, Lidral A, Jiang R. Expression of Wnt9b and activation of canonical Wnt signaling during midfacial morphogenesis in mice. Dev Dyn. 2006;235:1448–1454. doi: 10.1002/dvdy.20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieven O, Knobloch J, Rüther U. The regulation of Dkk1 expression during embryonic development. Dev Biol. 2010;340:256–268. doi: 10.1016/j.ydbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- Liu F, Millar SE. Wnt/β-catenin signaling in oral tissue development and disease. J Dent Res. 2010;89:318–330. doi: 10.1177/0022034510363373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, Andl T, Taketo MM, Dlugosz AA, Moon RT, Barlow LA, Millar SE. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007a;39:106–112. doi: 10.1038/ng1932. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Arron JR, Stankunas K, Crabtree GR, Longaker MT. Chemical rescue of cleft palate and midline defects in conditional GSK-3-beta mice. Nature. 2007b;446:79–82. doi: 10.1038/nature05557. [DOI] [PubMed] [Google Scholar]
- Liu F, Chu EY, Watt B, Zhang Y, Gallant NM, Andl T, Yang SH, Lu MM, Piccolo S, Schmidt-Ullrich R, Taketo MM, Morrisey EE, Atit R, Dlugosz AA, Millar SE. Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi M, Tucker AS, Sharpe PT. Expression of Axin2 indicates a role for canonical Wnt signaling in development of the crown and root during pre- and postnatal tooth development. Dev Dyn. 2010;239:160–167. doi: 10.1002/dvdy.22047. [DOI] [PubMed] [Google Scholar]
- Luning C, Rass A, Rozell B, Wroblewski J, Obrink B. Expression of E-cadherin during craniofacial development. J Craniofac Genet Biol. 1994;14:207–216. [PubMed] [Google Scholar]
- Mani P, Jarrell A, Myers J, Atit R. Visualizing canonical Wnt signaling during mouse craniofacial development. Dev Dyn. 2010;239:354–63. doi: 10.1002/dvdy.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Alvarez C, Bonelli R, Tudela C, Gato A, Mena J, O’Kane S, Ferguson MW. Bulging medial edge epitheliual cells and palatal fusion. Int J Dev Biol. 2000;44:311–335. [PubMed] [Google Scholar]
- Martinez-Alvarez C, Blanco MJ, Perez R, Rabadan MA, Aparicio M, Resel E, Martinez T, Nieto MA. Snail family members and cell survival in physiological and pathological cleft palates. Dev Biol. 2004;265:207–18. doi: 10.1016/j.ydbio.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming grwoth factor-beta 3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murillo J, Maldonado E, Barrio MC, Del Río A, López Y, Martínez-Sanz E, González I, Martín C, Casado I, Martínez-Álvarez C. Interactions between TGF-[beta]1 and TGF-[beta]3 and their role in medial edge epithelium cell death and palatal fusion in vitro. Differentiation. 2009;77:209–220. doi: 10.1016/j.diff.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Nawshad A. Palatal seam disintegration: To die or not to die? that is no longer the question. Dev Dyn. 2008;237:2643–2656. doi: 10.1002/dvdy.21599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawshad A, Hay ED. TGFβ3 signaling activates transcription of the LEF1 gene to induce epithelial mesenchymal transformation during mouse palatal development. J Cell Biol. 2003;163:1291–1301. doi: 10.1083/jcb.200306024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MW, Doetschman T. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions causes cleft palate. J Clin Invst. 2004;113:1692–1700. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Vraun KM, Watt FM. B-catenin and hedgehog signaling streng can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Song L, Li Y, Wang K, Wang YZ, Molotkov A, Gao L, Zhao T, Yamagami T, Wang Y, Gan Q, Pleasure DE, Zhou CJ. Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development. 2009;136:3161–3171. doi: 10.1242/dev.037440. [DOI] [PubMed] [Google Scholar]
- St Amand TR, Zhang Y, Semina EV, Zhao X, Hu YP, Nguyen L, Murray JC, Chen YP. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol. 2000;217:323–332. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- Taya Y, O'Kane S, Ferguson MW. Pathogenesis of cleft palate in TGF-beta3 knockout mice. Development. 1999;126:3869–3879. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Tudela C, Formoso MA, Martinez T, Perez R, Aparicio M, Maestro C, Del Rio A, Martinez E, Ferguson MW, Martinez-Alvarez C. TGF-beta3 is required for the adhesion and intercalation of medial edge epitheliual cells during palate fusion. Int J Dev Biol. 2002;46:333–336. [PubMed] [Google Scholar]
- Tunggal JA, Helfrich I, Schmitz A, Schwarz H, Gunzel D, Fromm M, Kemler R, Krieg T, Niessen CM. E-cadherin is essential for in vivo epidermial barrier function by regulating tihgt junctions. EMBO J. 2005;24:1146–1156. doi: 10.1038/sj.emboj.7600605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DR, Smith HS, Webb CL, Greene RM, Pisano MM. Expression of Wnts in the developing murine secondary palate. Int J Dev Biol. 2009;53:1105–12. doi: 10.1387/ijdb.082578dw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, He F, Morikawa Y, Yu X, Zhang ZY, Lan Y, Jiang R, Cserjesi P, Chen YP. Hand2 is required in the epithelium for palatogenesis in mice. Dev Biol. 2009;330:131–141. doi: 10.1016/j.ydbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Han J, Ito Y, Bringas P, Jr, Urata MM, Chai Y. Cell autonomous requirement for Tgfbr2 in the disappearance of medial edge epithelium during palatal fusion. Dev Biol. 2006;297:238–48. doi: 10.1016/j.ydbio.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Yamada W, Nagao K, Horikoshi K, Fujikura A, Ikeda E, Inagaki Y, Kakitani M, Tomizuka K, Miyazaki H, Suda T, Takubo K. Craniofacial malformation in R-spondin2 knockout mice. Biochem Biophys Res Commun. 2009;381:453–8. doi: 10.1016/j.bbrc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- Yang L, Kaartinen V. Tgfb1 expressed in the Tgfb3 locus partially rescues the cleft palate phenotype of Tgfb3 null mutants. Dev Biol. 2007;312:384–395. doi: 10.1016/j.ydbio.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YD, Zhang Z, Zhao X, Yu X, Hu Y, Ramamurthy R, Qiu MS, Chen YP. Msx1 is required for the induction of Patched by Sonic hedgehog in the mammalian tooth germ. Dev Dyn. 1999;215:45–53. doi: 10.1002/(SICI)1097-0177(199905)215:1<45::AID-DVDY5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen YP. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Altered expression of Tgfβ3 and Axin2 in the developing palatal shelves of K14Cre;CatnbF/F and K14Cre;CatnbF(ex3) mice. (A) Whole mount in situ hybridization shows Tgfβ3 expression in the MEE (arrows) of E13.5 wild type palatal shelves. (B) Whole mount in situ hybridization shows a down-regulated Tgfβ3 expression in the MEE (arrows) of E13.5 K14Cre;CatnbF/F palatal shelves. Note that the expression of Tgfβ3 in the molar mesenchyme remains unaltered. (C) Whole mount in situ hybridization shows ectopic Tgfβ3 expression (arrows) in an E13.5 K14Cre;CatnbF(ex3) palatal shelves. (D–F) Whole mount in situ hybridization shows Aixn2 expression in the palatal shelves (arrows) and molars of an E13.5 wild type embryo (D), downregulated Axin2 expression (arrows) in the palatal shelves (arrows) but not in the molar of an E13.5 K14Cre;CatnbF/F embryo (E), and ectopic activation of Axin2 in the palatal shelves (arrows) and the snout of an E13.5 K14Cre;CatnbF(ex3) embryo (F). M, molar; PS, palatal shelf.