Abstract
Synthetic hydrogels with tunable properties are appealing for regenerative medicine. A critical limitation in hydrogel design at low solids concentration is the formation of defects, which increase gelation times and swelling, and reduce elasticity. Here, we report that tri-functional crosslinking peptides applied to 4-arm poly-(ethylene glycol) (PEG) hydrogels decreased swelling and gelation time relative to bi-functional crosslinkers. In contrast to bi-functional peptides, the third cross-linking site on the peptide created a branch point if an intramolecular crosslink formed, which prevented non-functional “dangling-ends” in the hydrogel network and enhanced the number of elastically active cross-links. The improved network formation enabled mouse ovarian follicle encapsulation and maturation in vitro. Hydrogels with bi-functional crosslinkers resulted in cellular dehydration, likely due to osmosis during the prolonged gelation. For tri-functional crosslinkers, the hydrogels supported a 17-fold volumetric expansion of the tissue during culture, with expansion dependent on the ability of the follicle to rearrange its microenvironment, which is controlled through the sensitivity of the cross-linking peptide to the proteolytic activity of plasmin. The improved network design enabled ovarian follicle culture in a completely synthetic system, and can advance fertility preservation technology for women facing premature infertility from anticancer therapies.
Keywords: Hydrogel, tunable degradation, Michael type addition, ovarian follicle
Introduction
Hydrogels can have physical properties similar to native extracellular matrix[1], making these biomaterials appealing for numerous applications in regenerative medicine. The physical properties of these hydrogels can be manipulated through parameters such as the polymer composition and the cross-linking chemistry, and have emerged as essential design parameters in promoting cell differentiation and tissue formation [2–5]. Despite the range of available chemistries [6], many hydrogels have non-ideal physical properties due to defects formed during the cross-linking process, leading to high swelling and loss of mechanical integrity [2,3]. Defects arising from intramolecular cross-linking are termed primary loops, but other defects can be present due to an incomplete reaction of the cross-linking groups, or heterogeneity within the hydrogel [7, 8]. In ideal cross-linking, all cross-linking reactions are intermolecular, which is favored at high polymer concentrations [2]. However, the relatively low hydrogel solids content relevant to regenerative medicine applications leads to substantial intramolecular cross-linking between 2 functional groups on the same precursor molecule.
We investigated the design of cross-linking molecules that could form elastically active primary loops in order to enhance network cross-linking under conditions that favor defect formation, such as low solids content. Cross-linkers were designed that could provide a branch point regardless of whether an intra- or intermolecular reaction occurred. We hypothesized that cross-links that form elastically active primary loops would enhance network formation and attenuate the impact of defects. This impact of the network formation during cross-linking was characterized through hydrogel swelling and gelation time. We then applied the material data to design encapsulation conditions that simultaneously maintained the viability of the encapsulated cells and high cross-linking efficiency of the hydrogel.
The utility of the hydrogels in promoting tissue growth was investigated in a model of ovarian follicle maturation. In vitro systems for ovarian follicle maturation are being developed as a means to preserve fertility for females facing anticancer therapies [9]. In this clinical scenario, immature ovarian follicles would be isolated from the ovary prior to the exposure to a toxic treatment and cultured in vitro to produce mature oocytes for in vitro maturation (IVM) and subsequent in vitro fertilization (IVF). Creating an artificial environment for in vitro folliculogenesis that mimics native tissue presents a unique engineering challenge because the ovarian tissue exhibits spatiotemporal dynamics with respect to the developmental stage of the follicle [10]. In particular, follicles undergo rapid volumetric expansion during the final stages of maturation that culminates in ovulation. The hydrogel cross-linkers were designed to degrade in response to proteases secreted by the follicle, which can locally decrease the hydrogel mechanics to permit follicle expansion, while maintaining the global integrity of the hydrogel. Hydrogel degradation was tuned through plasmin-sensitive cross-linking peptides [4], which regulated somatic cell differentiation and successful germ cell maturation.
Materials and Methods
Hydrogel Materials
PEG diacrylate (3.5 and 10kDa) and 4-arm PEG acrylate (PEG-Ac) (20kDa) (Creative PEGworks: Winston Salem, NC) were used without further modification. PEG tetravinyl sulfone (PEG-VS) was synthesized from 4-arm PEG-OH 20kDa (Creative PEGworks) as previously published [3]. In all swelling studies, the difunctional (2 cysteine groups) cross-linking peptide used was MMP sensitive (Ac-GKCDGPQG↓IWGQDCKG) [11], and the trifunctional peptide was plasmin sensitive (e.g., Ac-GCYK↓NRGCYK↓NRCG) [4]. All peptides were synthesized on a Rink Amide MBHA resin using standard 9-fluorenylmethoxycarbonyl (Fmoc) solid phase peptide synthesis on a CSBio 136 automated peptide synthesizer. Plasmin sensitive 3-arm cross-linking peptides having fast Ac-GCYK↓NRGCYK↓NRCG, intermediate Ac-GCYK↓NSGCYK↓NSCG, and slow Ac-GCYK↓NDGCYK↓NDCG degradation kinetics were synthesized for ovarian follicle encapsulation. The non-degradable (or very slow degrading[12]) sequence was synthesized with D-isomers of tyrosine and asparagine surrounding the cleavage site of plasmin (Ac-GCYDKNDRGCYDKNDRCG).
PEG hydrogel preparation and swelling
Macromer functionality effect on hydrogel swelling (figure 2) was investigated using PEG-acrylates, which provided reaction rates that were sufficient slow to produce homogenous hydrogels at concentrations up to 20%. PEG hydrogels were prepared by cross-linking 4-arm PEG-Ac with MMP and plasmin sensitive peptides (4:2 and 4:3 gels, respectively) and PEG diacrylate was cross-linked with plasmin sensitive peptides (2:3 gel). Precursors were dissolved in triethanolamine (TEA) buffer at a pH empirically determined to give the lowest degree of swelling, which were found to be in agreement with published pH levels [4] (0.3M, pH=8.5 for 4:2 gel and pH=7.5–8 for 4:3 and 2:3 gels). The effect pH on reactivity, which has been studied in detail, is primarily influenced by the pKa of cysteine residues [13] on the peptide of interest. Gels (100 μL) were formed at varying final concentrations (3, 5, 10 and 20% w/v) and cross-linked for 1 hour in 37°C humidified incubator, and then were swollen in deionized water overnight. To investigate the swelling properties of hydrogels under conditions that maintain ovarian follicle viability, 4 arm PEG-VS and peptides were dissolved in an isotonic HEPES buffer (HEPES 0.1M, NaCl 0.1M, pH=7.4). Gels were formed at varying final concentrations (3, 4, 5 and 10%) and cross-linked for varying time 10, 30, 60 minutes and overnight (O/N) in 37°C humidified incubator, and then were swollen in deionized water overnight. Qm values were calculated as the ratio of the wet weight of the gel following swelling and its dry weight following lyophilization.
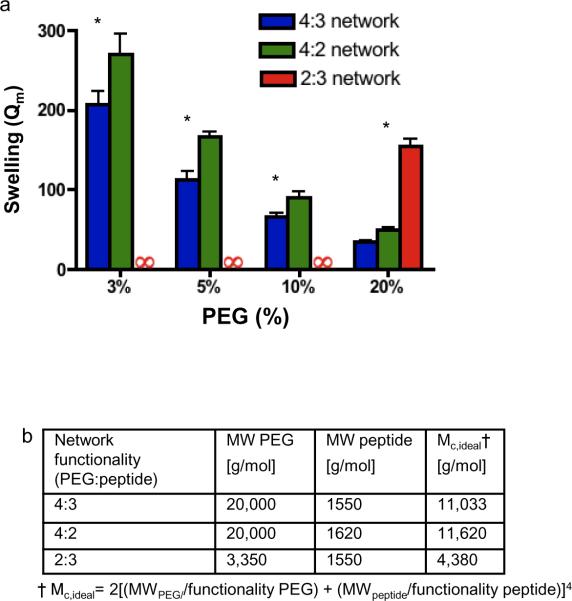
Figure 2. Swelling tests of the hydrogels formed from precursors with different functionalities.
a, Mass equilibrium swelling (Qm) of hydrogels with networks composed of different macromer functionalities (PEG-Ac : peptide functionality). At all concentrations, a 4:3 network swells the least, but at concentrations in which primary loop formation would be most favored (3%), the difference in swelling is greatest. Cross-linking reactions occurred at the pH with the lowest swelling, in agreement with published conditions[2, 3], to minimize effects of different peptide chemistries. An infinity symbol indicates that a hydrogel either did not form or dissolved during overnight swelling. Error bars are one standard deviation. *Indicates P < 0.001 relative to 4:3 network. (b) Ideal molecular weight between cross-links (Mc,ideal) for the hydrogels. Note that the trifunctional peptide has slightly asymmetric cross-links, which is not accounted for in this model.
Animals, follicle culture and imaging
Immature secondary follicles (140–150 μm in diameter) were mechanically isolated and selected [14] from 14 and 15 day old C57BL/6j×CBA/Ca mice and individually encapsulated in a 5% PEG hydrogel. The PEG-VS and peptides were dissolved in the isotonic HEPES buffer (pH=7.4), mixed at a 1:1 volumetric ratio, and cast between parafilm-coated glass slides after individual follicle addition to each 5 μL gel. Gels were mixed at a reactive group stoichometric ratio of 1:1.1 (PEG:peptide) to reduce the impact of disulfide bond formation in the peptide solution on the cross-linking efficiency during cell culture. The gels were allowed to cross-link for 5 minutes in a 37°C humidified incubator. Hydrogels were then transferred to a 96 well plate containing 150 μL follicle culture media (αMEM, 3 mg/mL bovine serum albumin [MP Biomedicals, Inc., SOLON, OH], 10mIU/mL rFSH [A.F. Parlow, NHPP, NIDDK], 1 mg/mL bovine fetuin, 5 μg/mL insulin, 5 μg/mL transferrin and 5 ng/mL selenium) [15]. Follicles were cultured for 10 days at 37°C in 5% CO2, and 75 μL of media was changed every 2 days. Follicle survival and diameter were assessed prior to every media change using an inverted Leica DM microscope with transmitted light and phase objectives. Follicle diameter was measured using ImageJ (NIH, Bethesda, Maryland). Follicles were mechanically retrieved at the end of the culture and matured in αMEM supplemented with 10% FCS, 1.5IU/mL human chorionic gonadotropin (hCG), and 5 ng/mL epidermal growth factor for 16 hours at 37°C, 5% CO2 [16].Maturation to a metaphase II (MII) stage was determined by visual confirmation of a polar body on the inverted Leica DM microscope. For confocal microscopy, MII eggs were fixed in 4% PFA and incubated in primary antibody (α/β tubulin cocktail 1:100; mouse; Sigma) in 4°C overnight, followed by 1-hour incubation of secondary antibody (Alexa 488 goat anti-mouse IgG 1:500; Molecular Probes), rhodamine-phalloidin (1:50; Molecular Probes), and DAPI (1:50, Molecular Probes) at room temperature. Eggs were mounted and imaged as previously described [17].
Statistics
Statistical analysis was performed using ANOVA with a Bonferroni post-test. P<0.05 was considered significant (Prism, GraphPad).
Results
Precursor functionality and network structure
End-linked cross-linking is illustrated with a widely used hydrogel in regenerative medicine: poly(ethylene glycol) (PEG) modified with acrylate groups, and cross-linked by Michael type addition (MTA) [18] with peptides containing reactive thiol groups on cysteine residues (fig. 1a,b) [3, 4, 6, 11, 19]. Previously described networks composed of multi-arm PEG macromers and peptides with functionalities of 4 and 2 [11, 19] or 2 and 3 [4], denoted as 4:2 and 2:3 networks, respectively, could form intramolecular reactions that lead to primary loops (fig. 1e,f) that are dead-ends within the context of network formation, as they neither branch nor support linear continuation. These primary loops are thus elastically inactive, and lead to hydrogel swelling, and a subsequent reduction in elastic modulus. A functionality greater than two on both molecules in the gelation reaction ensured that an intramolecular cross-link will contain a functional group that can serve as a branch point (fig. 1g), or at a minimum, a linear continuation of the network if two primary cycles occur within the same 4-arm PEG molecule. In our model hydrogel, the PEG macromer was a 4-arm star functionalized with 4 acrylate groups, whereas the peptide has 3 cross-linking sites in the form of cysteine residues that undergo MTA with the acrylate, creating a 4:3 network (fig. 1g).
Figure 1. Primary loop formation and swelling in end-linked PEG hydrogels.
a–d, Cross-linking of a 4-four arm PEG star and a difunctional peptide. (a) Ideally, reactions are intermolecular, which results in elastically active cross-links (blue shading) between PEG macromers, (c) and is favored at high solids content (represented by a grey circle). (b) Primary loop formation leads to elastically inactive cross-links (grey shading), (d) which is favored at low solids content. e–g, Primary loop structure in different networks. (e,f) In the 4:2 and 2:3 network, primary loops will be a “dangling-end” in the network, and thus elastically inactive. (g) In a 4:3 network, if a primary loop forms, a potential branch point is present to continue the network.
Precursor functionality and hydrogel swelling
The equilibrium mass swelling ratio of hydrogels, Qm, is indicative of defects in the network formation and we prepared PEG gels formed from precursors with different functionalities to test our hypothesis. At low PEG concentrations (≤10%), which most strongly favor intramolecular reactions (fig. 1c,d), the presence of the third reactive site on the cross-linker (4:3) substantially reduced swelling relative to networks that form dead-ends (4:2 and 2:3) (fig. 2a). The 2:3 network formed with linear PEG-acrylate precursor with Mw =3.5 kDa did not form hydrogels until the PEG content reached 20% w/v, suggesting that defect formation was limiting at lower concentrations. Note that the average molecular weight between cross-links, Mc,ideal was lowest in the 2:3 network (fig. 2b), despite having the greatest swelling ratio; therefore, the Flory-Rehner equation, which relates Mc,ideal and Qm in ideal cross-linked hydrogels, would predict this network to swell the least. A linear PEG-acrylate molecule with a greater molecular weight of 10 kDa cross-linked with a trifunctional peptide, which would have yielded a Mc,ideal comparable to the 4:3 and 4:2 network, did not form a hydrogel under any conditions. For the 4:3 and 4:2 hydrogels, the molecular weight of PEG was large relative to the cross-linking peptide; thus, the average molecular weight between cross-links predicted for ideal conditions was similar, (fig. 2b) and the Flory-Rehner equation predicts similar levels of swelling for these two networks. However, the 4:3 network had decreased swelling relative to the 4:2 network (fig. 2a), and as the PEG concentration was increased, the difference in swelling between the 4:3 and 4:2 hydrogels decreased, with the difference in swelling between the 4:3 network and the 4:2 network being 4-fold greater at 3% PEG content than at 20% PEG. This differential swelling supports the hypothesis that more elastically active primary loops enhanced network formation under cross-linking conditions that favor defect formation.
Hydrogel properties under physiological conditions
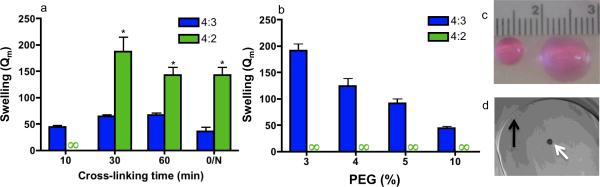
Designing hydrogels for cell encapsulation must balance the need to obtain the desired physical properties with the need to maintain cell viability, which may be contradictory. Synthetic hydrophilic polymers can be formed into hydrogels through end-linked cross-linking, under encapsulating conditions that are non-toxic to cells [11]. The enhancement of network formation at low solids content and short gelation time was investigated using physiological buffer conditions necessary for cell encapsulation, which reduces the cross-linking efficiency and can favor defect formation. For cell encapsulation, a PEG modified with vinyl sulfone (VS) was used along with adjustment of the buffer conditions (HEPES, pH 7.4) for decreased cytotoxicity to ovarian follicles. Swelling tests were performed for varying cross-linking times (fig. 3a). For a ten-minute cross-linking period, only hydrogels with a 4:3 network had sufficient integrity to withstand over-night swelling. For cross-linking times greater than 30 minutes, swollen hydrogels could be formed for both the 4:3 and 4:2 networks; however, the 4:2 network had greater swelling (≥ 2.1 fold) at all time points. Additionally, under the same physiological conditions, the 4:3 network formed hydrogels at lower PEG concentrations than the 4:2 network (fig. 3b, 3c). Taken together, these results demonstrate that the 4:3 network can produce hydrogels under physiological buffer conditions (fig. 3d), and decrease the gelation time. This decrease in gelation time is supported by Flory-Stockmayer statistics, which predict that increasing the functionality of both of the end-linked macromers will decrease the extent of reaction where the gel point will be reached during ideal cross-linking.
Figure 3. Swelling tests under cellular encapsulation conditions that support ovarian follicle viability.
a–d Hydrogel cross-linking in an isotonic HEPES buffer at pH 7.4 with PEG-VS. (a) A 4:3 network supported rapid hydrogel formation within 10 minutes, and swelling did not decrease appreciably with an increased cross-linking period, even after overnight (O/N) cross-linking at 37°C. PEG-VS content was 10% w/v. (b) A 10-minute cross-linking period was sufficient for 4:3 hydrogel formation at low PEG content, suggesting that these hydrogels are resilient to defect formation. An infinity sign indicates that a hydrogel did not form or dissolved during swelling. Error bars are one standard deviation (* indicates P<0.001). (c) Swelling of a 4:3 (left) and 4:2 (right) network of 5% PEG cross-linked for 1 hour. Both gels were formed with an initial volume of 20 μl. (d) To encapsulate ovarian follicles (white arrow) 5% PEG-VS hydrogel disks (black arrow) were cast between glass slides and allowed to gel for 5 minutes at 37°C.
Follicle development in a 4:3 network
For follicle encapsulation, PEG-VS based hydrogels were used because degradation will be solely proteolytic, whereas a PEG-Ac hydrogel will be hydrolytically labile. Follicles encapsulated in 4:2 network had a normal morphology after encapsulation in 10% and 20% solid content hydrogels if encapsulation was done in a TEA based buffer (fig 4a, d) that allows faster Michael-type addition cross-linking than a biological buffer such as HEPES. However, after 1 day of culture the encapsulated follicles appeared dark, the oocyte membrane was lost and follicles did not grow (fig 4 b,c,e,f). The deterioration of the follicle morphology may be due to the surfactant properties of TEA, which motivated the use of a less reactive buffer.
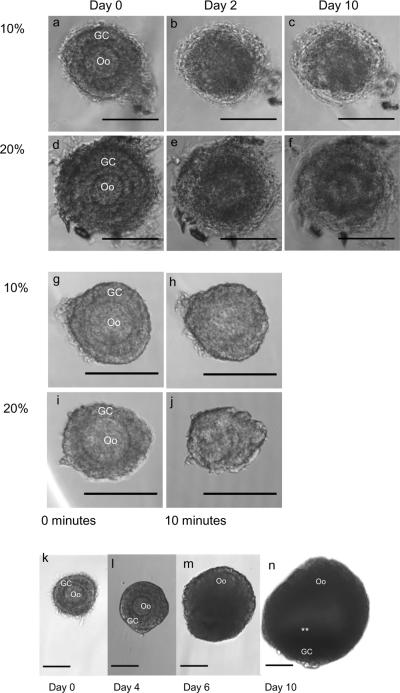
Figure 4. Influence of chemical and osmotic stress on follicles during encapsulation and follicle growth in optimized cross-linking conditions.
a,f Exposure of ovarian follicles to a triethanolamine (TEA) buffer, rather than HEPES, during encapsulation severely impacts viability. (a,d) Although follicles appear healthy immediately after encapsulation, (b,e) by day 2 the same follicles appear necrotic. (c,f Follicles do not recover from damage induced by TEA (day 10 of culture shown). g–j, Ovarian follicles suspended in (g,h) 10% or (i,j) 20% PEG solution without the cross-linking peptide lose their normal morphology within ten minutes, possibly due to osmotic pressure induced by the PEG. Notably, the membrane of the oocyte (Oo) (h,j) has lost it spherical shape. The extent of morphological change was dependent on the concentration of PEG and time of exposure, suggesting that a low concentration of PEG and a rapid gelation will be more permissive to ovarian follicle encapsulation. k–n, Morphology of a healthy ovarian follicle (k) following encapsulation: a central oocyte (Oo) surrounded by granulosa cells (GC). (l,m) After several days, proliferation of GCs is observed, (n) and after 10 days, follicles have formed an antral cavity in addition to the GC proliferation (white asterisks). The follicles were cultured in YKNS (intermediate degradation rate) condition. Scale bars are 100 μm.
However, a less reactive buffer will decrease the cross-linking efficiency during network formation. Therefore, to form hydrogels, a higher concentration of polymer must be used to obtain the desired material properties. Exposure to a 10% or 20% w/v PEG solution visibly diminished follicle morphology within 10 minutes (fig 4g–j), and it took up to 30 minutes to form a 10% w/v hydrogel with a 4:2 network (fig 3a), motivating the need for rapid gelation at low concentrations of PEG. PEG molecules are freely soluble before they become entrapped in a network and may act as a dehydrating agent [20]. Thus, a short cross-linking time at a low solid concentration is essential for maintaining follicle health and viability.
The use of a 4:3 network in HEPES buffer conditions enabled successful encapsulation of the ovarian follicles in lower solids concentration hydrogel (5% w/v) and follicles survived and developed to a normal appearing antral follicle in 10 days of culture (fig. 4 k–n). The 4:3 network permitted the use of both a mild buffer and a low polymer content with uncompromised rapid encapsulation (<5 minutes) and the desired material properties, while the 4:2 network did not form under these encapsulation conditions.
Degradation rate and follicle development
The sequence of the plasmin substrate was investigated as a means to tune the rate of hydrogel degradation to follicle expansion. Follicles were individually encapsulated in 5% w/v 4-arm PEG-VS and cross-linked with peptides containing 3 cysteine residues and two plasmin degradation sequences. Three plasmin substrate sequences (YKNR, YKNS, and YKND) with varying degradation rates were tested (fast, intermediate, and slow, respectively), as well as a non-degradable sequence containing D-isomeric amino acids (YDKNDR)[12]. Follicle growth and morphology was used to evaluate the degradation conditions. Successful follicle maturation was assessed by antrum formation, which is fluid-filled cavity whose presence correlates with effective oocyte maturation and fertilization in vitro[15]. After ten days of culture (fig. 5a), follicles encapsulated within the YKNS plasmin substrate (intermediate degradation rate) expanded 17-fold in volume and had consistently formed an antrum (69%), whereas the less degradable conditions (YKND and YDKNDR) had less than 12-fold volumetric expansion, and were unable to form antral cavities at a comparable rate (<17%) (fig. 5c–f). The YKNR plasmin substrate degraded too rapidly, resulting in displacement of the follicle from the hydrogel and subsequent adhesion to the culture plate. This adhesion to the culture plate induces migration of the somatic granulosa cells from the oocyte, disrupting the 3D follicular architecture [21] (fig. 5b). Oocytes from the YKNS condition, which demonstrated healthy follicle development throughout ten days of culture (fig. 4k–n), were competent to undergo in vitro maturation (IVM), a simulation of ovulation, to yield fertilizable eggs arrested at metaphase-II (MII) at a rate of 89% ± 10 (fig. 5g,h). This rate is comparable to IVM rates for oocytes grown to maturity in vivo following hormonal stimulation [22], suggesting that the biomaterial and encapsulation conditions supported oocyte development and its competence for maturation.
Figure 5. Follicle growth and development is dependent on proteolytic hydrogel degradation.
a, Follicles were encapsulated in 5% PEG-VS cross-linked with a peptide with two plasmin degradation sequences (YKNx), and cultured for 10 days. From day 6 to 10, the follicle expansion in the YKNS condition is significantly greater than the YKND and YDKNDR conditions (*, P<0.001). Error bars are SEM. b–e Hydrogels with the YKNR sequence did not maintain the follicle within the hydrogel beyond four days. (b) Follicles cultured in 2D adhere to and migrate on the culture plate, disrupting their 3D shape. (c–e) Follicles after 10 days of culture encapsulated within hydrogels cross-linked with peptides containing (c) YKNS, (d) YKND, and (e) YDKNDR sequences. (f) The YKNS condition had the greatest antral rate formation and volumetric expansion. Different superscripts indicate significant differences (P<0.01). g, Fertilizable eggs arrested at metaphase-II (MII) following IVM of cultured ooytes; egg (e) and polar body (white arrowheads). h, Confocal image of an egg showing a MII spindle from a perpendicular perspective (white arrow); actin (red), DNA (blue), and β-tubulin (green). All scale bars on light micrographs (b–g) are 100 μm and the scale bar on (h) is 25 μm.
Discussion
Hydrogels can have physical properties similar to native extracellular matrix and can promote tissue growth as a 3D environment for cellular encapsulation; however, finding conditions that allow gel formation while maintaining cellular viability is challenging. In this report we present that the functionality of the precursors can significantly enhance network formation, resulting in fewer defects, which is particularly critical for cross-linking conditions that maintain cell viability during encapsulation.
Ideal end-linked cross-linking during hydrogel formation (fig. 1) is described by the Flory-Rehner equation, which predicts mass-equilibrium swelling (Qm) based on the average molecular weight between cross-links, Mc,ideal [2]. PEG hydrogels, however, do not exhibit behavior that is predicted by the Flory-Rehner equation below a 50% PEG content [2], and the gel swelling increased monotonically as the polymer solids content decreased (fig. 2) and as the cross-linking time decreased (fig. 3). This trend in swelling is indicative of network defects, such as primary loops, because a decline in cross-linking efficiency induces swelling due to a reduction in network elasticity. Decreasing the solids content would be expected to increase the number of elastically inactive primary loops (fig. 1c,d), which results from the decreasing global concentration of reactive groups, yet a stable local concentration of intramolecular reactive groups (fig. 1a, b) [2]. Incorporation of a third cross-linking site on the peptide cross-linker introduced an additional branch point for the cross-linking reaction, rather than a network “dead end”, resulting in decreased swelling. Uncontrolled swelling that results from the defects in the network formation can affect the concentration of the incorporated biological cues, and influence the physical environment of the encapsulated cells. Taken together, the differential swelling between the hydrogel formulations and the theory illustrates the opportunity for network design in non-ideal cross-linking conditions.
Ovarian follicle culture is being investigated as a means to preserve fertility for females facing premature ovarian failure as a consequence of chemotherapy treatment or other reproductive pathology [9]. Previous culture systems have successfully utilized natural biomaterials, such as alginate, to encapsulate and culture immature mouse ovarian follicles to yield live, fertile offspring [23]. Alginate, however, is not degradable on the time scale of follicle culture [24]; thus, as follicle expansion displaces the surrounding hydrogel, the material will exert an elastic, compressive force on the follicle that may restrict its expansion [15]. During mouse folliculogenesis, the volumetric expansion of the follicle is approximately 300-fold, starting at 120 μm in diameter and reaching 400 μm in diameter at its mature stage. Human follicles start at a similar initial diameter, yet reach 5–18 mm in diameter, a volumetric increase 105-fold. Presently, human follicles are unable to expand larger than 700 μm in vitro [25], potentially due to the compressive force imparted by the non-degradable hydrogel as the follicle expands. New materials are required to accommodate the significant increase in follicle volume, while preserving the 3D architecture. In contrast to alginate, the PEG hydrogels degrade in response to proteases [19] secreted by the follicle during culture, thereby creating the space for follicular expansion without producing the compressive force that can limit growth. Synthetic environments with tunable properties provide a tool to shed light on the basic biology of follicle development and may enable the development of a culture system that can be translated between species.
Matrices for follicle culture must enable easy encapsulation with retention of viability, pore size that allows transport of nutrients and hormones toward and away from the follicle, permit follicle expansion, and enable follicle collection at the end of culture. Naturally occurring biomaterials, such as collagen and fibrin have the advantage of intrinsic biological activity, however, these materials are complex and difficult to modify for desired physical properties, such as degradation. UV crosslinked hydrogels have the advantage of fast and efficient network formation. However, the necessity of using photoinitiator and light source raises the question of germline mutagenesis and the difficulties in human epidemiological studies involving germ cells and animal data extrapolation [26]. PEG hydrogels formed by MTA at 4:2 functionality ratios presented several limitations, such as the PEG dehydration effect [20] during the extended time required for network formation, as well as the potentially harmful buffer conditions. Introduction of an increased functionality in the network (4:3) resulted in rapid gelation that reduced the time follicles were exposed to the unreacted PEG in solution, and enabled the use of a more cell compatible buffering condition.
Tunable degradation of the plasmin sensitive PEG gels was achieved by using peptide sequences with different plasmin sensitivity. The hydrogel degradation occurred only around the follicles forming a soft pocket inside otherwise rigid matrix, suggesting that the cell-mediated proteolysis was localized to the follicle-material interface. A tightly regulated balance between plasminogen that originated from media components and the activated plasmin could be responsible for the localized degradation. Building a library of peptides with varying plasmin sensitivity allows matrix design that can be adjusted not only to the follicle stage and culture duration, but also to different species with varying plasmin activity. Furthermore, peptide sequences sensitive to other proteases, such as MMP-1 or MMP-13 [27] can be utilized with the 3-arm peptide chemistry presented in this work.
Conclusions
While the chemistry of cross-linking has been a primary focus of hydrogel design, we report that the functionality of the polymer and cross-linker can enhance network formation leading to decreased swelling and gelation times. MTA cross-linked PEG hydrogels are typically formed under non-ideal conditions (e.g., low solids content, buffer conditions) that favor the formation of defects. The functionality of the polymer and cross-linker can be designed to provide elastically active primary loops, which maintain a reactive site regardless of whether intra- or intermolecular reactions occur. Using an ovarian follicle culture system, we demonstrated that the degradation rate in a synthetic material can regulate coordinated tissue development with degradation initiated by the follicle to influence its local environment. This enhanced network design can be extended to other hydrogel systems in order to regulate the physical properties and facilitate their application to regenerative medicine.
Acknowledgements
The authors thank Dr. Andrew Cheetam from the Chemistry Core Facility of the Institute for BioNanotechnology in Medicine at Northwestern University funded by the U.S. Army Research Office, the U.S. Army Medical Research and Materiel Command, and Northwestern University for peptide synthesis and purification. We would also like to thank Dr. Francesca Duncan for scientific discussion and assistance with confocal microscopy. This work was funded by NIH (U54HD41857 and PL1EB008542, a P30 Biomaterials Core within the Oncofertility Consortium Roadmap grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Lutolf MP, Hubbell J. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- [2].Metters A, Hubbell J. Network formation and degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- [3].Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–22. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- [4].Pratt AB, Weber FE, Schmoekel HG, Muller R. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86:27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- [5].Rydholm AE, Bowman CN, Anseth KS. Degradable thiol-acrylate photopolymers: polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials. 2005;26:4495–506. doi: 10.1016/j.biomaterials.2004.11.046. [DOI] [PubMed] [Google Scholar]
- [6].Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–56. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hiemenz PC, Lodge TP. Polymer Chemistry. 2 ed. CRC Press; Boca Raton, FL: 2007. [Google Scholar]
- [8].Elliott JE, Bowman CN. Monomer functionality and polymer network formation. Macromolecules. 2001;34:4642–9. [Google Scholar]
- [9].Jeruss JS, Woodruff TK. Current Concepts: Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902–11. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Pedersen T. Determination of follicle growth rate in ovary of immature mouse. J Reprod Fertil. 1970;21:81–93. doi: 10.1530/jrf.0.0210081. [DOI] [PubMed] [Google Scholar]
- [11].Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. Proc Natl Acad Sci USA. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schechte I, Berger A. On the size of the active site in proteases. I. Papain. Biochem Bioph Res Co. 1967;27:157–62. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- [13].Lutolf MP, Tirelli N, Cerritelli S, Cavalli L, Hubbell J. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug Chem. 2001;12:1051–6. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- [14].Cortvrindt R, Smitz J, Van Steirteghem AC. In-vitro maturation, fertilization and embryo development of immature oocytes from early preantral follicles from prepubertal mice in a simplified culture system. Hum Reprod. 1996;11:2656–66. doi: 10.1093/oxfordjournals.humrep.a019188. [DOI] [PubMed] [Google Scholar]
- [15].Xu M, West E, Shea L, Woodruff T. Identification of a stage-specific permissive in vitro culture environment for follicle growth and oocyte development. Biol Reprod. 2006;75:916–23. doi: 10.1095/biolreprod.106.054833. [DOI] [PubMed] [Google Scholar]
- [16].Smitz J, Cortvrindt R, Hu YX. Epidermal growth factor combined with recombinant human chorionic gonadotrophin improves meiotic progression in mouse follicle-enclosed oocyte culture. Hum Reprod. 1998;13:664–9. doi: 10.1093/humrep/13.3.664. [DOI] [PubMed] [Google Scholar]
- [17].Shikanov A, Xu M, Woodruff T, Shea L. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476–85. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31:487–531. [Google Scholar]
- [19].Raeber G, Lutolf M, Hubbell J. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys J. 2008;89:1374–88. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Money NP. Osmotic pressure of aqueous polyethelene glycols - relationship between molecular weight and vapor pressure deficit. Plant Physiol. 1989;91:766–9. doi: 10.1104/pp.91.2.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kreeger P, Deck J, Woodruff T, Shea L. The in vitro regulation of ovarian follicle development using alginate-extracellular matrix gels. Biomaterials. 2006;27:714–23. doi: 10.1016/j.biomaterials.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Combelles CMH, Albertini DF. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod. 2003;68:812–21. doi: 10.1095/biolreprod.102.008656. [DOI] [PubMed] [Google Scholar]
- [23].Xu M, Kreeger PK, Shea LD, Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–46. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Boontheekul T, Kong H, Mooney D. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials. 2005;26:2455–65. doi: 10.1016/j.biomaterials.2004.06.044. [DOI] [PubMed] [Google Scholar]
- [25].Xu M, Barrett S, West-Farrell E, Kondapalli L, Kiesewetter S, Shea L, et al. In vitro grown human ovarian follicles from cancer patients support oocyte growth. Hum Reprod. 2009;24:2531–40. doi: 10.1093/humrep/dep228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Xu GG, Intano GW, McCarrey JR, Walter RB, McMahan CA, Walter CA. Recovery of a low mutant frequency after ionizing radiation-induced mutagenesis during spermatogenesis. Mutat Res. 2008;654:150–7. doi: 10.1016/j.mrgentox.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hahn MS, McHale MK, Wang E, Schmedlen RH, West JL. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng. 2007;35:190–200. doi: 10.1007/s10439-006-9099-3. [DOI] [PubMed] [Google Scholar]