Summary
Myosin XVa (MyoXVa) and its cargo whirlin are implicated in deafness and vestibular dysfunction and have been shown to localize at stereocilia tips and to be essential for the elongation of these actin protrusions [1–4]. Given that whirlin has no known actin-regulatory activity, it remains unclear how these proteins work together to influence stereocilia length. Here we show that the actin-regulatory protein Eps8 [5] interacts with MyoXVa and that mice lacking Eps8 show short stereocilia comparable to MyoXVa and whirlin deficient mice. We show that Eps8 fails to accumulate at the tips of stereocilia in the absence of MyoXVa, that overexpression of MyoXVa results in both elongation of stereocilia as well as increased accumulation of Eps8 at stereocilia tips, and that the exogenous expression of MyoXVa in MyoXVa-deficient hair cells rescues Eps8 tip-localization. We find that Eps8 also interacts with whirlin and that the expression of both Eps8 and MyoXVa at stereocilia tips is reduced in whirlin-deficient mice. We conclude that MyoXVa, whirlin, and Eps8 are integral components of the stereocilia tip complex where Eps8 is a central actin-regulatory element for elongation of the stereocilia actin core.
Keywords: hair cells, Myosin XVa, Eps8, actin protrusion, filopodia, stereocilia, hearing, whirlin, deafness, motor protein, actin polymerization, EPS8, MYO15A
Results and Discussion
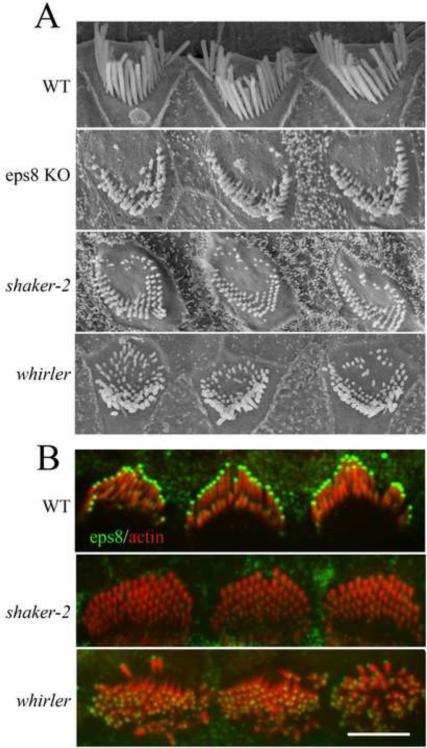
Using scanning electron microscopy, we found that cochlear hair cells from adult Eps8 KO mice [6] have very short stereocilia (Figure 1a). Mice deficient in MyoXVa (shaker-2 [3]) and whirlin (whirler [2]) also display very short stereocilia, although shaker-2 stereocilia are shorter, suggesting that yet to be identified MyoXVa cargoes other than whirlin are involved in regulating stereocilia length [4]. In order to compare stereocilia lengths we measured the lengths of the tallest row of stereocilia from medial turn cochlear outer hair cells of 6-week-old mice (Figures 1a and S1). Wild-type stereocilia were 4.2 ± 0.4 μm (mean length ± S.D., nstereocilia=39, nHC=6, nmice=1). In contrast, Eps8 KO mouse stereocilia were only 0.9 ± 0.6 μm (nstereocilia=229, nOHC=45, nmice=3). On average, Eps8 KO stereocilia are significantly (p<0.001) longer than shaker-2 stereocilia (0.3± 0.1 μm; nstereocilia=303, ncells=36, nmice=2) [3, 4]. Eps8 KO stereocilia are only slightly shorter (p<0.001) than whirler stereocilia (1.3 ± 0.4 μm; nstereocilia=305, ncells=64). Similar to the shaker-2 and whirler mice [4], we found that Eps8 KO mice are also deaf: in a Preyer's reflex test all adult Eps8 KO mice tested negative while all wild-type mice tested positive (n =14 each).
Figure 1.
Eps8 is essential for stereocilia elongation, and localizes to stereocilia tips in a MyoXVa-dependent manner.
(A) Scanning electron microscope images show that Eps8 KO mouse stereocilia are shorter than wild-type (WT) stereocilia, similar to shaker-2 and whirler mouse stereocilia.
(B) Eps8 (green) localizes to the tips of stereocilia in amounts scaled to length (see Figure S1), and also appears at the tips of microvilli in neighboring supporting cells. In shaker-2 stereocilia, Eps8 is undetectable above background, while in whirler mice Eps8 localizes to stereocilia tips, but in reduced amounts. Scale bars, 5 μm. See also Figure S1.
To better understand how Eps8 influences stereocilia lengths, we investigated the localization of Eps8 in hair cells. Immunofluorescence labeling of mouse and rat cochlear and vestibular sensory tissue shows localization of Eps8 at the tips of stereocilia (Figures 1b and S1). This tip-labeling persisted from the early stages of stereocilia elongation at birth through to adulthood, similar to MyoXVa [7] and whirlin [8]. Quantification of Eps8 immunofluorescence shows that Eps8 appears at stereocilia tips in concentrations proportional to stereocilia length (Figures 1b and S1), much like MyoXVa [7]. Exogenous expression of GFP-Eps8 or cherry-Eps8 (Figure S1) in transfected hair cells from four days old rats confirms the length proportional amounts of Eps8 at stereocilia tips. This also demonstrates that Eps8 is continuously targeted to stereocilia tips in concentrations that are actively maintained after stereocilia have already elongated and reached steady-state lengths. It is noteworthy that other than Eps8, espin-1, a MyoIIIa cargo [9, 10], is the only other actin-regulatory protein reported to be at the tips of stereocilia in amounts proportional to length. Interestingly, actin polymerization rates in stereocilia also show a similar relationship to stereocilia length [7, 11].
The striking similarity in both localization of MyoXVa [7], whirlin [12], and Eps8 labeling and the similar shortening of stereocilia when these proteins are absent or non-functional led us to hypothesize that MyoXVa transports Eps8 to stereocilia tips. Using the same antibody to Eps8 on shaker-2 and whirler mouse tissue, we found that Eps8 labeling was absent or reduced (respectively) at stereocilia tips in cochlear (Figure 1b) and vestibular (data not shown) hair cells. This indicates that Eps8 tip-localization is completely dependent on the presence of MyoXVa but can localize to the tips, albeit in smaller amounts, in the absence of whirlin. Interestingly, we found that MyoXVa labeling at the tips of whirler mouse stereocilia was reduced by ~50%, similarly to Eps8 (Figure S2). This result, along with the gradation in stereocilia length from whirler to Eps8 KO to shaker-2 mice, suggests that the scaffolding protein whirlin facilitates stereocilia elongation by stabilizing the MyoXVa:Eps8 complex at the tips of stereocilia.
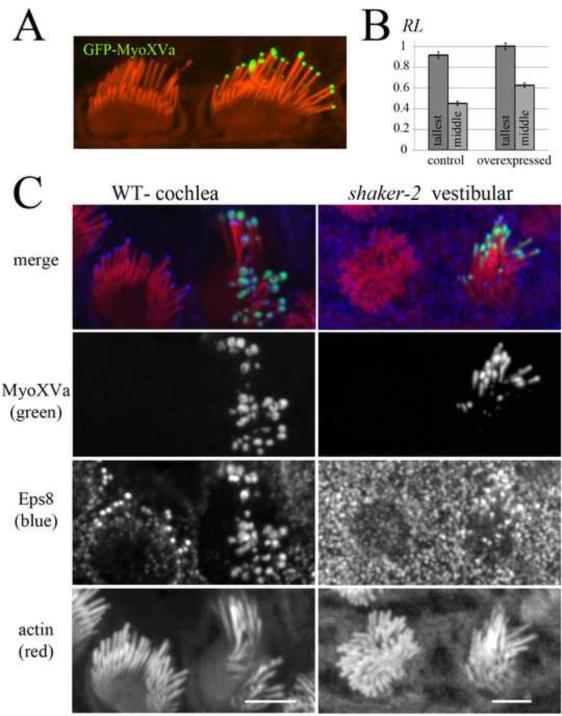
Further supporting the hypothesis that MyoXVa elongates stereocilia by transporting Eps8 to stereocilia tips, overexpression of GFP-MyoXVa in wild-type hair cells resulted in stereocilia elongation and concomitant enrichment of Eps8 at stereocilia tips (Figure 2). When compared to neighboring non-transfected control cells, hair cells transfected with MyoXVa showed slight elongation of the tallest row of stereocilia (9% increase, n=55, p<0.05) and even more elongation of the middle row of stereocilia (39%, n=48, p<0.0001). Thus, in hair cells overexpressing GFP-MyoXVa, the ratio of heights between the tallest and middle row of stereocilia significantly decreases (Figure 2a–b), demonstrating that the staircase patterning of the hair cell bundle is sensitive to the dynamic sorting of myosin concentrations in each row. These data suggest that not only is MyoXVa essential for the elongation of stereocilia during development [12], but MyoXVa can also dynamically regulate stereocilia length after elongation has terminated [13]. A previous study showed that there is no detectable increase in stereocilia length beyond wild-type lengths when MyoXVa is overexpressed in shaker-2 mutant vestibular hair cells [12]. However, shaker-2 stereocilia do not develop normally and have structural and mechanotransduction defects [14] caused by the lack of a functional myoXVa expression during their initial elongation. Furthermore, Shaker-2 hair cells express a motor-dead MyoXVa, which potentially has dominant-negative effects.
Figure 2.
Overexpression of MyoXVa elongates stereocilia and enhances Eps8 tip-localization.
(A) Overexpression of GFP-MyoXVa in a rat cochlear hair cell elongates stereocilia. The transfected cells showed elongation of both the top and middle row of stereocilia when compared to neighboring non-transfected control cells.
(B) The relative length (RL) of the tallest (dark gray) and middle (light gray) row of stereocilia in control versus GFP-MyoXVa transfected hair cells.
(C) A wild-type (WT) cochlear hair cell overexpressing GFP-MyoXVa (green) shows enhanced Eps8 labeling that colocalizes with MyoXVa. A shaker-2 vestibular hair cell expressing GFP-MyoXVa (green) shows Eps8 labeling at stereocilia tips, in contrast to neighboring non-transfected cells, which have undetectable levels of Eps8 labeling. Scale bars, 5 μm. See also Figure S2.
When comparing Eps8 labeling at the tips of the tallest row of stereocilia in GFP-MyoXVa transfected vs. non-transfected cells (Figure 2c), we found that Eps8 immunofluorescence is enhanced in transfected cells (transfected: relative intensity RI ± S.E.M. = 8.5 ± 1.8, n=13; non-transfected: 4.7 ± 0.9, n=20). We also observed that occasionally when MyoXVa is overexpressed, it appears in a “bulbous” pattern at stereocilia tips (Figure 2c) and when this happens, Eps8 also appears with the same pattern further suggesting that these two proteins strongly colocalize. Finally, when shaker-2 hair cells were transfected with GFP-MyoXVa, Eps8 tip-localization was rescued (Figure 2c).
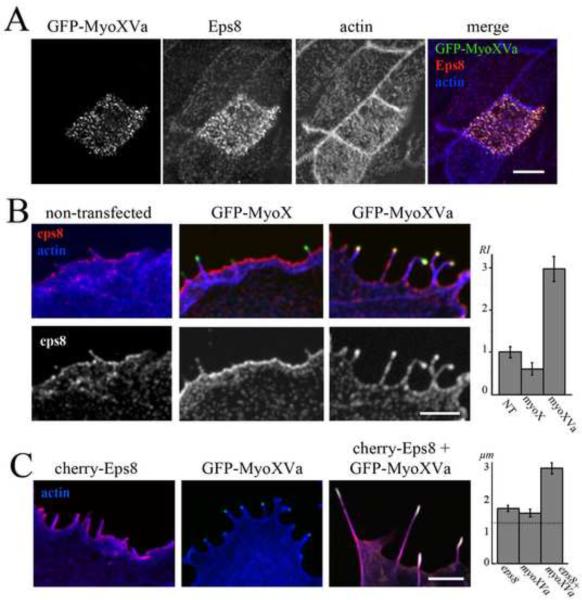
Heterologous expression of GFP-MyoXVa in organ of Corti and vestibular supporting cells increases the amount of Eps8 labeling at the tips of their microvilli (Figure 3a). Similarly, Eps8 often decorates the tips of filopodia in Cos-7 cells, but when cells were transfected with GFP-MyoXVa Eps8 labeling was increased (Figure 3b) from (RI ± S.E.M.) 1.1 ± 0.12 (nfilopodia=20, ncells=19) to 3.0 ± 0.30 (nfilopodia=25, ncells=14). In contrast, GFP-MyoX [15], an unconventional myosin implicated in actin protrusion elongation that also possesses a tail with a MyTH4-FERM domain, similar to the two MyTH4-FERM domains found in MyoXVa, failed to enhance Eps8 accumulation at filopodia tips (RI = 0.6 ± 0.14, nfilopodia=20, ncells=16) (Figure 3b). GFP-MyoIIIa, a hair cell myosin that elongates actin protrusions by transporting espin-1 to stereocilia tips [10], also failed to increase Eps8 amounts at filopodia tips (RI = 0.3 ± 0.15, nfilopodia=10, ncells=6). The enhanced accumulation of Eps8 at filopodia tips specifically in the presence of GFP-MyoXVa suggests that MyoXVa motor activity can maintain higher concentrations of Eps8 at the tips of actin protrusions. This should be particularly important in longer protrusions such as stereocilia, in which case passive diffusion over much longer distances could result in much lower concentrations of Eps8 available for binding at the tip, especially since Eps8 can also bind to the sides of actin filaments [16].
Figure 3.
MyoXVa enhances Eps8 accumulation and actin protrusion elongation activity in heterologous systems.
(A) A vestibular supporting cell expressing GFP-MyoXVa (green) shows enhanced labeling of Eps8 (red) in microvilli. Counterstained actin is shown in blue. Scale bar: 5 μm.
(B) In Cos-7 cells, the relative intensity (RI) of endogenous Eps8 labeling (red) at filopodia tips versus the cell cortex is significantly increased at filopodia tips in cells expressing GFP-MyoXVa (green) when compared to non-transfected or GFP-MyoX (green) transfected cells. Scale bar: 2 μm.
(C) Cos-7 cells overexpressing cherry-Eps8 (red) or MyoXVa (green) both show some elongation of filopodia, but when both are co-overexpressed, filopodia elongation is significantly enhanced. Scale bar: 2 μm. See also Figure S3.
Consistent with the hypothesis that Eps8 and MyoXVa cooperate to elongate stereocilia in hair cells, we found that co-overexpression of these two proteins in Cos-7 cells (Figure 3c) elongates actin protrusions in a cooperative manner. We found that Cos-7 cells overexpressing cherry-Eps8 and GFP-MyoXVa display actin protrusions almost twice as long (mean length ± S.E.M. = 3.0 ± 0.2 μm, nfilopodia=84, ncells=15) as cells overexpressing either cherry-Eps8 alone (1.7 ± 0.1 μm, nfilopodia=66, ncells=12) or GFP-MyoXVa alone (1.6 ± 0.1 μm, nfilopodia=81, ncells=16) (Figure 3c). In contrast, both GFP-MyoIIIa and GFPMyoX failed to further elongate filopodia when co-expressed with cherry-Eps8 (MyoIIIa: 1.4 ± 0.08 μm, nfilopodia=46, ncells=11; MyoX: 1.4 ± .05 μm, nfilopodia=53, ncells=19). This is consistent with the inability of these myosins to target Eps8 to filopodia tips, and is also consistent with the similar finding that GFP-MyoXVa failed to cooperate with the MyoIIIa cargo espin 1 in filopodia elongation [10]. It should be noted that our Eps8 overexpression experiments are within the context of endogenous Eps8 as well as Eps8 regulators, e.g. Abi-1, which could be influencing Eps8-overexpression mediated elongation activity via activation of Eps8 capping activity [17].
To further test the hypothesis that MyoXVa transports Eps8 to the tips of actin protrusions, we imaged live Cos-7 cells co-expressing cherry-MyoXVa and a GFP-Eps8 construct lacking the SH3, as well as actin capping and bundling activity (GFP-Eps8ΔSH3ΔCΔB). GFP-Eps8ΔSH3ΔCΔB, like full-length Eps8 (Figure 3b), colocalized with cherry-MyoXVa in transfected cells, and we observed co-migration of green and red fluorescent puncta in filopodia from the tip to the base of the filopodia at ~25 nm/s (Figure S3). Removing the actin-binding domains of Eps8 allowed us to rule out the possibility that co-migration of cherry-Eps8 and GFP-MyoXVa is due only to Eps8 binding to the treadmilling actin filaments. Removing the SH3 domain disrupts Eps8 binding to a number of potential interactors [18, 19]. Thus, the co-transport of these two proteins, even when Eps8 lacks actin-binding activity and its SH3 domain, further supports the hypothesis that MyoXVa transports Eps8.
The extreme tip localization of Eps8 led us to also question whether Eps8 is self-targeted to stereocilia tips by the activity of its actin capping domain which has a high affinity for F-actin barbed-ends [5, 17]. However, a GFP-Eps8 construct lacking its capping activity (Eps8ΔCapping [5]) or its SH3 domain (not shown) was still targeted to stereocilia tips in a length-dependent fashion (Figure S4), consistent with MyoXVa-dependent transport.
Notably, whirlin may also contribute to Eps8 targeting. Consistent with a model in which the N-terminus of Eps8 binding to whirlin enhances stereocilia tip targeting, a GFP-Eps8 construct lacking the first 280 amino acids in the Eps8 N-terminus (GFP-Eps8ΔPTBΔPR) targeted stereocilia tips much less efficiently (Figure S4). This result suggests that the first 280 amino acids of Eps8, which contains the phosphotyrosine binding domain (PTB) and the N-terminus proline rich domain (PR) of Eps8, is required for efficient targeting to stereocilia tips, perhaps via binding to whirlin or another protein.
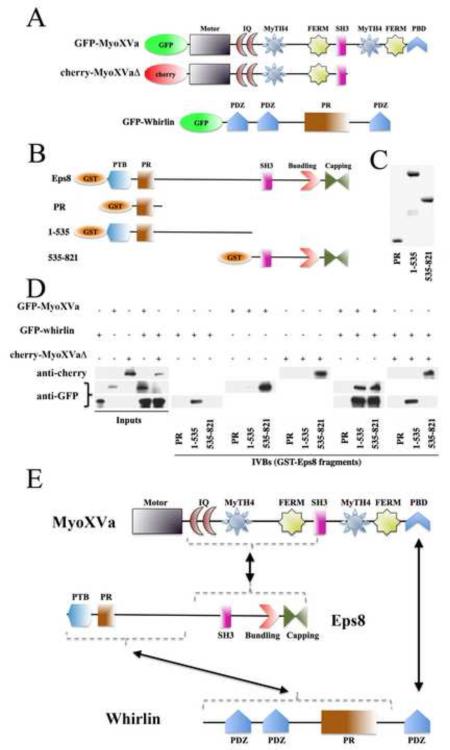
In order to determine whether interactions between Eps8, whirlin, and MyoXVa can occur in vitro, we performed GST-pull-down experiments on Eps8 fragments with GFP-MyoXVa and GFP-whirlin (Figure 4). We found that MyoXVa specifically binds to the C-terminus of Eps8, and perhaps weakly binds to the N-terminus. A yeast two-hybrid screen of human proteins revealed an interaction between the second MyTh4-FERM domain of the MyoXVa tail and Eps8 [20]. A MyoXVa construct with a tail truncated just past the SH3 domain was generated. This construct, which lacks the second MyTh4-FERM domain (cherry-MyoXVaΔ) as well as the C-terminus PDZ-binding domain (PBD) still interacted with Eps8 in our GST-pull-down experiment, suggesting that another region of the MyoXVa tail can interact with Eps8. We also found that the N-terminus of Eps8 interacts with whirlin. Consistent with previous results showing that MyoXVa needs its C-terminus PBD to interact with whirlin [12], we found that the co-expression of MyoXVa: whirlin, but not MyoXVaΔ:whirlin, allowed both the C- and N-termini of Eps8 to interact with the complex. These results suggest that whirlin and Eps8 interact with each other and with MyoXVa independently of one another, but that all three proteins can also form a complex (Figure 4e).
Figure 4.
MyoXVa, Eps8, and whirlin interact with one another in vitro.
(A) Cartoon diagrams showing the MyoXVa and whirlin constructs used in the in vitro binding assays (IVBs).
(B) Cartoon diagrams of the GST-tagged Eps8 fragments used in our binding assays.
(C) Ponceau staining of GST-tagged Eps8 fragments containing the proline-rich domain (PR), the first 535 amino acids (1–535), and amino acids 535–821.
(D) The proline-rich domain did not interact with GFP-MyoXVa, GFP-whirlin or cherry-MyoXVaΔ. The 1–535 fragment of Eps8 interacted with GFP-whirlin, while the 535–821 fragment interacted with both GFP-MyoXVa and cherry-MyoXVaΔ. When GFP-MyoXVa and GFP-whirlin were co-expressed, both halves (1–535 and 535–821) of Eps8 pulled down GFP-MyoXVa and GFP-whirlin. In contrast, when the cherry-MyoXVaΔ construct was co-expressed with GFP-whirlin, the 1–535 and 535–821 Eps8 fragments pulled down GFP-whirlin and cherry-MyoXVaΔ exclusively (respectively).
(E) Cartoon diagram showing the interacting regions of the MyoXVa:Eps8:whirlin tripartite complex. See also Figure S4.
Taken together, our results show that the MyoXVa:whirlin:Eps8 complex at stereocilia tips is essential for stereocilia elongation. The similarity between whirler and Eps8 KO mouse stereocilia (Figure 1) suggests that Eps8 is the molecule directly involved in whirlin's role in regulating stereocilia actin. Our results further suggest that one of the functions of whirlin in this process is to serve as a scaffold for the proper assembly, stability, and targeting of the MyoXVa:Eps8 complex. The identification of Eps8 as a key component of the MyoXVa:whirlin motor:cargo complex lends novel insight towards the physiopathology of the human deafness associated with mutations in MYO15A (DFNB3 [1]) and WHRL (DFNB31/USH2D [2, 21]) genes and furthermore explains the heretofore inexplicable effect of Myo15 and Whrl mutations on stereocilia length in the shaker-2 [3] and whirler [2] mice, respectively. However, it still remains to be elucidated how exactly Eps8 regulates actin protrusion length.
The observation that Eps8 capping activity is not necessary to restore proper intestinal and microvilli morphology in nematodes [22] supports the notion that it is not the capping activity of Eps8 that is primarily necessary for stereocilia elongation. This notwithstanding, the possibility still remains that Eps8 barbed-end capping may contribute to stereocilia elongation, perhaps through a “gated-capper” mechanism in which Eps8 protects the actin barbed ends from other capping proteins (e.g. twinfilin [23, 24]) but allows access for proteins (e.g. espin-1 [10] and/or other factors, such as perhaps Ena/VASP [25] or formins [26]) that would promote actin filament elongation. In any case, the potential for both capping and bundling activity in Eps8 raises intriguing possibilities for how Eps8 regulates stereocilia length and actin dynamics.
Experimental Procedures
Immunofluorescence labeling was performed as described previously [7]. Hair cell transfections were performed using a Helios Gene Gun (Biorad) on postnatal day 3–4 rat and mouse vestibular and cochlear tissue cultures. GFP-Eps8 was expressed for 18–24 hours, while GFP-MyoXVa was expressed for 48 hours before the tissues were fixed and processed for imaging. Cos-7 cells were transfected using GeneJuice (Novagen) for 48 hours. The relative amounts of fluorescence at the tips of stereocilia and filopodia were quantified by measuring the integrated pixel intensity of a 500-nm diameter circular area of interest, as described previously [7]. When comparing the relative lengths of stereocilia, we normalized to the tallest row of the longest stereocilia in the transfected hair cells. P-values were calculated using the Student's t-test in MATLAB (Mathworks).
Highlights.
Eps8 localizes to stereocilia tips and is required for elongation of stereocilia actin
Eps8 accumulates at stereocilia tips in proportion to length and myoXVa concentration
Eps8 interacts in vitro with both myoXVa and whirlin
MyoXVa, Eps8, and whirlin form a complex and cooperate to regulate stereocilia length
Supplementary Material
Acknowledgements
We would like to thank Nina Offenhauser for discussion, Charlotte Blanche Ekalle Soppo for technical assistance, and Agnieszka Rzadzinska for the early MyoXVa hair cell transfections. This work was supported by NIH intramural research fund Z01-DC000002-22 (B.K.); and by grants from the Associazione Italiana per la Ricerca sul Cancro, The Italian Ministries of Education, and the International Association for Cancer Research (A.D., G.S., and P.P.D.F.). H.L. current address is Department of Otolaryngology-Head and Neck Surgery, Massachusetts Eye and Ear Infirmary.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang A, Liang Y, Fridell RA, Probst FJ, Wilcox ER, Touchman JW, Morton CC, Morell RJ, Noben-Trauth K, Camper SA, et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3. Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]
- 2.Mburu P, Mustapha M, Varela A, Weil D, El-Amraoui A, Holme RH, Rump A, Hardisty RE, Blanchard S, Coimbra RS, et al. Defects in whirlin, a PDZ domain molecule involved in stereocilia elongation, cause deafness in the whirler mouse and families with DFNB31. Nat Genet. 2003;34:421–428. doi: 10.1038/ng1208. [DOI] [PubMed] [Google Scholar]
- 3.Probst FJ, Fridell RA, Raphael Y, Saunders TL, Wang A, Liang Y, Morell RJ, Touchman JW, Lyons RH, Noben-Trauth K, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene. Science. 1998;280:1444. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 4.Mustapha M, Beyer LA, Izumikawa M, Swiderski DL, Dolan DF, Raphael Y, Camper SA. Whirler mutant hair cells have less severe pathology than shaker 2 or double mutants. J Assoc Res Otolaryngol. 2007;8:329–337. doi: 10.1007/s10162-007-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, et al. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol. 2010;8:e1000387. doi: 10.1371/journal.pbio.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D'Angelo E, Frassoni C, Amadeo A, Tocchetti A, et al. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Rzadzinska AK, Schneider ME, Davies C, Riordan GP, Kachar B. An actin molecular treadmill and myosins maintain stereocilia functional architecture and self-renewal. J Cell Biol. 2004;164:887–897. doi: 10.1083/jcb.200310055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mburu P, Kikkawa Y, Townsend S, Romero R, Yonekawa H, Brown SDM. Whirlin complexes with p55 at the stereocilia tip during hair cell development. PNAS. 2006;103:10973–10978. doi: 10.1073/pnas.0600923103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider ME, Dose AC, Salles FT, Chang W, Erickson FL, Burnside B, Kachar B. A New Compartment at Stereocilia Tips Defined by Spatial and Temporal Patterns of Myosin IIIa Expression. J. Neurosci. 2006;26:10243–10252. doi: 10.1523/JNEUROSCI.2812-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salles FT, Merritt RC, Jr., Manor U, Dougherty GW, Sousa AD, Moore JE, Yengo CM, Dose AC, Kachar B. Myosin IIIa boosts elongation of stereocilia by transporting espin 1 to the plus ends of actin filaments. Nat Cell Biol. 2009;11:443–450. doi: 10.1038/ncb1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manor U, Kachar B. Dynamic length regulation of sensory stereocilia. Seminars in Cell & Developmental Biology. 2008;19:502–510. doi: 10.1016/j.semcdb.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, Friedman TB. Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol. 2005;7:148–156. doi: 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- 13.Lin HW, Schneider ME, Kachar B. When size matters: the dynamic regulation of stereocilia lengths. Current Opinion in Cell Biology. 2005;17:55–61. doi: 10.1016/j.ceb.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Stepanyan R, Frolenkov GI. Fast Adaptation and Ca2+ Sensitivity of the Mechanotransducer Require Myosin-XVa in Inner But Not Outer Cochlear Hair Cells. J. Neurosci. 2009;29:4023–4034. doi: 10.1523/JNEUROSCI.4566-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerber ML, Jacobs DT, Campagnola L, Dunn BD, Yin T, Sousa AD, Quintero OA, Cheney RE. A novel form of motility in filopodia revealed by imaging myosin-X at the single-molecule level. Curr Biol. 2009;19:967–973. doi: 10.1016/j.cub.2009.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naoz M, Manor U, Sakaguchi H, Kachar B, Gov NS. Protein Localization by Actin Treadmilling and Molecular Motors Regulates Stereocilia Shape and Treadmilling Rate. Biophysical Journal. 2008;95:5706–5718. doi: 10.1529/biophysj.108.143453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Disanza A, Carlier MF, Stradal TE, Didry D, Frittoli E, Confalonieri S, Croce A, Wehland J, Di Fiore PP, Scita G. Eps8 controls actin-based motility by capping the barbed ends of actin filaments. Nat Cell Biol. 2004;6:1180–1188. doi: 10.1038/ncb1199. [DOI] [PubMed] [Google Scholar]
- 18.Scita G, Nordstrom J, Carbone R, Tenca P, Giardina G, Gutkind S, Bjarnegard M, Betsholtz C, Di Fiore PP. EPS8 and E3B1 transduce signals from Ras to Rac. Nature. 1999;401:290–293. doi: 10.1038/45822. [DOI] [PubMed] [Google Scholar]
- 19.Lanzetti L, Rybin V, Malabarba MG, Christoforidis S, Scita G, Zerial M, Di Fiore PP. The Eps8 protein coordinates EGF receptor signalling through Rac and trafficking through Rab5. Nature. 2000;408:374–377. doi: 10.1038/35042605. [DOI] [PubMed] [Google Scholar]
- 20.Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–968. doi: 10.1016/j.cell.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 21.van Wijk E, van der Zwaag B, Peters T, Zimmermann U, te Brinke H, Kersten FFJ, Marker T, Aller E, Hoefsloot LH, Cremers CWRJ, et al. The DFNB31 gene product whirlin connects to the Usher protein network in the cochlea and retina by direct association with USH2A and VLGR1. Hum. Mol. Genet. 2006;15:751–765. doi: 10.1093/hmg/ddi490. [DOI] [PubMed] [Google Scholar]
- 22.Disanza A, Mantoani S, Hertzog M, Gerboth S, Frittoli E, Steffen A, Berhoerster K, Kreienkamp HJ, Milanesi F, Di Fiore PP, et al. Regulation of cell shape by Cdc42 is mediated by the synergic actin-bundling activity of the Eps8-IRSp53 complex. Nat Cell Biol. 2006;8:1337–1347. doi: 10.1038/ncb1502. [DOI] [PubMed] [Google Scholar]
- 23.Peng AW, Belyantseva IA, Hsu PD, Friedman TB, Heller S. Twinfilin 2 regulates actin filament lengths in cochlear stereocilia. J Neurosci. 2009;29:15083–15088. doi: 10.1523/JNEUROSCI.2782-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rzadzinska AK, Nevalainen EM, Prosser HM, Lappalainen P, Steel KP. MyosinVIIa interacts with Twinfilin-2 at the tips of mechanosensory stereocilia in the inner ear. PLoS One. 2009;4:e7097. doi: 10.1371/journal.pone.0007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wanner SJ, Miller JR. Regulation of otic vesicle and hair cell stereocilia morphogenesis by Ena/VASP-like (Evl) in Xenopus. J Cell Sci. 2007;120:2641–2651. doi: 10.1242/jcs.004556. [DOI] [PubMed] [Google Scholar]
- 26.Lynch ED, Lee MK, Morrow JE, Welcsh PL, Leon PE, King MC. Nonsyndromic deafness DFNA1 associated with mutation of a human homolog of the Drosophila gene diaphanous. Science. 1997;278:1315–1318. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.